Abstract
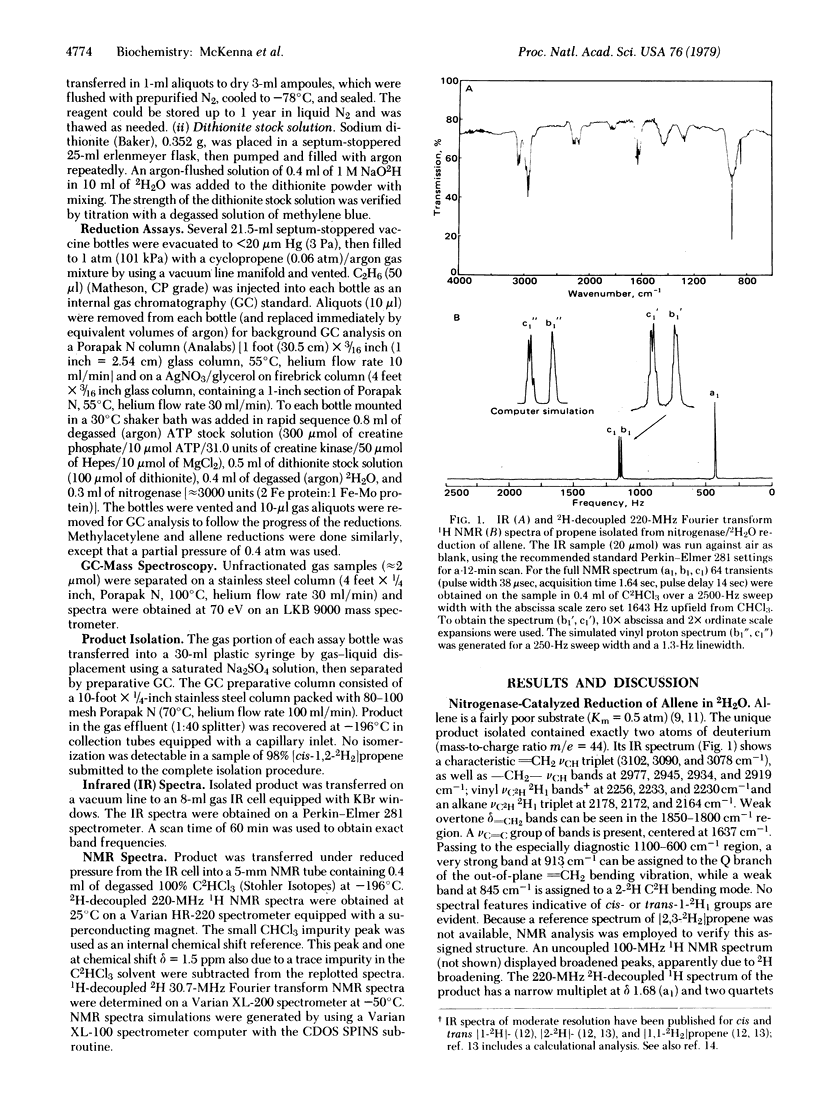
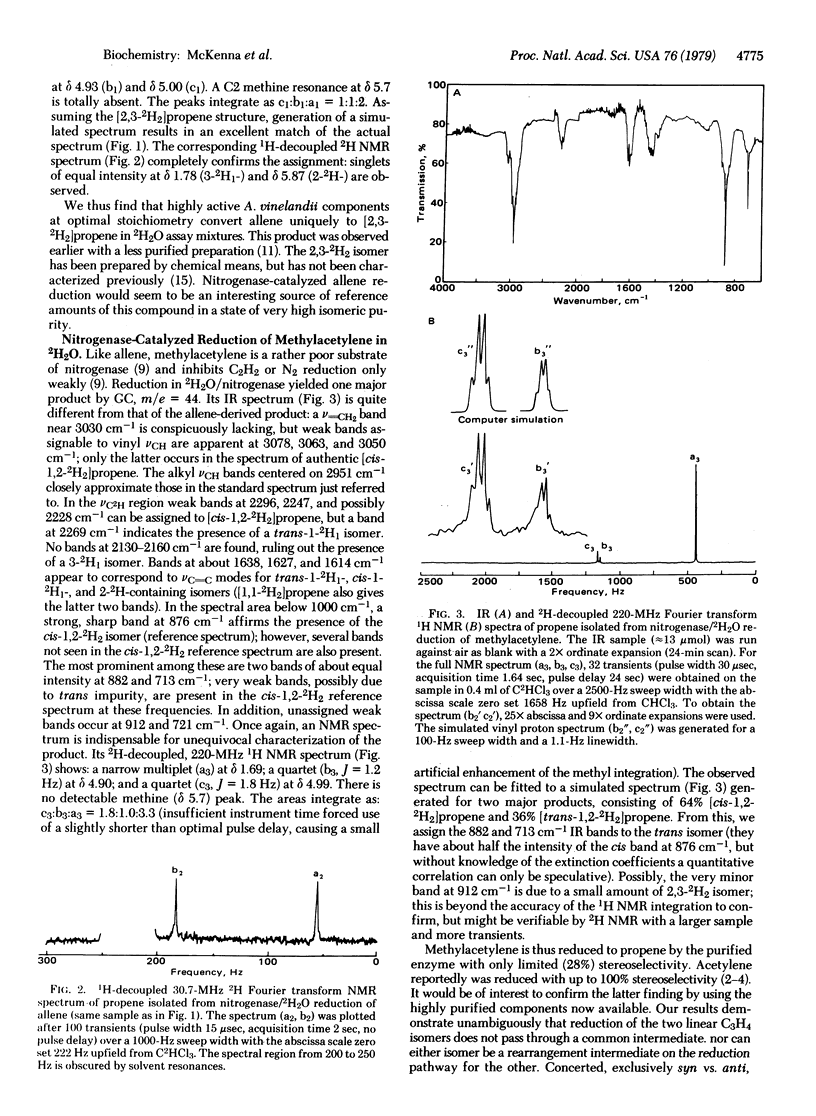
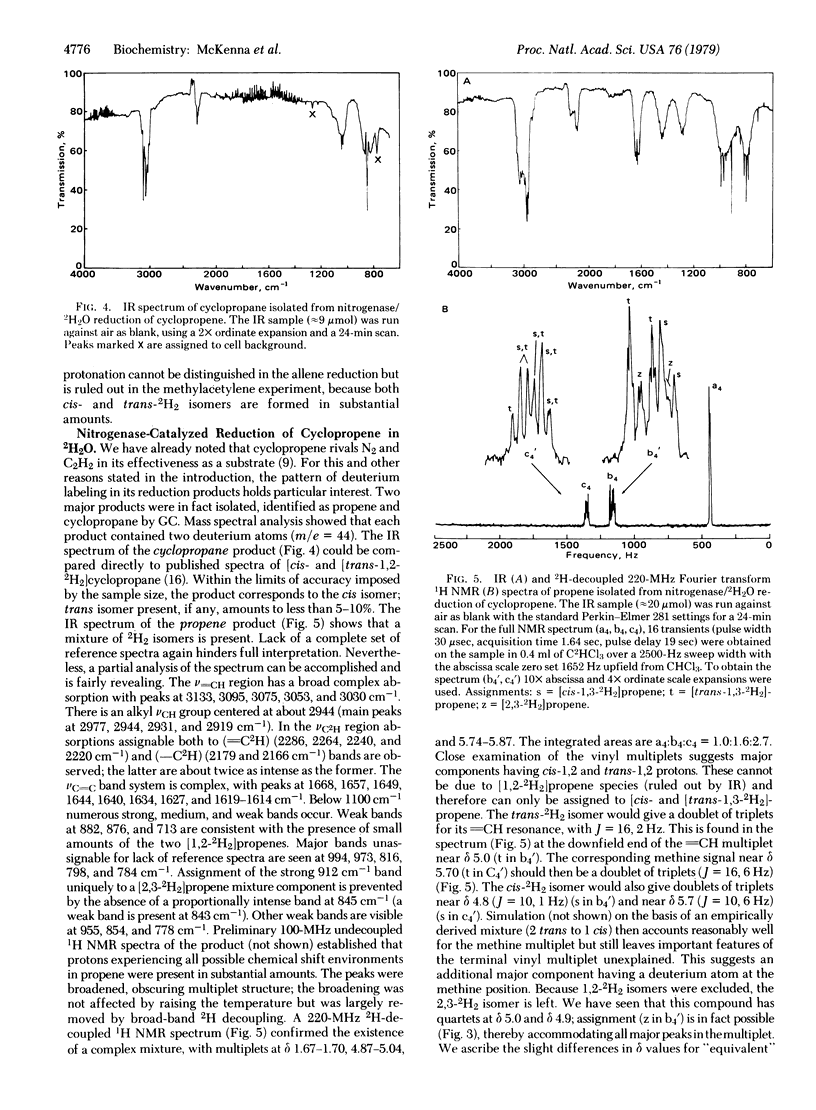
The stereochemistry of reductions catalyzed by nitrogenase in 2H2O has been investigated by using allene, methylacetylene, and cyclopropene as substrates. Deuterium labeling patterns in the reduction products were determined by mass spectroscopy, infrared spectroscopy, 2H-decoupled 220-MHz 1H NMR, and 1H-decoupled 30.7-MHz 2H NMR. Reduction of allene gave pure [2,3-2H2]propene. Reduction of methyl acetylene gave a 1.8:1.0 mixture of [cis- and [trans-1,2-2H2]propene. (Similar reduction of acetylene reportedly gave virtually all [cis-1,2-2H2]ethylene.) Reduction of cyclopropene gave [cis-1,2-2H2]cyclopropane and a mixture of [2H2]propenes. The major propene 2H2 isomers formed were [trans-1,3-2H2]-propene (approximately 2), [cis-1,3-2H2]propene (approximately 1) and [2,3-2H2]propene (approximately 1). Cyclopropene appears to be unique as a nitrogenase substrate