Abstract
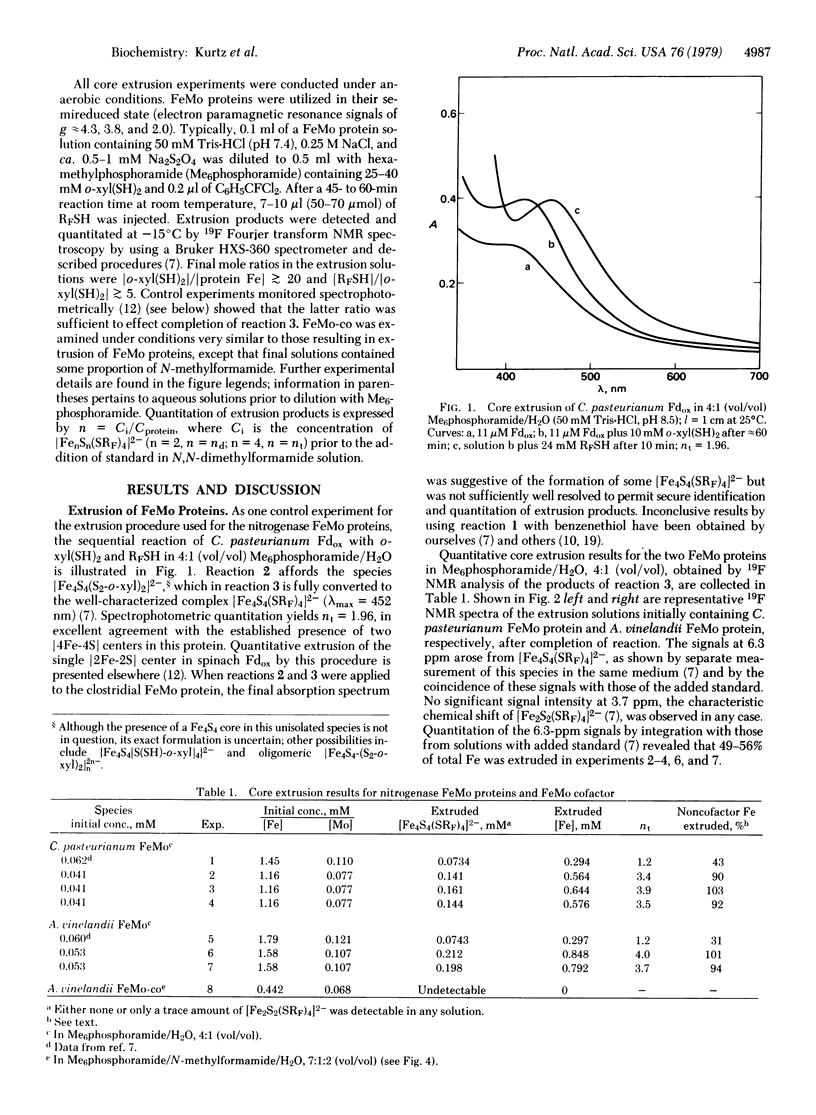
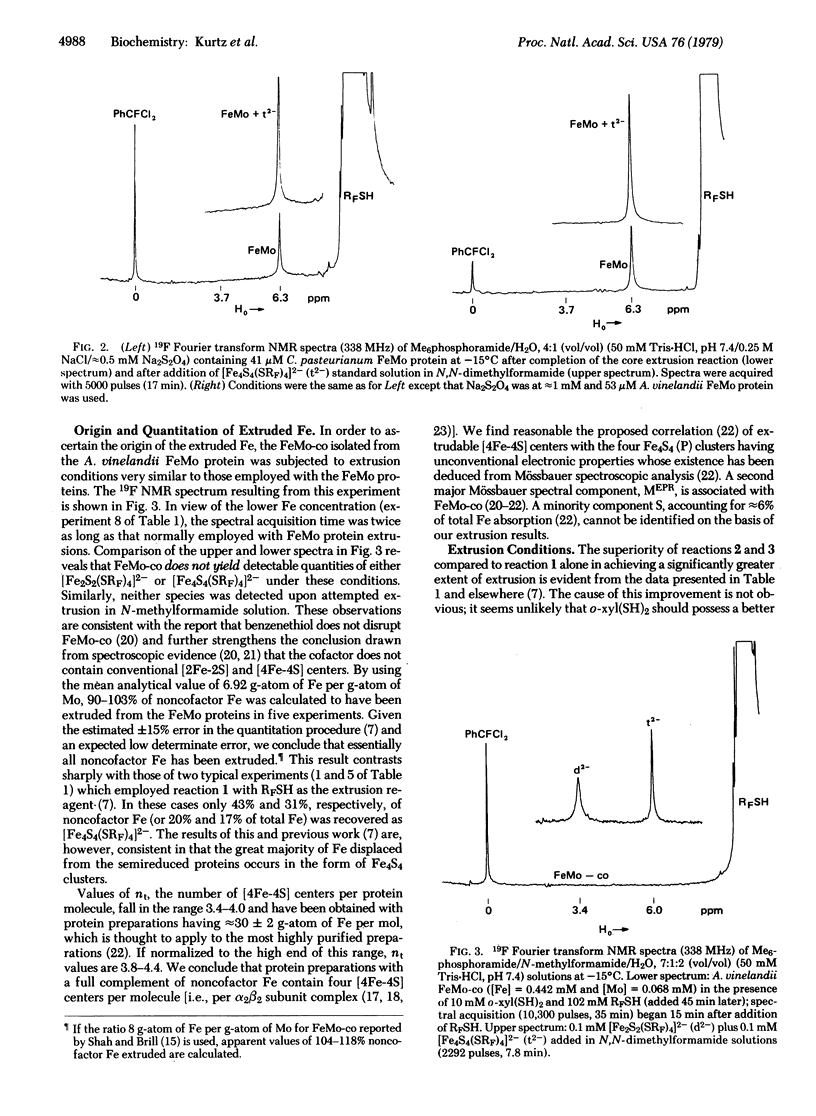
The core extrusion method has been applied to the determination of the type ([2Fe-2S], [4Fe-4S]) and number of iron-sulfur centers in the FeMo proteins of the nitrogenases from Clostridium pasteurianum and Azotobacter vinelandii. The method involves extrusion with o-xylyl-alpha, alpha'-dithiol, ligand exchange of the extrusion products with p-CF3C6H4SH (RFSH), and identification and quantitation of the resultant [FenSn(SRF)4]2- complexes (n = 2,4) by 19F NMR spectroscopy. In hexamethylphosphoramide/water, 4:1 (vol/vol), 49-56% of the Fe content was extruded as [Fe4S4(SRF)4]2-, corresponding to 3,4-4.0 Fe4S4 cores per alpha 2 beta 2 subunit complex. The extruded iron does not arise from the FeMo cofactor, separate examination of which detected no extrusion products, and corresponds to 90-103% of noncofactor iron. No significant quantity of Fe2S2 cores was extruded. These results indicate the presence of four [4Fe-4S] centers per alpha 2 beta 2 subunit complex in preparations undepleted in iron. There are two main structural populations of iron atoms in these proteins, those in the cubane-type Fe4S4 cores and those in the FeMo cofactor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coles C. J., Holm R. H., Kurtz D. M., Jr, Orme-Johnson W. H., Rawlings J., Singer T. P., Wong G. B. Characterization of the iron-sulfur centers in succinate dehydrogenase. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3805–3808. doi: 10.1073/pnas.76.8.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes G. R., Holm R. H. Synthetic analogs of the active sites of iron-sulfur proteins; X. Kinetics and mechanism of the ligand substitution reactions of arylthiols with the tetranuclear clusters [Fe4S4(SR)4]2 minus;. J Am Chem Soc. 1975 Feb 5;97(3):528–533. doi: 10.1021/ja00836a011. [DOI] [PubMed] [Google Scholar]
