Abstract
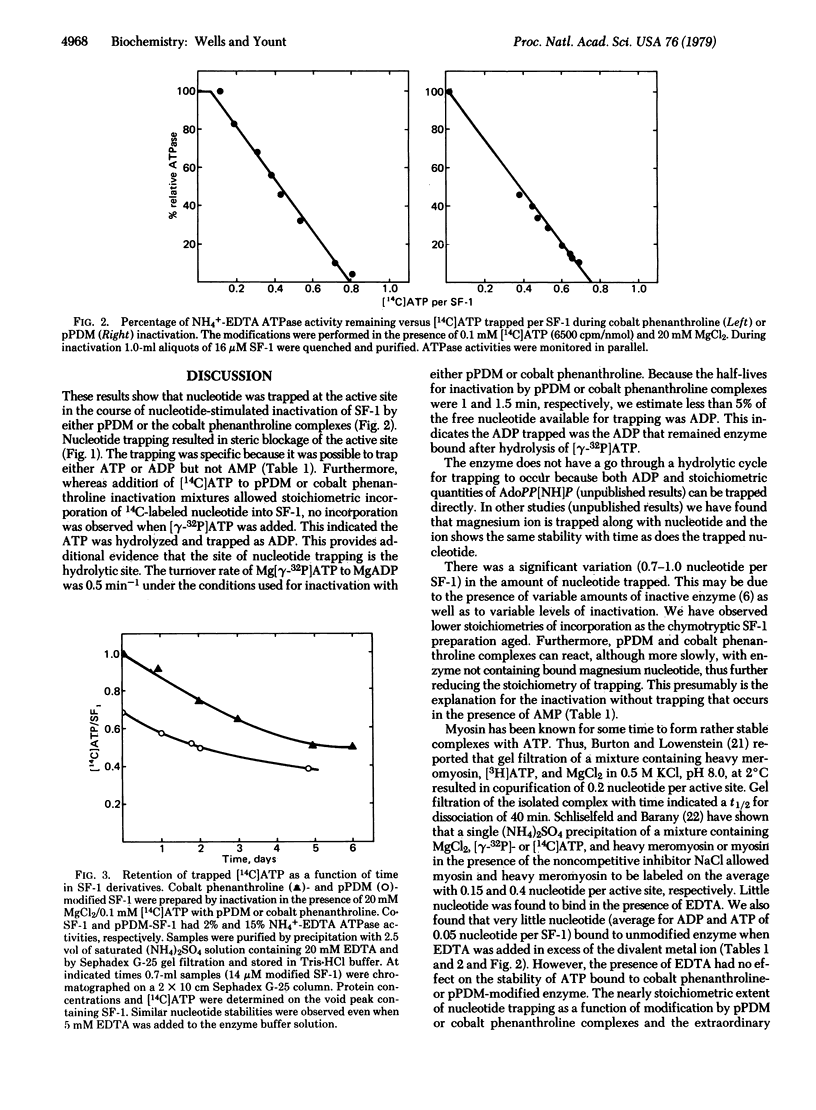
Studies with reagents that crosslink two thiol groups have shown that it is possible to trap nucleotides at the active site of myosin chymotryptic subfragment 1. Subfragment 1 incorporates nearly stoichiometric quantities of [14C]ATP or [14C]ADP in a manner that depends linearly on the extent of inactivation by either N,N'-p-phenylenedimaleimide or Co(II)phenanthroline/[Co(III)(phenanthroline)2CO3]+ complexes. The incorporated radioactive nucleotide is retained after gel filtration, even when the enzyme derivatives are stored in the presence of EDTA or nonradioactive nucleotides (t 1/2 approximately 5 days). The nucleotide incorporated is not covalently bound because HClO4 denaturation allows immediate release of bound nucleotide. The nucleotide retained is ADP because the gamma-phosphate of [gamma-32P]ATP is lost after trapping. Subfragment 1 inactivated as above does not bind the competitive inhibitor adenosine 5'-[beta, gamma-imido]triphosphate, indicating that the active site is blocked. It is proposed that a jawlike nucleotide cleft closes on MgADP or MgATP, which can be locked shut by crosslinking two thiol groups by reaction with N,N'-p-phenylenedimaleimide or cobalt phenanthroline complexes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. M., Zucker F. H., Steitz T. A. Space-filling models of kinase clefts and conformation changes. Science. 1979 Apr 27;204(4391):375–380. doi: 10.1126/science.220706. [DOI] [PubMed] [Google Scholar]
- Bagshow C. R., Reed G. H. Investigations of equilibrium complexes of myoxin subfragment 1 with the manganous ion and adenosine diphosphate using magnetic resonance techniques. J Biol Chem. 1976 Apr 10;251(7):1975–1983. [PubMed] [Google Scholar]
- Bennett W. S., Jr, Steitz T. A. Glucose-induced conformational change in yeast hexokinase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4848–4852. doi: 10.1073/pnas.75.10.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burke M., Reisler E. Effect of nucleotide binding on the proximity of the essential sulfhydryl groups of myosin. Chemical probing of movement of residues during conformational transitions. Biochemistry. 1977 Dec 13;16(25):5559–5563. doi: 10.1021/bi00644a026. [DOI] [PubMed] [Google Scholar]
- Burke M., Reisler E., Harrington W. F. Myosin ATP hydrolysis: a mechanism involving a magnesium chelate complex. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3793–3796. doi: 10.1073/pnas.70.12.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M., Reisler F., Harrington W. F. Effect of bridging the two essential thiols of myosin on its spectral and actin-binding properties. Biochemistry. 1976 May 4;15(9):1923–1927. doi: 10.1021/bi00654a020. [DOI] [PubMed] [Google Scholar]
- Bálint M., Wolf I., Tarcsafalvi A., Gergely J., Sréter F. A. Location of SH-1 and SH-2 in the heavy chain segment of heavy meromyosin. Arch Biochem Biophys. 1978 Oct;190(2):793–799. doi: 10.1016/0003-9861(78)90339-9. [DOI] [PubMed] [Google Scholar]
- Haugland R. P. Myosin structure. Proximity measurements by fluorescence energy transfer. J Supramol Struct. 1975;3(4):338–347. doi: 10.1002/jss.400030405. [DOI] [PubMed] [Google Scholar]
