Abstract
X-ray crystal structures for the soluble amino terminal and ligand binding domains of glutamate receptor ion channels, combined with a 3.6 Å resolution structure of the full length AMPA receptor GluA2 homotetramer, provide unique insights into the mechanisms of iGluR assembly and function. Increasingly sophisticated biochemical, computational and electrophysiological experiments are beginning to reveal the mechanism of action of partial agonists, and yield new models for the mechanism of action of allosteric modulators. Newly identified NMDA receptor ligands acting at novel sites offer hope for development of subtype selective modulators. Many issues remain unsolved, including the role of the ATD in AMPA receptor signaling, and the mechanisms by which auxiliary proteins regulate receptor activity. The structural basis for ion permeation and ion channel block also remain areas of uncertainty, and despite substantial progress, molecular dynamics simulations have yet to reveal how binding of glutamate opens the ion channel pore.
Keywords: Synaptic Transmission, AMPA Receptors, Kainate Receptors, NMDA Receptors, Crystallography
INTRODUCTION
Glutamate receptor ion channels (iGluRs) are abundantly expressed in the postsynaptic density at the large majority of excitatory synapses in the brain of vertebrates, where they mediate chemical neurotransmission (1–4). Genetic and post-translational controls generate iGluR subtypes with diverse kinetics, calcium ion permeability, and sensitivity to voltage-dependent ion channel block by extracellular magnesium and cytoplasmic polyamines (5–7). In combination, these properties shape the ion flux and post synaptic depolarization triggered by release of glutamate from nerve terminals, generating a signaling system which can mediate rapid information transfer on the ms time scale, but in addition control excitability over the much longer time scales necessary for regulation of synaptic plasticity (8–10). There is substantial evidence for the presence of iGluRs on nerve terminals (11–13), but although their effects on neurotransmitter release are well documented, presynaptic iGluRs are functionally less well characterized due to the difficulty of recording from these small structures.
During development of the CNS, the expression profile and extent of mRNA editing of individual iGluR genes is tightly controlled in a cell-specific manner, such that in the embryonic and adult CNS receptor subunit compositions are strikingly different. While the functional significance of this is not yet well understood, it is likely that, compared to the requirements for synaptic transmission in the adult CNS, receptors with different signaling properties and calcium permeability are required for the control of synapse formation and circuit development in the embryo. Also of note, although a neuron centric view of iGluR function is natural, given their major role in synaptic transmission, glial cells also express a variety of ligand gated ion channels (14–16). Glial iGluRs have been especially well studied in oligodendrocyte precursor cells present in the optic nerve and cerebellum (17), but much remains to be learned about the roles of iGluRs in other glial cell subtypes and brain regions.
THE THREE MAJOR GLUTAMATE RECEPTOR SUBTYPES
A major goal in the field of molecular neuroscience is to understand how the diverse functional activity of glutamate receptors and other ion channels arises from their structure. The first steps towards this began with the cloning of 18 individual mammalian iGluR cDNAs, followed by analysis of their genetic organization, leading to the discovery of subtype specific mRNA editing and alternative splicing (18). Coupled with this, amino acid sequence alignments, and analysis of ligand binding selectivity, led to the classification of iGluR subunits into three major families named AMPA, kainate and NMDA receptors, the preferred agonists for each subtype (19). In the subunit nomenclature proposed by IUPHAR in 2009 (20), AMPA receptors are formed by coassembly of GluA1-GluA4, kainate receptors by coassembly of GluK1-GluK5, and NMDA receptors by coassembly of GluN1 with GluN2A-GluN2D and GluN3A-GluN3B; with the exception of kainate receptors, for which GluK1-3 and GluK4-GluK5 are encoded by distinct gene families, for which the subunits were previously named GluR5-7 and KA1/KA2, the IUPHAR scheme nicely captures the underlying genetic substrates for iGluR assembly. A fourth iGluR gene family formed by GluD1 and GluD2 apparently does not generate glutamate or glycine activated ion channels, but is abundantly expressed in the cerebellum where its role in synaptic transmission is poorly understood.
The availability of cDNAs for iGluR genes triggered a large body of experiments using site directed mutagenesis for functional mapping. The cloning of iGluR cDNAs also made possible biochemical experiments which led to the overexpression, crystallization and X-ray diffraction analysis of an intact AMPA receptor (21), as well as structural studies on soluble domains from multiple iGluR subtypes (22–26). As more of these structures are solved it is now possible to begin to understand both the principles of iGluR gating, as well as the molecular mechanisms underlying their subtype specific properties. Central to this emerging structural biology of iGluRs, a variety of biophysical techniques in addition to crystallography are playing an increasingly important role, including analytical ultracentrifugation, calorimetry, computational chemistry, fluorescence and IR spectroscopy, NMR, and optogenetics, while functional studies on intact iGluRs using single channel recording, rapid perfusion with outside-out membrane patches, and whole-cell recording remain indispensable.
Subunit Structure
Amino acid sequence analysis revealed that the 18 iGluR genes have a unique conserved structural profile, that distinguishes them from all other neurotransmitter receptors and ion channels. The key features which identify vertebrate iGluR subunits include (i) a 500 residue long extracellular stretch of amino acids, starting at the amino terminus and preceding the first hydrophobic membrane spanning segment; (ii) an ion channel composed of a pore-loop helix flanked by three membrane spanning hydrophobic alpha helical segments, which includes a highly conserved SYTANLAAF motif in the M3 segment; (iii) interruption of the extracellular 300 residue ligand binding domain by insertion of the ion channel pore; (iv) a cytoplasmic carboxy-terminus of variable length, which includes sites for palmitoylation and phosphorylation; (v) separate amino terminal (ATD) and ligand binding (LBD) domains which have sequence and structural homology to bacterial periplasmic binding proteins, and which are connected to each other, and to the ion channel by short polypeptide linkers (Figure 1a).
Figure 1.
Domain organization and mechanism of iGluR activation and desensitization. (a) An iGluR subunit, with extracellular amino terminal (ATD) R1 R2 and ligand binding (LBD) S1 S2 domains interrupted by insertion of the ion channel pore; the highly conserved “SYTAN” motif present on the M3 TM helix is shaded in red. (b) Crystal structure of the full length GluA2 tetramer (3KG2) with each subunit colored differently. The extracellular and ion channel domains are arranged as layers, with subunit crossover taking place at the LBD layer for the B and D subunits. (c) Least squares superpositions of the AC and BD subunit pairs; distinct A/C and B/D subunit conformers result from subunit crossover. (d) Crystal structures of the GluA2 LBD dimer with a cartoon representation of the ion channel in resting (1FTO), glutamate activated (1FTJ) and desensitized (2I3V) states. A 21° rotation of the lower lobe on ligand binding increases separation of the S2 domains by ~5 Å compared to the apo structure, leading to ion channel opening, as shown by the position of glycine residue Cα atoms in the GT linker (red spheres). In the desensitized state, the dimer interface is broken due to a 14° rotation of domain S1, resulting in ~ 13Å separation of the upper lobes, depicted by blue spheres for the Ser741 Cα atoms on the top of helix J, which decouples LBD domain closure from channel opening.
BLAST searches which capitalize on this unique profile have led to identification of iGluRs in all classes of vertebrates, as well as in insects, nematodes and primitive eukaryotic organisms. The best characterized are a family of iGluRs in the nematode Caenorhabditis elegans which have many functional similarities with their vertebrate counterparts (27). For other iGluR related genes the ligand binding properties are more diverse. A protein from the rotifer Adineta vaga named AvGluR1 encodes a functional ion channel gated by glutamate, but also by serine and glutamine (28). Surprisingly, although the GYG pore loop motif of AvGluR1 predicts high potassium selectivity, both sodium and potassium ions are permeable (PNa/PK 0.3). A large family of additional iGluR related genes named ionotropic receptors (IRs), some of which function as chemosensory receptors, has been identified in the fruit fly Drosophila (29). Some IRs generate ligand-gated ion channels when expressed in Xenopus oocytes and can reconstitute odorant responses in olfactory receptor neurons (30), but these proteins have low amino acid sequence homology in the ligand binding domain, and are gated by ligands as diverse as phenylethylamine and propionic acid (30). Although some of the IR genes have overall structural homology with vertebrate iGluRs, other lack the amino terminal domain; also, the SYTANLAAF related motif in M3 that is present even in AvGluR1 and conventional drosophila iGluRs is strikingly absent in IRs. Likewise, numerous plant genes have been identified which are structurally related to vertebrate iGluRs (31); although their ligand binding properties are not well characterized, sequence analysis suggests that plant iGluRs also will recognize a diverse spectrum of ligands. Finally, several bacterial iGluR channels have been identified (32–34); these lack both the amino terminal domain and M4 segment compared to the characteristic subunit design conserved in vertebrate, nematode, insect and A. vaga iGluRs. Although these bacterial proteins have significance, in that they define the minimal elements required for assembly of a functional glutamate receptor, recent work has focused on unraveling the function of more complex eukaryotic and especially vertebrate iGluRs, for which interactions of the M4 segment with other TM domains is required for surface expression (35).
Domain Organization in Tetrameric iGluRs
After 10 years of structural studies on isolated iGluR ligand binding and amino terminal domains, the landmark full length structure of a GluA2 antagonist complex revealed how the unique sequence elements in iGluR subunits were arranged in a tetrameric assembly (21). As expected from sequence similarity with potassium channels, the ion channel has four fold rotational symmetry; consistent with work on the isolated ATDs and LBDs, the extracellular domains are arranged as a dimer of dimers (Figure 1b). However, unexpectedly, the GluA2 homotetramer is formed by pairs of conformationally distinct AC and BD subunits, and different subunit combinations form dimer pairs in the ATD and LBD layers, with the AB and CD subunits forming ATD dimers while the AD and BC subunits form LBD dimer pairs; as a result the tetramer has very complex geometry (Figure 1b, c). Each of the four ATDs has a clam shell like structure formed by upper (R1) and lower (R2) lobes, with a nearly identical conformation in each subunit; ATD dimers assemble via contacts mediated by both the upper R1 and lower R2 lobes, with a buried surface ≈ 1400 Å2 per subunit. These ATD dimer pairs form a tetramer via interactions of the lower lobes of subunits B and D, but with a much smaller buried surface ≈ 330 Å2. The ATD dimer pairs are tilted away from the overall axis of symmetry, such that subunits B and D lie proximal to the center of mass, while subunits A and C form the distal edges of the tetramer assembly. Within the LBD layer, dimer pairs like those crystallized for the isolated domains for numerous iGluRs, assemble via contacts mediated by the upper lobes, with a buried surface for each subunit of ≈ 900 Å2. Subunits A and C, which lie proximal to the center of mass, form the smaller dimer of dimers interface, buried surface ≈ 220 Å2. As a result, in contrast to previously published cartoon representations, the ATD and LBD layers in subunits B and D do not pack on top of each other in a linear arrangement, and instead there is a cross over between proximal and distal subunits in the ATD and LBD layers, generating a large cavity in the extracellular domain (Figure 1b). The peptide linkers which mediate these transitions between the ATD and LBD were shortened by deletion of six residues in the crystallized construct. As a result, it is possible that the ATD and LBD layers are even less closely packed with respect to each other than in the GluA2 crystal structure.
