Abstract
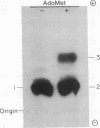
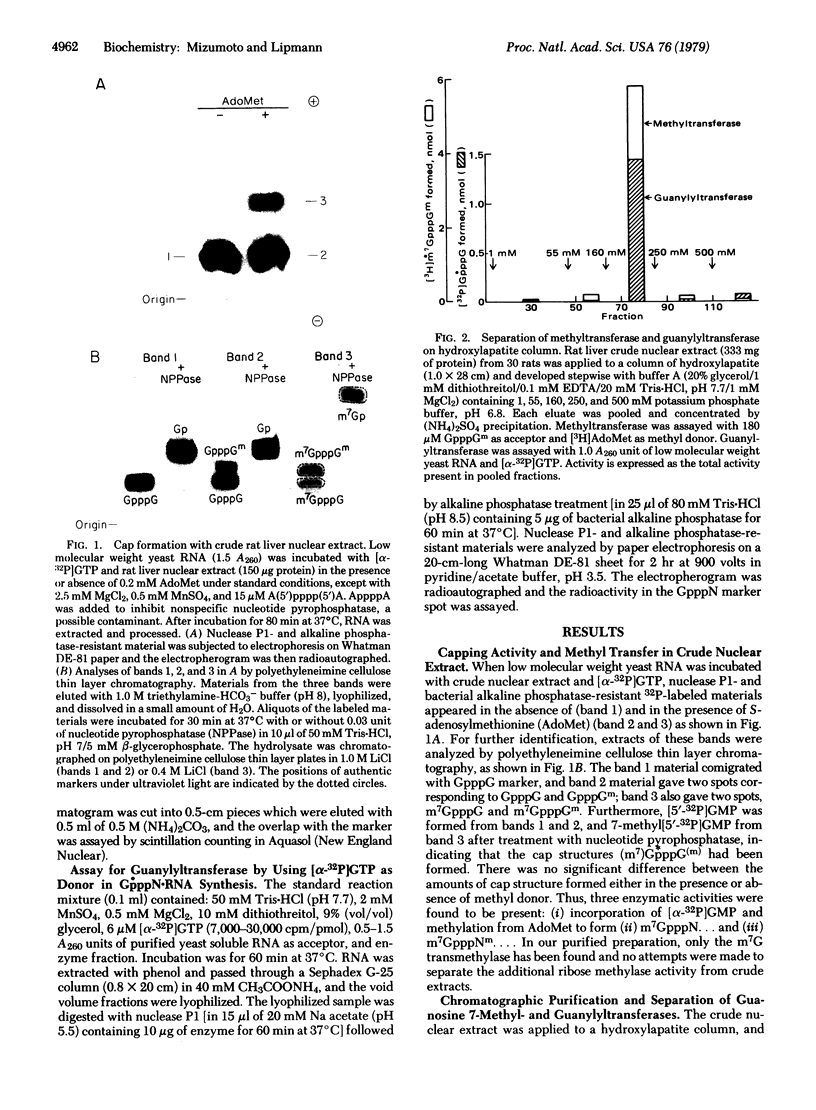
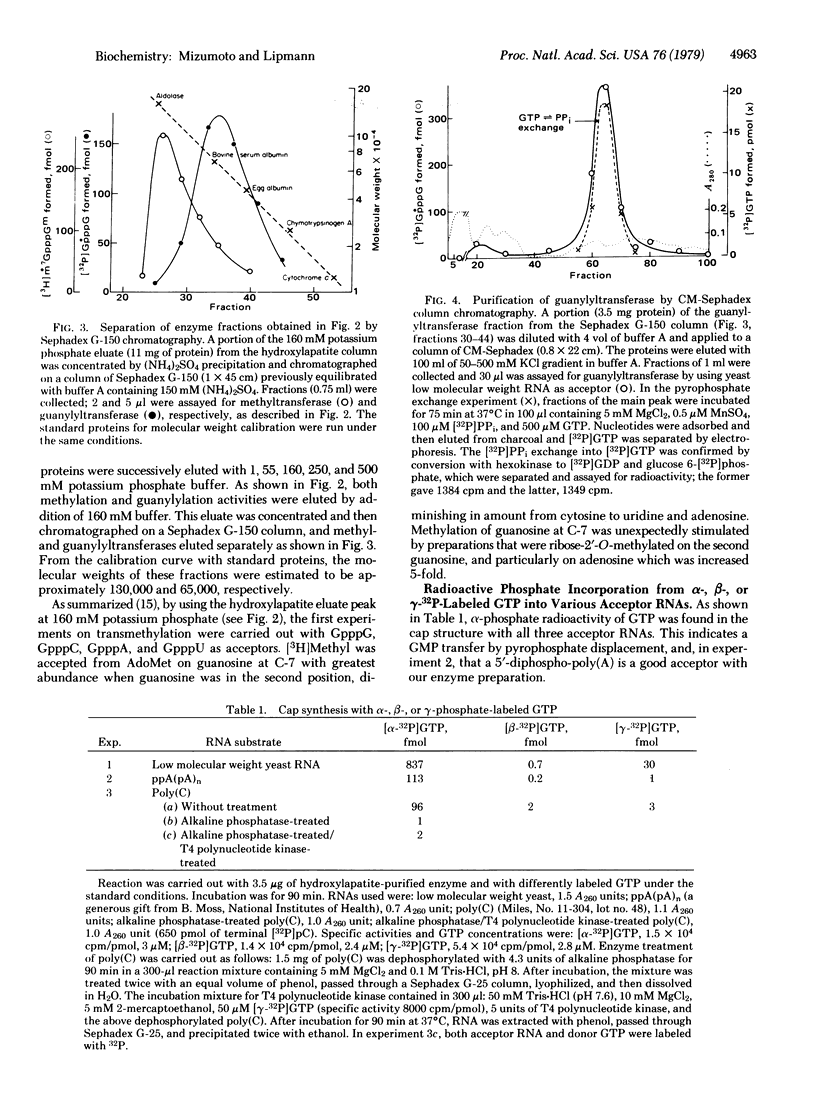
Rat liver nuclei were isolated and sonicated for extraction in order to study the capping of RNA. The guanosine 7-methyltransferase was purified from the extract by hydroxylapatite column chromatography with stepwise addition of phosphate buffer. It was assayed by using as methyl acceptor synthetic G(5')ppp(5')G and S-adenosylmethionine as donor. The enzyme appeared in a sharp peak at 160 mM. The same peak fraction was subsequently found to contain the enzyme that guanylylates short synthetic polynucleotides and low molecular weight yeast RNA as acceptors. The two enzymatic activities were separated on Sephadex G-150 chromatography, yielding guanylyltransferase and guanosine 7-methyltransferase with molecular weights of approximately 65,000 and 130,000 respectively. Guanylyltransferase was further purified by CM-Sephadex chromatography, whereby G-7-methyltransferase was completely removed. Dithiothreitol was essential for guanylylation, and 2 mM Mn2+ (optimum) was twice as active as 8 mM Mg2+ (optimum). The alpha-32P of [32P]GTP, but not its beta- or gamma-32P was incorporated into the cap structure. By using unlabeled GTP with [beta-32P]ppGpCpC-poly(A2,U2,G) as acceptor, [beta'-32P]-GpppG... was formed. Our purified transguanylylation enzyme was found to catalyze a [32P]pyrophosphate exchange with GTP, which may be useful as a rapid assay for transguanylylation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Rhodes D. P., Banerjee A. K. The 5' terminal structure of the methylated mRNA synthesized in vitro by vesicular stomatitis virus. Cell. 1975 May;5(1):51–58. doi: 10.1016/0092-8674(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Barbosa E., Moss B. mRNA(nucleoside-2'-)-methyltransferase from vaccinia virus. Purification and physical properties. J Biol Chem. 1978 Nov 10;253(21):7692–7697. [PubMed] [Google Scholar]
- Both G. W., Furuichi Y., Muthukrishnan S., Shatkin A. J. Effect of 5'-terminal structure and base composition on polyribonucleotide binding to ribosomes. J Mol Biol. 1976 Jul 5;104(3):637–658. doi: 10.1016/0022-2836(76)90126-1. [DOI] [PubMed] [Google Scholar]