- Erbes D. L., Burris R. H., Orme-Johnson W. H. On the iron-sulfur cluster in hydrogenase from Clostridium pasteurianum W5. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4795–4799. doi: 10.1073/pnas.72.12.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum W. O., Mortenson L. E., Chen J. S., Holm R. H. Quantitative extrusions of the Fe4S4 cores of the active sites of ferredoxins and the hydrogenase of Clostridium pasteurianum. J Am Chem Soc. 1977 Jan 19;99(2):584–595. doi: 10.1021/ja00444a044. [DOI] [PubMed] [Google Scholar]
- Hatchikian E. C., Bruschi M., Le Gall J. Characterization of the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Commun. 1978 May 30;82(2):451–461. doi: 10.1016/0006-291x(78)90896-3. [DOI] [PubMed] [Google Scholar]
- Hill C. L., Steenkamp D. J., Holm R. H., Singer T. P. Identification of the iron-sulfur center in trimethylamine dehydrogenase. Proc Natl Acad Sci U S A. 1977 Feb;74(2):547–551. doi: 10.1073/pnas.74.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. C., Zumft W. G., Mortenson L. E. Structure of the molybdoferredoxin complex from Clostridium pasteurianum and isolation of its subunits. J Bacteriol. 1973 Feb;113(2):884–890. doi: 10.1128/jb.113.2.884-890.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh B. H., Münck E., Orme-Johnson W. H. Nitrogenase XI: Mössbauer studies on the cofactor centers of the MoFe protein from Azotobacter vinelandii OP. Biochim Biophys Acta. 1979 Jan 25;576(1):192–203. doi: 10.1016/0005-2795(79)90497-5. [DOI] [PubMed] [Google Scholar]
- Kurtz D. M., Holm R. H., Ruzicka F. J., Beinert H., Coles C. J., Singer T. P. The high potential iron-sulfur cluster of aconitase is a binuclear iron-sulfur cluster. J Biol Chem. 1979 Jun 25;254(12):4967–4969. [PubMed] [Google Scholar]
- Lundell D. J., Howard J. B. Isolation and partial characterization of two different subunits from the molybdenum-iron protein of Azotobacter vinelandii nitrogenase. J Biol Chem. 1978 May 25;253(10):3422–3426. [PubMed] [Google Scholar]