By contrast to the 2-fold symmetry of the LBD and ATD, the ion channel pore of GluA2 has 4-fold rotational symmetry, with the M4 segment of each subunit packed against M1 and M3 from an adjacent subunit, in a counterclockwise rotation when viewed from above. Lying parallel to the plane of the membrane a pre-M1 cuff helix wraps around the exterior of the ion channel assembly. A bundle crossing of the M3 helices forms a barrier to ion permeation, and above the SYTANLAAF motif the M3 helices of subunits A and C are extended by an extra turn compared to the B and D subunits. Three sets of polypeptide linkers, which have different conformations in the AC and BD subunit pairs, connect the M1, M3 and M4 transmembrane alpha helixes with the LBD; these linkers mediate the 2-fold to 4-fold symmetry transition between the ion channel and ligand binding domain, and likely form entrance pathways for permeant ions and pore blockers. There are large fenestrations in the ion channel assembly which allow lipids intimate access to the pore; these windows possibly form binding sites for iGluR auxiliary proteins, and are potential sites for the action of lipid soluble drugs. Within the ion channel, there was no electron density for the pore loop; likewise, on the cytoplasmic side of the membrane, the linkers between the TM domains, which form the turret structure in structurally related potassium channels, were also disordered. As a result the GluA2 structure provides no insight into the structure of the binding sites for permeant ions or polyamines which block Ca2+ permeable AMPA receptors.
While there are likely to be functionally important differences in the structure of other iGluR subtypes, cysteine mutant cross linking experiments reveal that the domain organization found in the GluA2 homotetramer is a general feature conserved across kainate and NMDA receptors (36–39), and that in the obligate heteromeric assemblies of GluK2/GluK5, and GluN1/GluN2, the ATD and LBD layers are assembled as pairs of heterodimers (37, 39, 40). Single molecule fluorescence counting techniques for NMDA receptors expressed in Xenopus oocytes indicate that when coexpressed GluN1, GluN2 and GluN3 assemble with a fixed stoichiometry which excludes triheteromeric assemblies, consistent with formation of GluN1:GluN2 and GluN1:GluN3 heterodimers (41). Asymmetry revealed by the GluA2 homotetramer has profound consequences for receptor activation, since the different conformations of the proximal and distal subunit pairs impacts their influence on ion channel gating. This has greatest significance in obligate heteromeric receptors, for which the GluN1 and GluK4/GluK5 subunits occupy proximal positions in the LBD layer of NMDA and kainate receptor tetramer assemblies, with GluN2, GluN3, and GluK1-GluK3 forming the distal subunits. In these receptors, due to differences in sequence, asymmetry extends to the ion channel, and although no structural data is available, homology models suggest that interactions between the TM domains of the GluN1 and GluN2 subunits underlie subtype specific Mg2+ block and Ca2+ permeation (42).
MODELS FOR ACTIVATION AND DESENSITIZATION
The availability of a full length iGluR structure provides for the first time a template that can be used to model the molecular events underlying activation and desensitization; obviously, experimental verification of such models is essential but likely to take substantial effort. Starting from the antagonist bound complex of the closed ion channel, the active and desensitized states have been modeled by two related approaches. The first was to perform rigid body manipulations, using glutamate bound crystal structures of the isolated LBD (Figure 1d) as a template for the activation process (21); to model desensitization a disulfide cross linked LBD structure (Figure 1d) was used as a surrogate for the desensitized state (43). In a related approach, similar models were used as targets for steered molecular dynamics of a GluA2 assembly in which the ATD was deleted to reduce computational overhead (44). The goal was to ask what resulting conformational changes would occur in the ion channel domain during activation and desensitization. Although both approaches have limitations, they provide preliminary structural insights, and suggest hypotheses that can be experimentally tested by site directed mutagenesis, prior to direct structural studies on other conformational states. Notably, because the distal lobes of the LBD are anchored on top of the membrane, they prevent domain closure from pulling the LBD downwards instead of opening the ion channel, and thus convert domain closure into rotational and translational movement of M3, which expands outwards, opening the ion channel pore. The process of desensitization, in which the upper lobes of the LBD dimers separate, partially reverses glutamate triggered movement of the M3 helices. Related to this, the Arg628 side chains in the M3-S2 linker move towards the lipid bilayer during desensitization, such that it is plausible that attenuation of desensitization observed for the GluA2 R628E mutation (45) could be due to electrostatic repulsion between the mutant side chain carboxyl group and negatively charged lipid phosphate groups (44). The structural changes occurring during desensitization in NMDA receptors are much less well understood, and paradoxically, Cys mutant cross links at positions which abolish desensitization in AMPA and kainate receptors have no effect on NMDA receptor desensitization (46, 47).
Both of these modeling studies dramatically highlight the unequal role of proximal and distal subunits in the activation mechanism. At identical reference positions the M3-S2 linker moves by ≈ 7Å following glutamate triggered domain closure for the distal subunits, but only 4 Å for the proximal subunits. It is intriguing that in heteromeric kainate and NMDA receptors, the GluK1-GluK3 and GluN2 subunits likely occupy distal positions in the LBD (21, 40). For NMDA receptors it is well known that glycine binding to the proximal GluN1 LBDs is not sufficient to activate ion channel gating, which requires in addition glutamate binding to the distal GluN2 subunits. However, for kainate receptors, selective binding of agonists to just the proximal GluK5 subunits produces partial activation, with desensitization when the distal GluK2 subunits in addition bind glutamate (48). Apparently different functional roles for kainate receptor proximal and distal subunits were reported previously (49), with selective binding of dysiherbaine to just the distal GluK1 subunit producing weakly desensitizing responses, with strong desensitization of ion channel activity when the proximal GluK5 subunits in addition bind glutamate. In combination, these experiments indicate that kainate receptor activation without substantial desensitization occurs irrespective of whether the proximal or distal subunits in diagonally apposed subunits in adjacent dimers bind agonist, while strong desensitization requires binding of agonists to both subunits in a LBD dimer assembly. Further experiments with additional LBD mutants are required to test whether separate activation of just the proximal or distal subunits in AMPA and kainate receptor LBD dimer assemblies produce equal extents of activation of ion channel gating, but clearly, for reasons that are not understood, NMDA receptors behave differently.
The most significant limitations of these computational experiments are that the templates used for modeling activation do not consider possible movements of the LBD dimers relative to each other, and that the MD study completely ignores conformational changes in the ATD tetramer assembly during desensitization. This is significant because models based on rigid body manipulation of the LBD predict that ATDs will undergo a separation during the process of desensitization (21), and that restricting this movement should limit the extent of desensitization. To date, only limited experiments have been performed to test this, and surprisingly the results showed that intermolecular ATD cross links inhibit receptor activation rather than receptor desensitization (36). In addition, the steered MD analysis of the glutamate bound active state fails to open the pore sufficiently to permit permeation of hydrated Na+ and K+ ions; in fact the change in radius, from 0.7 Å in the resting state, to 1.3 Å in the glutamate bound ‘activated state’, is much smaller than the experimentally measured open radius of 3.8 Å (50, 51) raising major questions about the nature of additional conformational changes that must occur in an intact receptor, and why the steered MD analysis failed to open the ion channel.
THE AMINO TERMINAL DOMAIN
Role of the ATD in iGluR Assembly
The large size, layered domain organization, assembly of both the ATD and LBD as a dimer of dimers, and subunit cross over in the extracellular domains of iGluRs revealed by the full length GluA2 structure, impacts our thinking about how this complex structure is assembled during the process of receptor biogenesis. Synthesis of individual subunits, which begins with the amino terminal domain and S1 segment of the LBD, requires insertion of the M1–M3 ion channel segments through the translocon and into the lipid bilayer, before growth of the LBD polypeptide chain continues with the S2 segment, followed by M4 and the cytoplasmic carboxy terminus. At what stage individual subunits begin to interact in the process of tetramer assembly is unknown; possibly the extracellular domains begin to assemble as dimers before subunit synthesis is complete; perhaps there are membrane protein chaperones which shield the TM segments of isolated full length subunits before they assemble as oligomers. Due to subunit cross over in the ATD and LBD layers, it is very unlikely that the tetramer is zipped up in a cooperative process involving all four subunits.
Convincing evidence for assembly of iGluRs as dimer intermediates comes from synchronized induction of protein expression combined with negative stain single particle EM studies for GluA2 (52). These experiments revealed that synthesis of GluA2 dimers precedes the formation of tetramers, and that in the dimers the ATD and TM segments are closely apposed while the LBDs are too far apart to interact; by contrast, for the LBD dimer stabilizing L483Y mutant, all three segments are closely apposed, but subsequent tetramer formation is strongly inhibited. The most reasonable interpretation is that subunit cross over observed in the full length GluA2 structure cannot occur when the LBD dimer pairs are unable to separate. Relevant to this, sedimentation analysis by AUC reveals that the isolated GluA2 ATDs form dimers at sub micromolar protein concentrations (53, 54). By contrast, the isolated LBDs do not measurably interact in solution at the highest protein concentrations compatible with AUC, such that we can only set lower limits, of 5–10 mM, on the Kd for LBD dimer and tetramer assembly (46, 55). Thus, while it seems very unlikely that the LBD drives assembly in vertebrate iGluRs, subunit cross over between the ATD and LBD layers requires that the LBD plays a critical role in tetramer formation. Of note, for prokaryotic iGluRs which lack the ATD, LBD dimer assembly occurs at micromolar protein concentrations (33), suggesting that in the absence of the ATD, tighter association of the remaining extracellular domain becomes important for ion channel assembly.