in that it simultaneously undergoes parallel reductions, one of which proceeds with high stereoselectivity while the other proceeds with low stereoselectivity. The weakly selective stereochemistry observed in these reductions is not consistent with a completely concerted dual proton-dual electron transfer mechanism. The results provide a basis to probe stereochemical effects in nitrogenase and in biomimetic model systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dilworth M. J. Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim Biophys Acta. 1966 Oct 31;127(2):285–294. doi: 10.1016/0304-4165(66)90383-7. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. Comparisons and cross reactions of nitrogenase from Klebsiella pneumoniae, Azotobacter chroococcum and Bacillus polymyxa. Biochim Biophys Acta. 1969;191(3):527–540. doi: 10.1016/0005-2744(69)90346-5. [DOI] [PubMed] [Google Scholar]
- McKenna C. E., McKenna M. C., Higa M. T. Letter: Chemical probes of nitrogenase. 1. Cyclopropene. Nitrogenase-catalyzed reduction to propene and cyclopropane. J Am Chem Soc. 1976 Jul 21;98(15):4657–4659. doi: 10.1021/ja00431a059. [DOI] [PubMed] [Google Scholar]
- McKenna C. E., Nguyen H. T., Huang C. W., Smith S. A. A convenient apparatus and tracking dye for anaerobic analytical polyacrylamide-gel electrophoresis. Anal Biochem. 1977 Dec;83(2):337–345. doi: 10.1016/0003-2697(77)90042-2. [DOI] [PubMed] [Google Scholar]
- Stephens P. J., McKenna C. E., Smith B. E., Nguyen H. T., McKenna M. C., Thomson A. J., Devlin F., Jones J. B. Circular dichroism and magnetic circular dichroism of nitrogenase proteins. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2585–2589. doi: 10.1073/pnas.76.6.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiefel E. I. Proposed molecular mechanism for the action of molybedenum in enzymes: coupled proton and electron transfer. Proc Natl Acad Sci U S A. 1973 Apr;70(4):988–992. doi: 10.1073/pnas.70.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]