- Mendelson R., Putnam S., Morales M. Time-dependent fluorescence depolarization and lifetime studies of myosin subfragment-one in the presence of nucleotide and actin. J Supramol Struct. 1975;3(2):162–168. doi: 10.1002/jss.400030209. [DOI] [PubMed] [Google Scholar]
- Mendelson R., Putnam S., Morales M. Time-dependent fluorescence depolarization and lifetime studies of myosin subfragment-one in the presence of nucleotide and actin. J Supramol Struct. 1975;3(2):162–168. doi: 10.1002/jss.400030209. [DOI] [PubMed] [Google Scholar]
- Morita F. Interaction of heavy meromyosin with substrate. I. Difference in ultraviolet absorption spectrum between heavy meromyosin and its Michaelis-Menten complex. J Biol Chem. 1967 Oct 10;242(19):4501–4506. [PubMed] [Google Scholar]
- Murphy A. J. Circular dichroism of the adenine and 6-mercaptopurine nucleotide complexes of heavy meromyosin. Arch Biochem Biophys. 1974 Jul;163(1):290–296. doi: 10.1016/0003-9861(74)90479-2. [DOI] [PubMed] [Google Scholar]
- Pope B. J., Wagner P. D., Weeds A. G. Heterogeneity of myosin heavy chains in subfragment-1 isoenzymes rabbit skeletal myosin. J Mol Biol. 1977 Jan 25;109(3):470–473. doi: 10.1016/s0022-2836(77)80024-7. [DOI] [PubMed] [Google Scholar]
- Reisler E., Burke M., Himmelfarb S., Harrington W. F. Spatial proximity of the two essential sulfhydryl groups of myosin. Biochemistry. 1974 Sep 10;13(19):3837–3840. doi: 10.1021/bi00716a001. [DOI] [PubMed] [Google Scholar]
- SEKINE T., YAMAGUCHI M. EFFECT OF ATP ON THE BINDING OF N-ETHYLMALEIMIDE TO SH GROUPS IN THE ACTIVE SITE OF MYOSIN ATPASE. J Biochem. 1963 Aug;54:196–198. [PubMed] [Google Scholar]
- Schaub M. C., Watterson J. G., Waser P. G. Conformational differences in myosin, IV.[1-3] Radioactive labeling of specific thiol groups as influenced by ligand binding. Hoppe Seylers Z Physiol Chem. 1975 Mar;356(3):325–339. doi: 10.1515/bchm2.1975.356.1.325. [DOI] [PubMed] [Google Scholar]
- Schliselfeld L. H., Bárány M. The binding of adenosine triphosphate to myosin. Biochemistry. 1968 Sep;7(9):3206–3213. doi: 10.1021/bi00849a024. [DOI] [PubMed] [Google Scholar]
- Seidel J. C., Chopek M., Gergely J. Effect of nucleotides and pyrophosphate on spin labels bound to S1 thiol groups of myosin. Biochemistry. 1970 Aug 4;9(16):3265–3272. doi: 10.1021/bi00818a021. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Eccleston J. F., Bagshaw C. R. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976 May;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Yount R. G. Stoichiometry of labeling of myosin's proteolytic fragments by a purine disulfide analog of adenosine triphosphate. Biochemistry. 1975 May 6;14(9):1900–1907. doi: 10.1021/bi00680a015. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Werber M. M., Szent-Györgyi A. G., Fasman G. D. Fluorescence studies on heavy meromyosin-substrate interaction. Biochemistry. 1972 Jul 18;11(15):2872–2883. doi: 10.1021/bi00765a021. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Sekine T. Sulfhydryl groups involved in the active site of myosin A adenosine triphosphatase. I. Specific blocking of the SH group responsible for the inhibitory phase in "B phasic response" of the catalytic activity. J Biochem. 1966 Jan;59(1):24–33. doi: 10.1093/oxfordjournals.jbchem.a128254. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]