More direct evidence for a role of the ATD in AMPA and kainate receptor assembly comes from AUC experiments combined with electrophysiological studies, which reveal that mutations in the ATD dimer interface alter the balance of assembly of homomeric versus heteromeric AMPA and kainate receptor assemblies (40, 54). Surprisingly, AUC experiments reveal that the GluA2 ATD forms homomeric dimer assemblies at low nM protein concentrations, and that heterodimer assembly with GluA2 is only modestly favored over the formation of GluA2 homodimers for GluA1/GluA2, GluA3/GluA2 and GluA4/GluA2. Formation of heteromeric assemblies containing GluA2, assayed by changes in polyamine block under conditions where GluA1 is present in excess, is modestly facilitated by mutations which likely decrease the strength of GluA2 ATD homodimer assembly, suggesting that if AMPA receptors initially assemble as homodimers, tetramer assembly from pairs of heterodimers might require subunit exchange (54). These results raise important questions which remain unresolved. In native AMPA receptors are the subunits arranged as pairs of homodimers, or pairs of heterodimers; does subunit exchange occur between homodimers and heterodimers during biosynthesis; if so how is this accomplished?
By contrast the role of the ATD in the initial assembly of NMDA and kainate receptors is much easier to understand. For those iGluR subunits which are obligate heteromers, namely all NMDA receptors subunits, and the kainate receptor GluK4 and GluK5 subunits, the ATDs interact very weakly; however, the monomer-dimer Kd for heterodimer assembly is at least 1000-fold stronger for the GluN1/GluN2 and GluK2/GluK5 combinations (Figure 2a), providing a simple affinity based mechanism for assembly of heteromeric NMDA and kainate receptor assemblies (38, 40). Consistent with this, ATD Cys mutant cross linking experiments for full length NMDA and kainate receptors confirm formation of GluN1/GluN2 and GluK2/GluK5 heterodimers, while by contrast, forced GluN1 homodimer assembly abolishes formation of full length GluN1/GluN2 tetramers (37, 40, 56). Surprisingly, based on a complex series of cell biology experiments in which complementation by the isolated GluN1ATD was used to study coassembly of full length GluN2 with a GluN1ATD deletion mutant, a critical role for formation of GluN1 homodimers as a key assembly intermediate prior to formation of GluN1/GluN2 heteromers has been proposed (56). Perhaps consistent with this, single particle EM studies reveal a small population of GluN1 homodimers in solution, and two groups have reported GluN1 ATD homodimer crystal structures (38, 56). However, by contrast to AMPA and kainate receptors, for which the ATDs interact very strongly in solution, oligomerization of the GluN1 ATD was undetectable by AUC, apparently at conflict with the results of single particle EM analysis.
Figure 2.
ATD structure and function. (a) Superimposed size exclusion chromatography profiles for the isolated GluR6 and KA2 ATD domains injected separately (dashed lines) or as a mixture of the two proteins at equal concentrations (solid lines); the increase in dimer peak and corresponding decrease in monomer peak indicates formation of GluR6/KA2 ATD heterodimers; red data points show mass values of the eluted peaks estimated by multi angle light scattering. (b) Crystal structure of GluR6/KA2 ATD heterotetramer assembly; two GluR6/KA2 heterodimer pairs are arranged such that the dimer of dimers interface is mediated by the GluR6 subunit (green), while KA2 subunits (red) occupy the lateral edges of the tetramer. (c) Stereoview of the cleft between the R1 and R2 domains of the GluA2 ATD (3HSY) showing an omit map contoured at 7σ revealing a characteristic tetrahedral density in which a phosphate ion present in crystallization condition could be easily modeled along with a well ordered water molecule.
ATD Crystal Structures
Studies on the isolated ATDs have provided important insight into the processes of assembly and allosteric modulation, and provide much higher resolution structural information than the full length GluA2 structure, but many questions have emerged from this body of data. Crystal structures at resolutions as high as 1.4Å have been solved for ATD homodimer assemblies of GluA1 (57), GluA2 (53, 54, 58), GluA3 (59), GluK2 (60), GluK3 (61), GluK5 (61) and GluN1 (25, 56); for the Zn complex of a GluN2B ATD monomer (62); and for heterodimers of GluK2/GluK5 (40) and GluN1/GluN2B (38). For AMPA and kainate receptors the majority of these structures contain symmetrical assemblies in which the upper (R1) and lower (R2) lobes contribute nearly equally to dimer formation; for the GluA3 ATD, multiple homodimer structures were solved, and for some of these there is a modest separation of the lower lobes in the dimer assembly (59), while for the GluK5 homodimer the upper lobes have separated due to a 16° tilt, leaving only the R2 domains in contact (61). For the GluK2/GluK5 combination a dimer of heterodimers assembly that closely matches that found in the full length GluA2 tetramer structure has been crystallized (Figure 2b); for GluA2, GluK2 and GluK3 ATD homodimers, crystallographic symmetry operations generate a similar tetramer assembly (40, 53, 58, 60, 61). Since these ATD tetramers form in the absence of the LBD and ion channel, it removes concerns that the hyper extended LBD found in the full length GluA2 complex with the competitive antagonist ZK 200775 somehow drives the ATD into an alternative conformation, as was proposed on the basis of low resolution single particle EM analysis (63).
A remarkable feature of all of these ATD structures is that the R1R2 clam shell adopts a partially closed conformation compared to structurally related periplasmic proteins, which by contrast undergo a robust 50° closure on binding ligands. In the GluK5 ATD structure a glycosylation site at the entrance to the inter lobe cleft generates a steric wedge preventing further closure (61), but no such feature is present in the other ATD structures. Limited variations in the extent of ATD domain closure across iGluR subtypes, and in different crystal forms for GluA2, GluA3 and GluK2, as well as the results of normal mode analysis and fluorescence correlation spectroscopy (59, 64), suggest only small fluctuations around this partially closed conformation in AMPA and kainate receptors compared to the robust conformation changes observed in structurally related periplasmic proteins (59, 60, 64). Whether these small amplitude changes are functionally significant, and whether non NMDA receptor ATDs can undergo larger conformational changes is an important unresolved issue relevant to allosteric modulation. Related to this, is the issue of whether AMPA and kainate receptor ATDs can even bind allosteric modulators. Small molecules and ions present in crystallization solutions have been found bound in the inter lobe clefts for some GluA2 and GluK2 ATD structures (59, 60); the most definitive structural evidence is for GluA2 crystallized in the presence of 200 mM ammonium phosphate (PDB 3HSY) for which a phosphate ion and water molecules are well resolved in the 1.75 Å resolution electron density maps (59). The bound anion forms direct and solvent mediated interdomain contacts with two Arg side chains which are conserved in AMPA receptor ATDs (Figure 2c). Functional studies to test for modulation of GluA2 by multivalent anions have not been reported, but should give insight into the potential for allosteric modulation of GluA2. However, related to this, the extent of domain closure in the phosphate bound GluA2 ATD structure is similar to that for other PDB entries for which no phosphate or sulfate was present in the crystallization solutions; for example, a GluA2 ATD dimer was refined with good statistics to a resolution of 2.3 Å (PDB 3H5V), and although 368 water molecules were modeled, difference maps, especially within the clamshell-like cleft or subunit interfaces, did not show electron density for other bound molecules or ions (53). Thus, the AMPA receptor ATD structures solved to date seem to provide no evidence for a ligand induced conformational change, and although all the available evidence argues against this possibility, it remains a tantalizing hypothesis due to the impact it could have on AMPA receptor function.
Crystal structures for the GluN1 and GluN2B ATDs differ substantially from those of AMPA and kainate receptors in two important aspects: (i) the relative orientation of the upper and lower lobes has undergone a substantial 40–50° twist (56, 62); (ii) due to this different conformation, the dimer interface is mediated exclusively by the upper R1 lobe in the GluN1 homodimer (56), while in the GluN1/GluN2B heterodimer the interface is asymmetric and mediated by a combination of both R1:R1 and R1:R2 interactions (38). Surprisingly, the ATDs of both GluN1 and GluN2 also adopt a partially closed conformation, in which the cleft formed by apposition of the upper and lower lobes of the clamshell remains open to solvent. The GluN2B subunit was in addition crystallized as a monomer in the apo state and in a complex with Zn, which binds in the entrance to the cleft between the upper and lower lobes of the ATD clamshell without producing substantial conformation changes compared to the apo state (62). The most surprising finding from this series of ATD structures was the discovery, discussed in the following section, that GluN2B specific phenylethanolamine negative allosteric modulators of NMDA receptor activity bind at the interface between the GluN1 and GluN2B subunits in the heterodimer assembly, and not within the clam shell of the GluN2B subunit, as had been proposed on the basis of site directed mutagenesis and molecular modeling. Notably, even for this complex, the GluN2B subunit retains a stubbornly fixed partially closed conformation with only minor differences from the crystal structure of the monomeric apo state, such that it is now essential to obtain direct experimental evidence for the open state conformation.