- Busch H. The function of the 5' cap of mRNA and nuclear RNA species. Perspect Biol Med. 1976 Summer;19(4):549–567. doi: 10.1353/pbm.1976.0064. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Singer M. F. Deoxyadenosine diphosphate as a substrate and inhibitor of polynucleotide phosphorylase of Micrococcus luteus. I. Deoxyadenosine diphosphate as a substrate for polymerization and the exchange reaction with inorganic 32 P. J Biol Chem. 1971 Dec 25;246(24):7486–7496. [PubMed] [Google Scholar]
- Darnell J. E., Jr Transcription units for mRNA production in eukaryotic cells and their DNA viruses. Prog Nucleic Acid Res Mol Biol. 1979;22:327–353. doi: 10.1016/s0079-6603(08)60803-x. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Muthukrishnan S., Tomasz J., Shatkin A. J. Mechanism of formation of reovirus mRNA 5'-terminal blocked and methylated sequence, m7GpppGmpC. J Biol Chem. 1976 Aug 25;251(16):5043–5053. [PubMed] [Google Scholar]
- Gilboa E., Soreq H., Aviv H. Initiation of RNA synthesis in isolated nuclei. Eur J Biochem. 1977 Jul 15;77(2):393–400. doi: 10.1111/j.1432-1033.1977.tb11679.x. [DOI] [PubMed] [Google Scholar]
- Groner Y., Hurwitz J. Synthesis of RNA containing a methylated blocked 5' terminus by HeLa nuclear homogenates. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2930–2934. doi: 10.1073/pnas.72.8.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9330–9335. [PubMed] [Google Scholar]
- Monroy G., Spencer E., Hurwitz J. Purification of mRNA guanylyltransferase from vaccinia virions. J Biol Chem. 1978 Jun 25;253(12):4481–4489. [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Specific nucleolar and nucleoplasmic RNA polymerases. Proc Natl Acad Sci U S A. 1970 Mar;65(3):675–682. doi: 10.1073/pnas.65.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Tomasz J., Allende J. E. Synthesis of pppGpN type dinucleotide derivatives: the 5' end sequence of some RNAs. Nucleic Acids Res. 1975 Feb;2(2):257–263. doi: 10.1093/nar/2.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Moss B. 5'-Terminal capping of RNA by guanylyltransferase from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3758–3761. doi: 10.1073/pnas.74.9.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winicov I., Perry R. P. Synthesis methylation, and capping of nuclear RNA by a subcellular system. Biochemistry. 1976 Nov 16;15(23):5039–5046. doi: 10.1021/bi00668a014. [DOI] [PubMed] [Google Scholar]