- Que L., Jr, Holm R. H., Mortenson L. E. Letter: Extrusion of Fe2S2 and Fe4S4 cores from the active sites of ferredoxin proteins. J Am Chem Soc. 1975 Jan 22;97(2):463–464. doi: 10.1021/ja00835a064. [DOI] [PubMed] [Google Scholar]
- Rawlings J., Shah V. K., Chisnell J. R., Brill W. J., Zimmermann R., Münck E., Orme-Johnson W. H. Novel metal cluster in the iron-molybdenum cofactor of nitrogenase. Spectroscopic evidence. J Biol Chem. 1978 Feb 25;253(4):1001–1004. [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. The soluble "high potential" type iron-sulfur protein from mitochondria is aconitase. J Biol Chem. 1978 Apr 25;253(8):2514–2517. [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P. J., McKenna C. E., Smith B. E., Nguyen H. T., McKenna M. C., Thomson A. J., Devlin F., Jones J. B. Circular dichroism and magnetic circular dichroism of nitrogenase proteins. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2585–2589. doi: 10.1073/pnas.76.6.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher R. H., Landt M. L., Reithel F. J. The molecular weight of, and evidence for two types of subunits in, the molybdenum-iron protein of Azotobacter vinelandii nitrogenase. Biochem J. 1977 Jun 1;163(3):427–432. doi: 10.1042/bj1630427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Münck E., Brill W. J., Shah V. K., Henzl M. T., Rawlings J., Orme-Johnson W. H. Nitrogenase X: Mössbauer and EPR studies on reversibly oxidized MoFe protein from Azotobacter vinelandii OP. Nature of the iron centers. Biochim Biophys Acta. 1978 Dec 20;537(2):185–207. doi: 10.1016/0005-2795(78)90504-4. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Mortensson L. E. Evidence for a catalytic-centre heterogeneity of molybdoferredoxin from Clostridium pasteurianum. Eur J Biochem. 1973 Jun 15;35(3):401–409. doi: 10.1111/j.1432-1033.1973.tb02852.x. [DOI] [PubMed] [Google Scholar]