Allosteric Modulation by the ATD
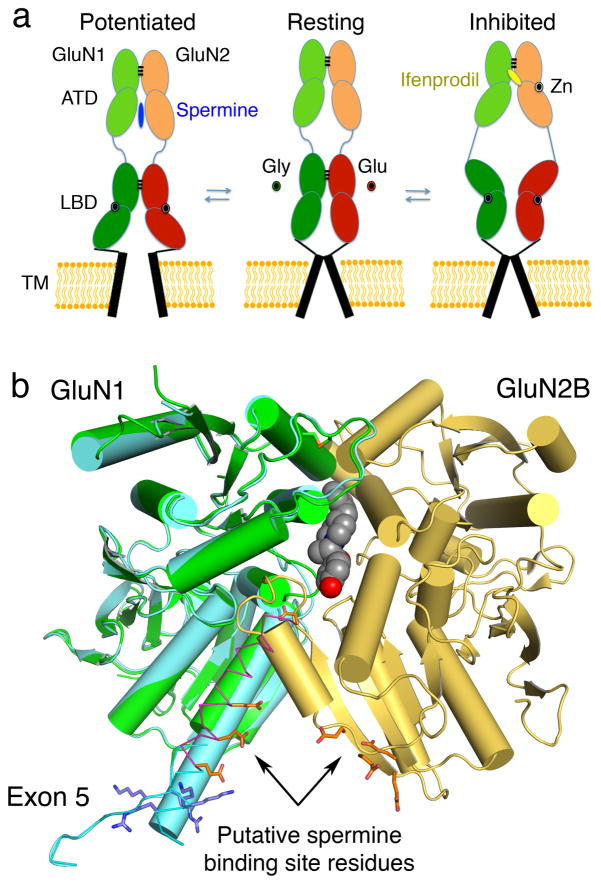
The GluN2A-GluN2D subunits, which bind glutamate, impart vastly different open probabilities, agonist potencies, deactivation time courses and single-channel conductance on NMDA receptor subtypes. It is believed that the GluN2 ATD domain plays a key role in many of these intrinsic receptor properties. Open probability is highest for GluN1/GluN2A, around 0.5, and 5-fold lower for GluN1/GluN2B (65), while GluN2C and GluN2D open with much lower open probabilities of 0.01 and 0.04 (66–68). To a large extent the GluN2 ATD, together with its short but highly divergent 16-residue ATD-LBD linker, determines these diverse channel open probabilities. Based on analysis of GluN2 chimeric receptors it was shown that exchange of the ATD, along with the ATD-LBD linker, shifts the open probability, response time course, and agonist potency in the direction of the subunit contributing the ATD, highlighting their role in regulating channel function (68, 69). NMDA receptor ATDs also mediate allosteric effects by directly binding endogenous ligands such as polyamines, Zn2+ and also synthetic ligands such as ifenprodil and related drugs. The first insights into this role came from studies which revealed that NMDA receptor ATDs are structurally related to periplasmic binding proteins (70, 71); that the GluN2A and GluN2B ATDs harbor nM and μM affinity binding site for Zn2+ respectively (71–74); and that ifenprodil binds to the GluN2B ATD (75). The high affinity and >100 fold selectivity of ifenprodil for GluN2B versus GluN2A, GluN2C or GluN2D has led to its extensive use as a lead compound for drug development and as a pharmacological tool to dissect NMDA receptor function (76). Zinc binding to the ATD also makes the channels more sensitive to proton inhibition, however the mechanism is not well understood (72, 73). In contrast to the negative allosteric action of Zn2+ and ifenprodil, polyamines like spermine also bind to the ATD, but act as positive allosteric modulators. This allosteric mechanism of spermine potentiation is specific to the combination of GluN2B with GluN1 subunits that lack exon 5 (77, 78). Surprisingly, recent work suggests that despite their opposite allosteric activity, both ifenprodil and spermine bind at the interface of the GluN1/GluN2 ATD assembly (38, 79).
A very appealing unified model of bidirectional regulation explains these effects (Figure 3a), and also the control of open probability by the ATDs (79, 80); this model depends in large part on elegant functional experiments that however have yet to be validated by structural studies. The need for the latter cannot be over stated given that the 1st crystal structure for an NMDA receptor ifenprodil complex (Figure 3b) revealed a totally unexpected mechanism of binding (38), while novel binding site residues identified by the crystal structure of the GluN2B Zn2+ complex (62) were missed by earlier studies based on ATD models derived from periplasmic proteins (74), which did not capture the characteristic twisted conformation of NMDA receptor ATDs (62, 81). In the new model of bidirectional regulation, GluN1/GluN2 NTD dimers alternate between open-cleft or ‘active’ and closed-cleft or ‘desensitized-like’ conformational states (69). The equilibrium between these states varies for individual GluN2 subunits, with the GluN2A ATD being open more frequently than GluN2C or GluN2D. The closed-cleft ATD conformation puts strain on the LBD dimer assemblies, causing then to separate, as occurs during the process of desensitization (46, 55, 80). Stabilization of the open-cleft conformation by polyamines, whereby GluN1 and GluN2B ATDs have their lower lobes extended and close to each other, would propagate little or no strain to the LBD dimers. By contrast, stabilization by zinc, ifenprodil, and its analogues, of a closed-cleft conformation, where the lower lobes have separated, introduces strain on the ATD-LBD linkers that leads to channel inhibition. Currently, it is unclear whether the GluN1 subunit ATD can alternate between open and closed cleft conformations, or whether this is limited to GluN2 subunits. Thus, although recent structures and biochemical evidence gives new insights into a central role of the ATDs in allosteric modulation of NMDA receptor function, a complete understanding of these complex mechanisms requires further exploration, and especially structures for different conformational states.
Figure 3.
Models for allosteric modulation by NMDA receptor ATD domains. (a) Schematic representation of a model for bidirectional allosteric regulation of NMDA receptors; spermine binding to the lower lobes of the ATD heterodimer interface stabilizes the open channel state, potentiating receptor function, whereas ifenprodil binding to the upper lobe dimer interface leads to channel inhibition. (b) Crystal structure of the GluN1b (exon5 variant) and GluN2B heterodimer complexed with ifenprodil (3QEL); a composite model generated after least-squares superpositions of GluN1 subunit homodimer crystal structures (3QEK) shows the position of Arg and Lys residues inserted due to exon 5 splicing (blue sticks), preceded by Glu and Asp residues which form the putative polyamine binding site.
LIGAND BINDING DOMAINS
More than 120 crystal structures of AMPA, kainate and NMDA receptor ligand binding domain (LBD) complexes with various agonists, partial agonists and antagonists provide a rich substrate for analysis of receptor function (23, 24, 82). They provide a structural basis for the mechanisms underlying both ligand selectivity, and the link between ligand induced conformational changes in the LBD domain and channel opening. Recent advances include identification of glycine and D-serine as ligands for the GluD2 LBD (83, 84); the first structural studies on NMDA receptor GluN2D agonist complexes (85); and new structures for AMPA receptor GluA3 and GluA4 and kainate receptor GluK3 LBDs (86–89).
Partial Agonist Activity in AMPA and Kainate Receptors
As described above the LBD domains are arranged as back-to-back dimer assemblies mediated by D1 domain contacts; on neurotransmitter binding the two lobes of LBD close to envelope the ligand resulting in separation of the D2 domains, which in turn pulls open the ion channel. Although a strong correlation between the degree of LBD cleft closure and agonist efficacy was observed early in the structural analysis of AMPA receptors (90–92), especially for willardiines, these partial agonists activate the same series of subconductance states as glutamate, but with different probabilities, raising questions about the link between domain closure and subconductance states that at present have no structural explanation (91, 93–95). Recently, it has been shown by NMR spectroscopy, fluorescence resonance energy transfer (FRET) measurements, and molecular dynamics (MD) simulations that the LBD domains can sample a range of conformational states on ligand binding, including but not limited to those observed in crystal structures (96–102). All-atom MD simulations using umbrella sampling to calculate the free energy landscape that governs the conformational change of the GluR2 LBD in the apo, agonist (Glu, AMPA, ACPA), partial agonist (kainate, thio-ATPA, 4-AHCP) and antagonist (ATPO, CNQX, DNQX) bound states revealed that the energy landscapes differed even within individual ligand classes, and that the trajectories traversed from apo to ligand-bound states vary (97, 101). Further calculations of LBD conformational distributions in the context of an intact GluA2 receptor revealed significant differences between the calculated thermal averages and crystal structures of the apo, AMPA and kainate bound GluA2 LBD, suggesting that crystal structures of isolated LBD complexes may not quantitatively account for the amount of conformational change transmitted to pore domain in an intact receptor. Additional evidence for a multistate equilibrium, similar to that predicted by MD simulations, has come from single-molecule FRET analysis of the GluA2 LBD bound to glutamate, which showed that it explores a range of conformations in solution, albeit on time scales much slower than required for receptor activation by glutamate (102).
Recent work has explored the idea that for channel activation the LBD adopts a closed cleft conformation that is the same for all ligands, and that the relative stability of this conformational state contributes to agonist efficacy. Measurements of LBD lobe orientation by NMR spectroscopy showed that the average solution conformations for willardiine partial agonist complexes was not strongly correlated with agonist efficacy and lies between that observed in different crystal forms (98). Using X-ray crystallography and NMR under reducing and oxidizing conditions, disulfide trapping experiments for a GluA2 LBD double cysteine A452C/S652C mutant, which locks the cleft in a closed conformation (103), reveals that the partial agonists 5-iodowillardine, kainate, even the antagonists CNQX and DNQX, can all promote nearly full cleft closure in oxidizing conditions (Figure 4a, b). Crystal structures with the partial agonists kainate, 5-iodowillardine and CNQX show that they are accommodated in the closed cleft conformation by rotation of side chains surrounding the ligand binding pocket, resulting in changes in cavity volume necessary to accommodate structurally diverse ligands (Figure 4c). Although the 5-iodowillardine, kainate and CNQX bound structures are 0.9°, 3° and 1.3° more open compared to the glutamate complex, two intra lobe hydrogen bonds characteristic of a closed cleft flipped conformation are observed for the kainate and 5-iodowillardiine, but not CNQX complex. However, for the kainate complex, cleft closure is insufficient to close the cavity, which remains open to bulk solvent. A caveat of these experiments is that the crosslinks were made in the isolated LBD domain, which is free from the constraints in an intact receptor, and that functional assays of changes in efficacy for the A452C/S652C mutant were not possible. Nonetheless, these results suggest that a closed cleft conformation is sampled by LBD when bound to partial agonists. However, although a closed cleft conformation may be attained by LBD when bound to partial agonists, MD simulations for AMPA and kainate receptors suggest this is not energetically favorable (101, 104), and thus at equilibrium agonist efficacy likely reflects both the frequency of sampling of the LBD closed state, and its stability, both of which are largest for glutamate and other full agonists. Experiments on intact receptors, for instance crystal structures of receptors in different conformational states complexed with all three classes of ligands, single molecule FRET measurements, or long duration MD simulations on intact receptors will likely be necessary to fully understand the seemingly complex mechanisms of partial agonism and ion channel substate activity.
Figure 4.
Partial agonists induce nearly full LBD closure. (a) Plot of mean domain closure for the GluA2 LBD for either WT or disulfide trapping mutants in complex with glutamate, kainate, 5-iodowillardine and CNQX, measured with respect to the apo LBD crystal structure (1FTO); the “error” bars indicate minimum and maximum domain closure values in individual subunits. (b) Least squared superimposition of GluA2 LBD disulfide trapping mutant in complex with CNQX, crystallized in either oxidizing (yellow 3T9U) or reducing conditions (dark brown 3T9V), showing an average cleft closure of ~17° in the oxidizing condition. (c) Stereoview of the oxidized state ligand binding cavities for the glutamate (green 3T93), kainate (cyan 3T9H), 5-iodowillardine (red 3T96) and CNQX (yellow 3T9U) complexes; note that despite similar domain closure the cavity sizes differ to accommodate diverse ligand molecules, and that in the kainate complex the cavity is continuous with bulk solvent; sticks show the location of the A452C and S652C mutant disulfide bond that traps the closed cleft conformation.
Partial agonism in NMDA receptors
NMDA receptors are obligate heteromeric coincidence detectors requiring binding of both glycine and glutamate to activate the ion channel. Unlike non-NMDA receptors, where binding of neurotransmitter to less than four subunits can trigger channel opening, albeit to lower sub-conductance states, NMDA receptor gating requires that all four-ligand binding sites be occupied, following which the channel is opened in a concerted fashion resulting in a uniform unitary conductance level (105). It has been shown that both full and partial agonists for GluN1 and GluN2 subunits elicit single channel currents with similar amplitudes consistent with a tight functional coupling between subunits in the heterodimer assembly. However, the open probability for partial agonists is lower due to longer closures and shorter openings (106, 107). An LRET based investigation, to measure conformational changes in GluN1/GluN2A LBD domains of full-length NMDA receptors, revealed no significant differences in cleft closure of the GluN1 subunit for full and partial agonists, consistent with the single conformation observed in agonist and partial agonist crystal structures for the isolated GluN1 LBD (108). However, a graded cleft closure for the GluN2A subunit was observed by LRET for partial agonists (109). So, again the question arises as to whether full-cleft closure of GluN2 subunits can be achieved with partial agonists, as might be expected for a concerted mechanism of channel opening. Related to this, locking the GluN2A LBD in a closed cleft conformation using engineered disulfide bonds spanning the cleft increases open probability, suggesting that glutamate may not be a full agonist (110). A caveat in these experiments could be that a non-natural LBD conformation due to disulfide cross-linking mediates these effects. However, at present there are no crystal structures of either GluN2 subunits with partial agonists, or cross linked closed cleft mutants like those for AMPA receptors.
NOVEL ALLOSTERIC MODULATORS
Structural studies have given great insight into the mechanism of action and location of binding sites for iGluR allosteric modulators. In addition to the binding of ifenprodil and zinc to NMDA receptor ATD dimers, a large body of crystallographic work reveals that the LBD dimers of AMPA receptors bind allosteric drugs which either attenuate desensitization or slow deactivation, while kainate receptor LBD dimers bind modulatory ions. For AMPA receptors more than 20 crystals structures have been solved for GluA2 and GluA3 complexes with a chemically diverse range of novel allosteric modulators, including ligands which differentiate flip and flop splice isoforms (55, 111–118). All of these ligands bind to a series of five overlapping hydrophobic and hydrophilic sites at the base of the LBD dimer assembly, but the stoichiometry varies from 1:1 or 2:1 drug molecules per dimer, depending on the ligand structure and binding sites targeted. These allosteric binding sites also form the structural determinants of deactivation, i.e. cleft closure and movement of the hinge regions, and in addition overlap with those of desensitization, i.e. subunit movements around the LBD dimer interface. Although many of these ligands bind to multiple sites in these regions, some degree of selectivity is apparent in crystallographic and functional studies (111, 117). Although this work has generated great anticipation for new classes of drug, clinical trials have met with limited success (119). No similar drugs have been reported for kainate receptors, although their disclosure likely remains only a matter of time. However, crystal structures for LBD dimer assemblies of GluK1, GluK2 and GluD2 reveal novel binding sites for Na+, Ca2+ and Cl− ions (84, 120–123), which act as counter charges at polar sites on the dimer interface, thereby increasing LBD dimer stability and reducing desensitization (124).
For NMDA receptors, although structural details remain vague, recent work has identified multiple classes of novel subtype specific allosteric ligands which potentiate or inhibit activity. In addition to ifenprodil and polyamines, neurosteroids (125–127) have been identified as potential leads for GluN2A and GluN2B containing receptors. Recently, the quinazolin-4-one analogue QNZ46 and the dihydroquinolone-pyrazoline derivative DQP-1105 were identified as examples of two classes of noncompetitive inhibitors selective for GluN2C/D containing receptors (128, 129). Using QNZ46 as a representative of this class, site directed mutagenesis suggests that the putative binding site is close to the membrane proximal part of the LBD and the linkers to the TM domains (130). Related to this Cys scanning mutagenesis reveals that modification of LBD-TM linkers in NMDA receptors profoundly affects ion channel activity (131, 132). More recently, the tetrahydroisoquinoline ClQ has been identified as a subunit selective potentiator for GluN2C/D (133). However, its mechanism of action seems to be more complex, requiring both the ATD-LBD linker and residue Thr592 in the M3 TM helix for potentiation. Napthoic and phenanthroic acid derivatives also display varied subunit selectivity and have both potentiating as well as inhibitory activity (134, 135). Although further development of these new subtype selective compounds will likely provide important pharmacological tools to analyze receptor function, and are potential lead compounds for drug development, structural investigations are required to fully elucidate their mechanism of action.
COMPUTATIONAL STUDIES
All atom molecular dynamics simulations for the isolated ligand binding domains of iGluRs are addressing increasingly fundamental questions, yielding substantial insight into the molecular mechanisms for ligand selectivity, partial agonist activity and the process of agonist triggered domain closure (101, 104, 136, 137). In a recent study, unbiased MD simulations were used to analyze cleft-closing mechanisms triggered by the spontaneous binding of glutamate to GluA2; this required 256 MD runs, each of 2 ns duration, for which six binding events were captured, only one of which lead to domain closure (138). Insights from this work include an analysis of side chain rearrangements during transitions between open, semi-open and closed-cleft LBD conformations, the existence of which had been proposed previously on the basis of hydrogen-deuterium exchange NMR experiments (100), and which were observed also in steered MD simulations with umbrella sampling (101). Cation-π interactions mediated by Tyr450, a highly conserved residue in iGluR LBDs, help to steer glutamate into the cleft during the initial docking process prior to stabilization of the closed-cleft conformation by a series of protein-ligand and interdomain contacts triggered by the coordinated movement of Glu705, Asp728 and Lys730 (138, 139). MD studies also give insights into the mechanism of modulation of kainate receptors by allosteric ions, revealing that the binding of Na+ ions lowers the barrier height and increases the well depth for Cl− ions which bind in the LBD dimer interface of kainate receptors (122). Water molecules are found in the ligand binding pocket of numerous iGluR LBD crystal structures, and appear to be trapped together with glutamate in the closed cleft conformation. MD studies reveal how these water molecules contribute to crystallographically observed ligand conformations (140), and show that the trapped waters are mobile and either rearrange on the ns time scale, or are replaced by water molecules that enter the binding site through transient water channels while glutamate remains bound (141).
SUMMARY POINTS.
Glutamate receptor ion channels are tetrameric assemblies with a unique architecture distinct from other ion channels. The amino terminal and ligand binding domains can be expressed as soluble proteins, yielding high resolution crystal structures, while a 3.6 Å resolution full length AMPA receptor structure reveals the overall architecture.
Biochemical techniques, including analytical ultracentrifugation, NMR, and numerous other forms of spectroscopy, together with increasingly sophisticated molecular dynamics calculations, are providing complimentary insights to X-ray crystal structures.
The information obtained by combining these results is beginning to give insight into the mechanisms for receptor activation, partial agonists, desensitization, and allosteric regulation.
FUTURE ISSUES.
There has been essentially no progress in understanding how an expanding family of glutamate receptor auxiliary membrane proteins modulates receptor function; given that most native iGluRs are likely to exist as complexes with auxiliary membrane proteins overcoming this limitation is essential for further progress.
Additional high resolution structures of novel ligand complexes and allosteric modulators, as well as lower resolution structures of full length AMPA, kainate and NMDA receptors in different conformational states, remain essential goals.
Sustained efforts in medicinal chemistry, which played a key role early in the discovery of iGluR receptor subtypes, will likely remain very important in the immediate future, as judged by the recent development using classical high throughput screening techniques of ligands which likely target novel structural motifs.
Microsecond and longer duration MD simulations will eventually give insight into the mechanisms of activation and desensitization, but the large size, and conformational freedom of iGluRs, which result from their unique architecture, will make these challenging computational targets.
iGluR Auxiliary subunits.
Ionotropic glutamate receptors in vivo interact with several auxiliary proteins that regulate their trafficking, turnover, subcellular localization, synaptic stabilization, and kinetics. Diverse families of auxiliary subunits have been identified for each receptor subtype including transmembrane AMPA receptor regulatory proteins (TARPS), Cystine-knot AMPAR modulating protein (CKAMP44), Cornichon homologs-2 and -3 (CNIH-2 and CNIH-3), Synapse differentially induced gene 1 (SynDIG1); neuropilin tolloid-like 1 (NETO1) and neuropilin tolloid-like 2 (NETO2) for kainate receptors; and NETO1 for NMDA receptors, respectively. Accumulating experimental evidence indicates that native iGluRs function as supra-molecular complexes with these various auxiliary subunits, which fine tunes receptor function; for instance while TARPs & Cornichons enhance AMPA receptor surface expression and increase receptor responses, CKAMP44 on the other hand has an opposite effect. The presence of auxiliary subunits has also helped to reconcile differences in channel kinetics observed between native and recombinant AMPA and kainate receptors, e.g. TARPs and NETO2 slow down both the deactivation and desensitization of AMPA and kainate receptors respectively. However, understanding the mechanisms of modulation of receptor function by these auxiliary subunits is still in its nascent stages and is likely to improve only with structural and biochemical information.
Acknowledgments
This work was supported by the intramural research program of NICHD, NIH, DHHS.
Key terms/definitions and acronyms
- mRNA EDITING
Enzymatic modification of mRNA, frequently adenosine to inosine, which changes the genetic code and controls Ca permeability in AMPA receptors
- IUPHAR
International Union of Basic & Clinical Pharmacology maintains receptor database and issues nomenclature guidelines
- Analytical ultracentrifugation (AUC)
A Powerful technique for characterizing the solution-state behavior of macromolecules by sedimentation velocity or sedimentation equilibrium
- ATD and LBD
Extracellular iGluR domains which can be separately expressed as soluble proteins and crystallized
- BLAST searches
Basic Local Alignment Search Tool used to query DNA and protein sequence data bases for bioinformatic analysis
- Buried surface
Surface area not accessible to solvent. An important tool for structural analysis of intermolecular contacts in protein assemblies
- Desensitization
A decrease in receptor response on prolonged exposure to agonist. In iGluRs mediated by separation of the upper lobes of LBD dimer
- Translocon
A large protein assembly which mediates translocation of polypeptide chains across membranes during biosynthesis of nascent membrane proteins
- Negative stain single particle EM
Proteins are embedded in an electron dense medium to generate a three-dimensional outline by combining two-dimensional images
- Normal mode analysis
Analysis of large-scale macromolecule motions carried out by studying harmonic oscillations around a local energy minimum
- Allosteric modulation
A mechanism for receptor regulation by the binding of ligands to regulatory sites distinct from the active site
- Conductance and subconductance
Refers to the measure of current flowing through ion channels in their fully and partially open states
- Open probability (Po)
The amount of time an ion channel is in the open state
- Partial agonist
Compounds that elicit less than maximal responses at full occupancy relative to full agonists
- Agonist efficacy
Refers to the magnitude of a receptor response following ligand binding. See also partial agonist
- LRET
Luminescence resonance energy transfer is a modification of conventional FRET using a luminescent chelate as donor
LITERATURE CITED
- 1.Petralia RS, Wang YX, Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol. 1994;349:85–110. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- 2.Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J Neurosci. 1996;16:4457–67. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29:436–52. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Winters C, Azzam R, Li X, Galbraith JA, et al. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci U S A. 2008;105:4453–8. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–96. doi: 10.1124/pr.109.002451. A comprehensive compilation of recent iGluR literature and a good reference resource. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 7.Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, et al. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–68. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postlethwaite M, Hennig MH, Steinert JR, Graham BP, Forsythe ID. Acceleration of AMPA receptor kinetics underlies temperature-dependent changes in synaptic strength at the rat calyx of Held. J Physiol. 2007;579:69–84. doi: 10.1113/jphysiol.2006.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–99. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 10.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–45. [PubMed] [Google Scholar]
- 11.Schmitz D, Mellor J, Frerking M, Nicoll RA. Presynaptic kainate receptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2001;98:11003–8. doi: 10.1073/pnas.191351498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF. Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J Neurosci. 2003;23:8013–9. doi: 10.1523/JNEUROSCI.23-22-08013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9:423–36. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- 14.Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, et al. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992;256:1566–70. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- 15.Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron. 1994;12:357–71. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 16.Lipton SA. NMDA receptors, glial cells, and clinical medicine. Neuron. 2006;50:9–11. doi: 10.1016/j.neuron.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Zonouzi M, Renzi M, Farrant M, Cull-Candy SG. Bidirectional plasticity of calcium-permeable AMPA receptors in oligodendrocyte lineage cells. Nat Neurosci. 2011;14:1430–8. doi: 10.1038/nn.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollmann M. Structure of ionotropic glutamate receptors. In: Jonas P, Monyer H, editors. Ionotropic glutamate receptors in the CNS. Berlin: Springer-Verlag; 1999. pp. 3–98. [Google Scholar]
- 19.Watkins JC, Jane DE. The glutamate story. Br J Pharmacol. 2006;147(Suppl 1):S100–8. doi: 10.1038/sj.bjp.0706444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–56. doi: 10.1038/nature08624. The only intact iGluR receptor crystal structure solved till date gave unprecedented insights into receptor architecture and the mechanism of receptor function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer ML. Structure and mechanism of glutamate receptor ion channel assembly, activation and modulation. Curr Opin Neurobiol. 2011;21:283–90. doi: 10.1016/j.conb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stawski P, Janovjak H, Trauner D. Pharmacology of ionotropic glutamate receptors: A structural perspective. Bioorg Med Chem. 2010;18:7759–72. doi: 10.1016/j.bmc.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Pohlsgaard J, Frydenvang K, Madsen U, Kastrup JS. Lessons from more than 80 structures of the GluA2 ligand-binding domain in complex with agonists, antagonists and allosteric modulators. Neuropharmacology. 2011;60:135–50. doi: 10.1016/j.neuropharm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa H. Structure and function of glutamate receptor amino terminal domains. J Physiol. 2011;590:63–72. doi: 10.1113/jphysiol.2011.213850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer ML. Emerging models of glutamate receptor ion channel structure and function. Structure. 2011;19:1370–80. doi: 10.1016/j.str.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brockie PJ, Maricq AV. Ionotropic glutamate receptors in Caenorhabditis elegans. Neurosignals. 2003;12:108–25. doi: 10.1159/000072159. [DOI] [PubMed] [Google Scholar]
- 28.Janovjak H, Sandoz G, Isacoff EY. A modern ionotropic glutamate receptor with a K(+) selectivity signature sequence. Nat Commun. 2011;2:232. doi: 10.1038/ncomms1231. [DOI] [PubMed] [Google Scholar]
- 29.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–62. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, Benton R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69:44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davenport R. Glutamate receptors in plants. Ann Bot. 2002;90:549–57. doi: 10.1093/aob/mcf228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen GQ, Cui C, Mayer ML, Gouaux E. Functional characterization of a potassium-selective prokaryotic glutamate receptor. Nature. 1999;402:817–21. doi: 10.1038/45568. [DOI] [PubMed] [Google Scholar]
- 33.Mayer ML, Olson R, Gouaux E. Mechanisms for ligand binding to GluR0 ion channels: crystal structures of the glutamate and serine complexes and a closed apo state. J Mol Biol. 2001;311:815–36. doi: 10.1006/jmbi.2001.4884. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Kang GB, Lim HH, Jin KS, Kim SH, et al. Crystal structure of the GluR0 ligand-binding core from Nostoc punctiforme in complex with L-glutamate: structural dissection of the ligand interaction and subunit interface. J Mol Biol. 2008;376:308–16. doi: 10.1016/j.jmb.2007.10.081. [DOI] [PubMed] [Google Scholar]
- 35.Salussolia CL, Corrales A, Talukder I, Kazi R, Akgul G, et al. Interaction of the M4 segment with other transmembrane segments is required for surface expression of mammalian alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. J Biol Chem. 2011;286:40205–18. doi: 10.1074/jbc.M111.268839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das U, Kumar J, Mayer ML, Plested AJ. Domain organization and function in GluK2 subtype kainate receptors. Proc Natl Acad Sci USA. 2010;107:8463–8. doi: 10.1073/pnas.1000838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CH, Gouaux E. Amino terminal domains of the NMDA receptor are organized as local heterodimers. PLoS One. 2011;6:1–6. doi: 10.1371/journal.pone.0019180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karakas E, Simorowski N, Furukawa H. Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature. 2011;475:249–53. doi: 10.1038/nature10180. Structure of a GluN1/GluN2B ATD heterodimer complexed with the allosteric modulator ifenprodil that was unexpectedly found to bind in the heterodimer interface. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salussolia CL, Prodromou ML, Borker P, Wollmuth LP. Arrangement of Subunits in Functional NMDA Receptors. J Neurosci. 2011;31:11295–304. doi: 10.1523/JNEUROSCI.5612-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar J, Schuck P, Mayer ML. Structure and assembly mechanism for heteromeric kainate receptors. Neuron. 2011;71:319–31. doi: 10.1016/j.neuron.2011.05.038. Molecular mechanisms and energetics of assembly of heteromeric kainate receptors by their amino terminal domains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulbrich MH, Isacoff EY. Rules of engagement for NMDA receptor subunits. Proc Natl Acad Sci U S A. 2008;105:14163–8. doi: 10.1073/pnas.0802075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Retchless BS, Gao W, Johnson JW. A single GluN2 subunit residue controls NMDA receptor channel properties via intersubunit interaction. Nat Neurosci. 2012;15:406–13. doi: 10.1038/nn.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell. 2006;127:85–97. doi: 10.1016/j.cell.2006.08.037. Structure of a crosslinked GluA2 LBD mutant suggests a mechanism for decoupling agonist binding and ion channel gating during desensitization. [DOI] [PubMed] [Google Scholar]
- 44.Dong H, Zhou HX. Atomistic mechanism for the activation and desensitization of an AMPA-subtype glutamate receptor. Nat Commun. 2011;2:354. doi: 10.1038/ncomms1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yelshansky MV, Sobolevsky AI, Jatzke C, Wollmuth LP. Block of AMPA receptor desensitization by a point mutation outside the ligand-binding domain. J Neurosci. 2004;24:4728–36. doi: 10.1523/JNEUROSCI.0757-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weston MC, Schuck P, Ghosal A, Rosenmund C, Mayer ML. Conformational restriction blocks glutamate receptor desensitization. Nature Structural and Molecular Biology. 2006;13:1120–7. doi: 10.1038/nsmb1178. [DOI] [PubMed] [Google Scholar]
- 47.Borschel WF, Murthy SE, Kasperek EM, Popescu GK. NMDA receptor activation requires remodelling of intersubunit contacts within ligand-binding heterodimers. Nat Commun. 2011;2:498. doi: 10.1038/ncomms1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher JL, Mott DD. Distinct functional roles of subunits within the heteromeric kainate receptor. J Neurosci. 2011;31:17113–22. doi: 10.1523/JNEUROSCI.3685-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swanson GT, Green T, Sakai R, Contractor A, Che W, et al. Differential activation of individual subunits in heteromeric kainate receptors. Neuron. 2002;34:589–98. doi: 10.1016/s0896-6273(02)00676-1. [DOI] [PubMed] [Google Scholar]
- 50.Burnashev N, Villarroel A, Sakmann B. Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J Physiol (Lond) 1996;496:165–73. doi: 10.1113/jphysiol.1996.sp021674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bähring R, Bowie D, Benveniste M, Mayer ML. Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J Physiol (Lond) 1997;502:575–89. doi: 10.1111/j.1469-7793.1997.575bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shanks NF, Maruo T, Farina AN, Ellisman MH, Nakagawa T. Contribution of the global subunit structure and stargazin on the maturation of AMPA receptors. J Neurosci. 2010;30:2728–40. doi: 10.1523/JNEUROSCI.5146-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin R, Singh SK, Gu S, Furukawa H, Sobolevsky AI, et al. Crystal structure and association behaviour of the GluR2 amino-terminal domain. EMBO J. 2009;28:1812–23. doi: 10.1038/emboj.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossmann M, Sukumaran M, Penn AC, Veprintsev DB, Babu MM, Greger IH. Subunit-selective N-terminal domain associations organize the formation of AMPA receptor heteromers. Embo J. 2011;30:959–71. doi: 10.1038/emboj.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–53. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- 56.Farina AN, Blain KY, Maruo T, Kwiatkowski W, Choe S, Nakagawa T. Separation of domain contacts is required for heterotetrameric assembly of functional NMDA receptors. J Neurosci. 2011;31:3565–79. doi: 10.1523/JNEUROSCI.6041-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao G, Zong Y, Gu S, Zhou J, Xu H, et al. Crystal structure of the glutamate receptor GluA1 amino-terminal domain. Biochem J. 2011;438:255–63. doi: 10.1042/BJ20110801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clayton A, Siebold C, Gilbert RJ, Sutton GC, Harlos K, et al. Crystal structure of the GluR2 amino-terminal domain provides insights into the architecture and assembly of ionotropic glutamate receptors. J Mol Biol. 2009;392:1125–32. doi: 10.1016/j.jmb.2009.07.082. [DOI] [PubMed] [Google Scholar]
- 59.Sukumaran M, Rossmann M, Shrivastava I, Dutta A, Bahar I, Greger IH. Dynamics and allosteric potential of the AMPA receptor N-terminal domain. Embo J. 2011;30:972–82. doi: 10.1038/emboj.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar J, Schuck P, Jin R, Mayer ML. The N-terminal domain of GluR6-subtype glutamate receptor ion channels. Nat Struct Mol Biol. 2009;16:631–8. doi: 10.1038/nsmb.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar J, Mayer ML. Crystal Structures of the Glutamate Receptor Ion Channel GluK3 and GluK5 Amino-Terminal Domains. J Mol Biol. 2010;404:680–96. doi: 10.1016/j.jmb.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karakas E, Simorowski N, Furukawa H. Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J. 2009;28:3910–20. doi: 10.1038/emboj.2009.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Midgett CR, Gill A, Madden DR. Domain architecture of a calcium-permeable AMPA receptor in a ligand-free conformation. Front Mol Neurosci. 2012;4:56. doi: 10.3389/fnmol.2011.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jensen MH, Sukumaran M, Johnson CM, Greger IH, Neuweiler H. Intrinsic motions in the N-terminal domain of an ionotropic glutamate receptor detected by fluorescence correlation spectroscopy. J Mol Biol. 2011;414:96–105. doi: 10.1016/j.jmb.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 65.Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signaling profiles. J Physiol. 2005 doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dravid SM, Prakash A, Traynelis SF. Activation of recombinant NR1/NR2C NMDA receptors. J Physiol. 2008;586:4425–39. doi: 10.1113/jphysiol.2008.158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wyllie DJ, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol. 1998;510:1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuan H, Hansen KB, Vance KM, Ogden KK, Traynelis SF. Control of NMDA receptor function by the NR2 subunit amino-terminal domain. J Neurosci. 2009;29:12045–58. doi: 10.1523/JNEUROSCI.1365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gielen M, Siegler Retchless B, Mony L, Johnson JW, Paoletti P. Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature. 2009;459:703–7. doi: 10.1038/nature07993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masuko T, Kashiwagi K, Kuno T, Nguyen ND, Pahk AJ, et al. A regulatory domain (R1–R2) in the amino terminus of the N-methyl-D-aspartate receptor: effects of spermine, protons, and ifenprodil, and structural similarity to bacterial leucine/isoleucine/valine binding protein. Mol Pharmacol. 1999;55:957–69. doi: 10.1124/mol.55.6.957. [DOI] [PubMed] [Google Scholar]
- 71.Paoletti P, Perin-Dureau F, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J. Molecular organization of a zinc binding n-terminal modulatory domain in a NMDA receptor subunit. Neuron. 2000;28:911–25. doi: 10.1016/s0896-6273(00)00163-x. [DOI] [PubMed] [Google Scholar]
- 72.Low CM, Zheng F, Lyuboslavsky P, Traynelis SF. Molecular determinants of coordinated proton and zinc inhibition of N-methyl-D-aspartate NR1/NR2A receptors. Proc Natl Acad Sci USA. 2000;97:11062–7. doi: 10.1073/pnas.180307497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi YB, Lipton SA. Identification and mechanism of action of two histidine residues underlying high-affinity Zn2+ inhibition of the NMDA receptor. Neuron. 1999;23:171–80. doi: 10.1016/s0896-6273(00)80763-1. [DOI] [PubMed] [Google Scholar]
- 74.Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci. 2005;25:308–17. doi: 10.1523/JNEUROSCI.3967-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perin-Dureau F, Rachline J, Neyton J, Paoletti P. Mapping the binding site of the neuroprotectant ifenprodil on NMDA receptors. J Neurosci. 2002;22:5955–65. doi: 10.1523/JNEUROSCI.22-14-05955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hansen KB, Furukawa H, Traynelis SF. Control of assembly and function of glutamate receptors by the amino-terminal domain. Mol Pharmacol. 2010;78:535–49. doi: 10.1124/mol.110.067157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams K. Mechanisms influencing the stimulatory effects of spermine at recombinant N-methyl-D-aspartate receptors. Mol Pharmacol. 1994;46:161–8. [PubMed] [Google Scholar]
- 78.Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268:873–6. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- 79.Mony L, Zhu S, Carvalho S, Paoletti P. Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines. EMBO J. 2011;30:3134–46. doi: 10.1038/emboj.2011.203. Molecular basis of polyamine allosteric activity via binding to a site at the GluN1/GluN2 ATD heterodimer interface. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gielen M, Le Goff A, Stroebel D, Johnson JW, Neyton J, Paoletti P. Structural rearrangements of NR1/NR2A NMDA receptors during allosteric inhibition. Neuron. 2008;57:80–93. doi: 10.1016/j.neuron.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stroebel D, Carvalho S, Paoletti P. Functional evidence for a twisted conformation of the NMDA receptor GluN2A subunit N-terminal domain. Neuropharmacology. 2011;60:151–8. doi: 10.1016/j.neuropharm.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 82.Mayer ML. Glutamate receptors at atomic resolution. Nature. 2006;440:456–62. doi: 10.1038/nature04709. [DOI] [PubMed] [Google Scholar]
- 83.Naur P, Hansen KB, Kristensen AS, Dravid SM, Pickering DS, et al. Ionotropic glutamate-like receptor delta2 binds D-serine and glycine. Proc Natl Acad Sci USA. 2007;104:14116–21. doi: 10.1073/pnas.0703718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hansen KB, Naur P, Kurtkaya NL, Kristensen AS, Gajhede M, et al. Modulation of the dimer interface at ionotropic glutamate-like receptor delta2 by D-serine and extracellular calcium. J Neurosci. 2009;29:907–17. doi: 10.1523/JNEUROSCI.4081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vance KM, Simorowski N, Traynelis SF, Furukawa H. Ligand-specific deactivation time course of GluN1/GluN2D NMDA receptors. Nat Commun. 2011;2:294. doi: 10.1038/ncomms1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kasper C, Frydenvang K, Naur P, Gajhede M, Pickering DS, Kastrup JS. Molecular mechanism of agonist recognition by the ligand-binding core of the ionotropic glutamate receptor 4. FEBS Lett. 2008;582:4089–94. doi: 10.1016/j.febslet.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 87.Gill A, Birdsey-Benson A, Jones BL, Henderson LP, Madden DR. Correlating AMPA receptor activation and cleft closure across subunits: crystal structures of the GluR4 ligand-binding domain in complex with full and partial agonists. Biochemistry. 2008;47:13831–41. doi: 10.1021/bi8013196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ahmed AH, Wang Q, Sondermann H, Oswald RE. Structure of the S1S2 glutamate binding domain of GLuR3. Proteins. 2009;75:628–37. doi: 10.1002/prot.22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Venskutonyte R, Frydenvang K, Gajhede M, Bunch L, Pickering DS, Kastrup JS. Binding site and interlobe interactions of the ionotropic glutamate receptor GluK3 ligand binding domain revealed by high resolution crystal structure in complex with (S)-glutamate. J Struct Biol. 2011;176:307–14. doi: 10.1016/j.jsb.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 90.Jin R, Horning M, Mayer ML, Gouaux E. Mechanism of activation and selectivity in a ligand-gated ion channel: structural and functional studies of GluR2 and quisqualate. Biochemistry. 2002;41:15635–43. doi: 10.1021/bi020583k. [DOI] [PubMed] [Google Scholar]
- 91.Jin R, Banke TG, Mayer ML, Traynelis SF, Gouaux E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nature Neurosci. 2003;6:803–10. doi: 10.1038/nn1091. [DOI] [PubMed] [Google Scholar]
- 92.Hogner A, Kastrup J, Jin R, Liljefors T, Mayer M, et al. Structural Basis for AMPA Receptor Activation and Ligand Selectivity: Crystal Structures of Five Agonist Complexes with the GluR2 Ligand-binding Core. J Mol Biol. 2002;322:93. doi: 10.1016/s0022-2836(02)00650-2. [DOI] [PubMed] [Google Scholar]
- 93.Poon K, Nowak LM, Oswald RE. Characterizing single-channel behavior of GluA3 receptors. Biophys J. 2010;99:1437–46. doi: 10.1016/j.bpj.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Poon K, Ahmed AH, Nowak LM, Oswald RE. Mechanisms of Modal Activation of GluA3 Receptors. Mol Pharmacol. 2011;80:49–59. doi: 10.1124/mol.111.071688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prieto ML, Wollmuth LP. Gating modes in AMPA receptors. J Neurosci. 2010;30:4449–59. doi: 10.1523/JNEUROSCI.5613-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gonzalez J, Rambhadran A, Du M, Jayaraman V. LRET investigations of conformational changes in the ligand binding domain of a functional AMPA receptor. Biochemistry. 2008;47:10027–32. doi: 10.1021/bi800690b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lau AY, Roux B. The free energy landscapes governing conformational changes in a glutamate receptor ligand-binding domain. Structure. 2007;15:1203–14. doi: 10.1016/j.str.2007.07.015. Free energy calculations of GluA2 LBD conformational states using umbrella sampling for a panel of ligands highlighting complex mechanisms of ligand binding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maltsev AS, Ahmed AH, Fenwick MK, Jane DE, Oswald RE. Mechanism of partial agonism at the GluR2 AMPA receptor: Measurements of lobe orientation in solution. Biochemistry. 2008;47:10600–10. doi: 10.1021/bi800843c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahmed AH, Loh AP, Jane DE, Oswald RE. Dynamics of the S1S2 glutamate binding domain of GluR2 measured using 19F NMR spectroscopy. J Biol Chem. 2007;282:12773–84. doi: 10.1074/jbc.M610077200. [DOI] [PubMed] [Google Scholar]
- 100.Fenwick MK, Oswald RE. On the mechanisms of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor binding to glutamate and kainate. J Biol Chem. 2010;285:12334–43. doi: 10.1074/jbc.M109.086371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lau AY, Roux B. The hidden energetics of ligand-binding and activation in a glutamate receptor. Nat Struct Mol Biol. 2011;18:283–7. doi: 10.1038/nsmb.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Landes CF, Rambhadran A, Taylor JN, Salatan F, Jayaraman V. Structural landscape of isolated agonist-binding domains from single AMPA receptors. Nat Chem Biol. 2011;7:168–73. doi: 10.1038/nchembio.523. Conformational dynamics of the isolated GluA2 LBD analyzed by single molecule FRET studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahmed AH, Wang S, Chuang HH, Oswald RE. Mechanism of AMPA receptor activation by partial agonists: disulfide trapping of closed lobe conformations. J Biol Chem. 2011;286:35257–66. doi: 10.1074/jbc.M111.269001. Crystallographic evidence for a nearly closed cleft conformation of the isolated GluA2 LBD when bound to partial agonists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Postila PA, Ylilauri M, Pentikainen OT. Full and Partial Agonism of Ionotropic Glutamate Receptors Indicated by Molecular Dynamics Simulations. J Chem Inf Model. 2011;51:1037–47. doi: 10.1021/ci2000055. [DOI] [PubMed] [Google Scholar]
- 105.Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat Neurosci. 2003;6:144–52. doi: 10.1038/nn1000. [DOI] [PubMed] [Google Scholar]
- 106.Kussius CL, Popescu AM, Popescu GK. Agonist-specific gating of NMDA receptors. Channels (Austin) 2010;4:78–82. doi: 10.4161/chan.4.2.10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kussius CL, Popescu GK. Kinetic basis of partial agonism at NMDA receptors. Nat Neurosci. 2009;12:1114–20. doi: 10.1038/nn.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Inanobe A, Furukawa H, Gouaux E. Mechanism of partial agonist action at the NR1 subunit of NMDA receptors. Neuron. 2005;47:71–84. doi: 10.1016/j.neuron.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 109.Rambhadran A, Gonzalez J, Jayaraman V. Conformational changes at the agonist binding domain of the N-methyl-D-aspartic acid receptor. J Biol Chem. 2011;286:16953–7. doi: 10.1074/jbc.M111.224576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kussius CL, Popescu GK. NMDA receptors with locked glutamate-binding clefts open with high efficacy. J Neurosci. 2010;30:12474–9. doi: 10.1523/JNEUROSCI.3337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jin R, Clark S, Weeks AM, Dudman JT, Gouaux E, Partin KM. Mechanism of positive allosteric modulators acting on AMPA receptors. J Neurosci. 2005;25:9027–36. doi: 10.1523/JNEUROSCI.2567-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaae BH, Harpsoe K, Kastrup JS, Sanz AC, Pickering DS, et al. Structural proof of a dimeric positive modulator bridging two identical AMPA receptor-binding sites. Chem Biol. 2007;14:1294–303. doi: 10.1016/j.chembiol.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 113.Ptak CP, Ahmed AH, Oswald RE. Probing the allosteric modulator binding site of GluR2 with thiazide derivatives. Biochemistry. 2009;48:8594–602. doi: 10.1021/bi901127s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahmed AH, Oswald RE. Piracetam defines a new binding site for allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors. J Med Chem. 2010;53:2197–203. doi: 10.1021/jm901905j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ahmed AH, Ptak CP, Oswald RE. Molecular mechanism of flop selectivity and subsite recognition for an AMPA receptor allosteric modulator: structures of GluA2 and GluA3 in complexes with PEPA. Biochemistry. 2010;49:2843–50. doi: 10.1021/bi1000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ward SE, Harries M, Aldegheri L, Austin NE, Ballantine S, et al. Integration of lead optimization with crystallography for a membrane-bound ion channel target: discovery of a new class of AMPA receptor positive allosteric modulators. J Med Chem. 2011;54:78–94. doi: 10.1021/jm100679e. [DOI] [PubMed] [Google Scholar]
- 117.Timm DM, Benveniste M, Weeks AM, Nisenbaum ES, Partin KM. Structural and Functional Analysis of Two New Positive Allosteric Modulators of GluA2 Desensitization and Deactivation. Mol Pharmacol. 2011 doi: 10.1124/mol.110.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krintel C, Frydenvang K, Olsen L, Kristensen MT, de Barrios O, et al. Thermodynamics and structural analysis of positive allosteric modulation of the ionotropic glutamate receptor GluA2. Biochem J. 2012;441:173–8. doi: 10.1042/BJ20111221. [DOI] [PubMed] [Google Scholar]
- 119.Ward SE, Bax BD, Harries M. Challenges for and current status of research into positive modulators of AMPA receptors. Br J Pharmacol. 2010;160:181–90. doi: 10.1111/j.1476-5381.2010.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Plested AJ, Mayer ML. Structure and mechanism of kainate receptor modulation by anions. Neuron. 2007;53:829–41. doi: 10.1016/j.neuron.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 121.Plested AJ, Vijayan R, Biggin PC, Mayer ML. Molecular basis of kainate receptor modulation by sodium. Neuron. 2008;58:720–35. doi: 10.1016/j.neuron.2008.04.001. This study identifies allosteric ion binding sites and the mechanism of kainate receptor modulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vijayan R, Plested AJ, Mayer ML, Biggin PC. Selectivity and cooperativity of modulatory ions in a neurotransmitter receptor. Biophys J. 2009;96:1751–60. doi: 10.1016/j.bpj.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nayeem N, Mayans O, Green T. Conformational flexibility of the ligand-binding domain dimer in kainate receptor gating and desensitization. J Neurosci. 2011;31:2916–24. doi: 10.1523/JNEUROSCI.4771-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chaudhry C, Plested AJ, Schuck P, Mayer ML. Energetics of glutamate receptor ligand binding domain dimer assembly are modulated by allosteric ions. Proc Natl Acad Sci U S A. 2009;106:12329–34. doi: 10.1073/pnas.0904175106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Malayev A, Gibbs TT, Farb DH. Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. Br J Pharmacol. 2002;135:901–9. doi: 10.1038/sj.bjp.0704543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kussius CL, Kaur N, Popescu GK. Pregnanolone sulfate promotes desensitization of activated NMDA receptors. J Neurosci. 2009;29:6819–27. doi: 10.1523/JNEUROSCI.0281-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kostakis E, Jang MK, Russek SJ, Gibbs TT, Farb DH. A steroid modulatory domain in NR2A collaborates with NR1 exon-5 to control NMDAR modulation by pregnenolone sulfate and protons. J Neurochem. 2011;119:486–96. doi: 10.1111/j.1471-4159.2011.07442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mosley CA, Acker TM, Hansen KB, Mullasseril P, Andersen KT, et al. Quinazolin-4-one derivatives: A novel class of noncompetitive NR2C/D subunit-selective N-methyl-D-aspartate receptor antagonists. J Med Chem. 2010;53:5476–90. doi: 10.1021/jm100027p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Acker TM, Yuan H, Hansen KB, Vance KM, Ogden KK, et al. Mechanism for noncompetitive inhibition by novel GluN2C/D N-methyl-D-aspartate receptor subunit-selective modulators. Mol Pharmacol. 2011;80:782–95. doi: 10.1124/mol.111.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hansen KB, Traynelis SF. Structural and mechanistic determinants of a novel site for noncompetitive inhibition of GluN2D-containing NMDA receptors. J Neurosci. 2011;31:3650–61. doi: 10.1523/JNEUROSCI.5565-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Talukder I, Borker P, Wollmuth LP. Specific sites within the ligand-binding domain and ion channel linkers modulate NMDA receptor gating. J Neurosci. 2010;30:11792–804. doi: 10.1523/JNEUROSCI.5382-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Talukder I, Wollmuth LP. Local constraints in either the GluN1 or GluN2 subunit equally impair NMDA receptor pore opening. J Gen Physiol. 2011;138:179–94. doi: 10.1085/jgp.201110623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mullasseril P, Hansen KB, Vance KM, Ogden KK, Yuan H, et al. A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nat Commun. 2010;1:90. doi: 10.1038/ncomms1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, et al. A novel family of negative and positive allosteric modulators of NMDA receptors. J Pharmacol Exp Ther. 2010;335:614–21. doi: 10.1124/jpet.110.174144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, et al. Structure-activity relationships for allosteric NMDA receptor inhibitors based on 2-naphthoic acid. Neuropharmacology. 2012;62:1730–6. doi: 10.1016/j.neuropharm.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, et al. Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors. J Neurosci. 2010;30:2741–54. doi: 10.1523/JNEUROSCI.5390-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frydenvang K, Lash LL, Naur P, Postila PA, Pickering DS, et al. Full domain closure of the ligand-binding core of the ionotropic glutamate receptor iGluR5 induced by the high affinity agonist dysiherbaine and the functional antagonist 8,9-dideoxyneodysiherbaine. J Biol Chem. 2009;284:14219–29. doi: 10.1074/jbc.M808547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Okada O, Odai K, Sugimoto T, Ito E. Molecular dynamics simulations for glutamate-binding and cleft-closing processes of the ligand-binding domain of GluR2. Biophys Chem. 2012;162:35–44. doi: 10.1016/j.bpc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 139.Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: Crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–81. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 140.Sahai MA, Biggin PC. Quantifying water-mediated protein-ligand interactions in a glutamate receptor: a DFT study. J Phys Chem B. 2011;115:7085–96. doi: 10.1021/jp200776t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vijayan R, Sahai MA, Czajkowski T, Biggin PC. A comparative analysis of the role of water in the binding pockets of ionotropic glutamate receptors. Phys Chem Chem Phys. 2010;12:14057–66. doi: 10.1039/c004336b. [DOI] [PubMed] [Google Scholar]
Related articles
- 1.Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–99. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Straub C, Hunt DL, Yamasaki M, Kim KS, Watanabe M, et al. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat Neurosci. 2011;14:866–73. doi: 10.1038/nn.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, et al. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–96. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuzaki M. Cbln1 and its family proteins in synapse formation and maintenance. Curr Opin Neurobiol. 2011;21:215–20. doi: 10.1016/j.conb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 5.McMahon SA, Diaz E. Mechanisms of excitatory synapse maturation by trans-synaptic organizing complexes. Curr Opin Neurobiol. 2011;21:221–7. doi: 10.1016/j.conb.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Straub C, Tomita S. The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.09.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]