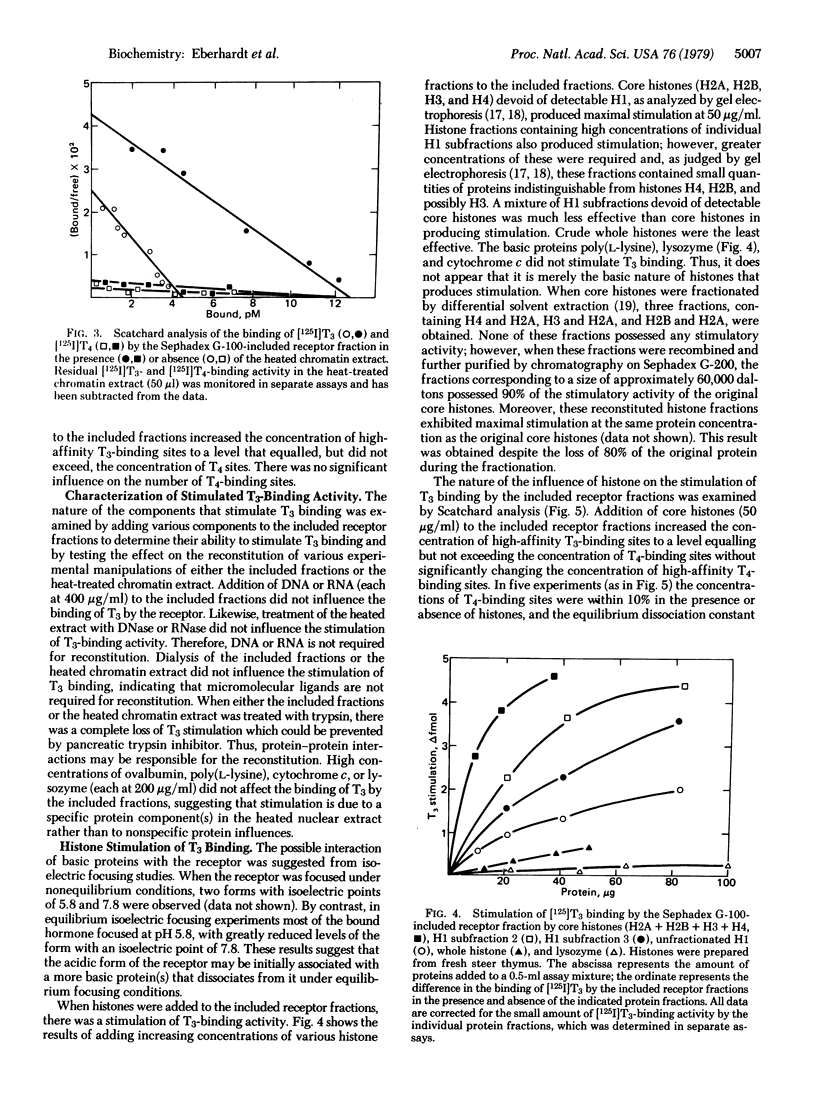
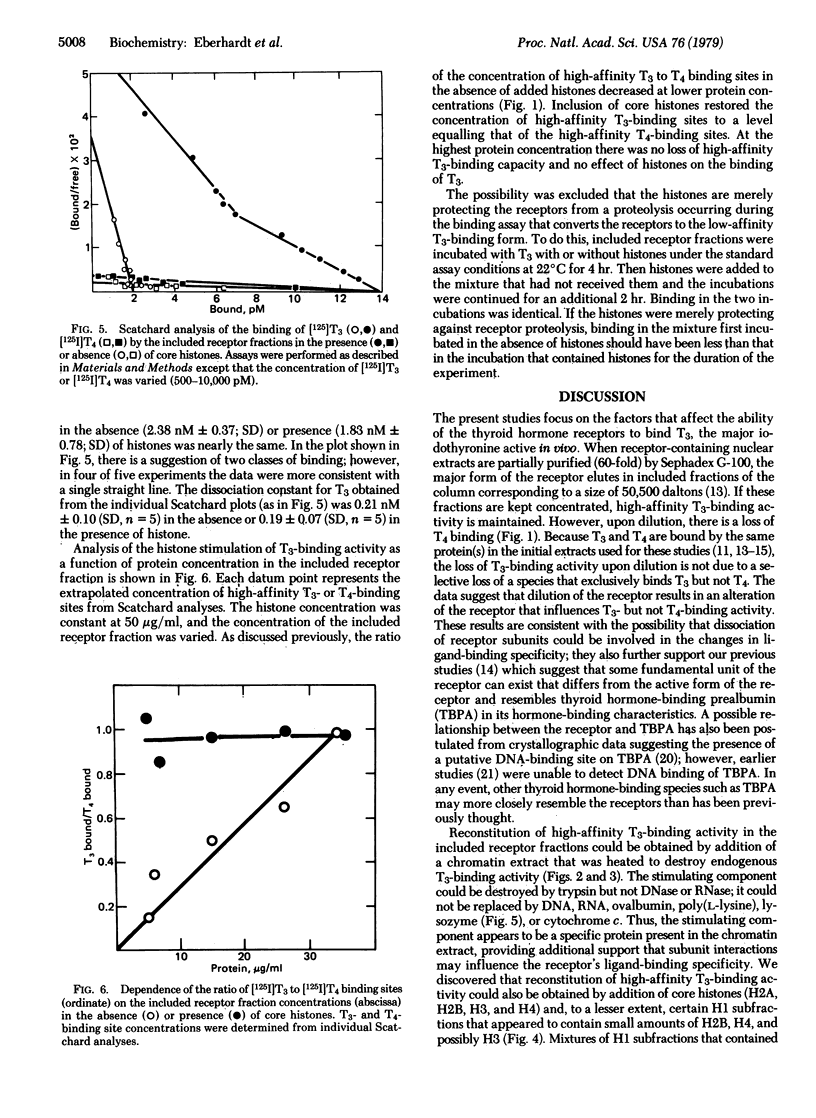
Abstract
Thyroid hormone receptors lose their capability for high-affinity binding of the biologically active triiodothyronine after solubilization and separation from other chromatin proteins. The high-affinity triiodothyronine-binding capacity can be reconstituted by addition of a histone-containing extract of chromatin of purified core histones (H2A, H2B, H3, and H4); a number of other acidic or basic proteins tested were ineffective. The data support a model of the receptor in which a "core" receptor subunit that contains a thyroid hormone-binding site interacts with a regulatory subunit, which is possibly a histone or histone-like species. This interaction with the "core" subunit enables the resulting "holo" receptor to bind biologically active hormones. These data also suggest that histones or related proteins can modulate the activity of nonhistone chromosomal proteins that are involved in regulating the expression of specific genes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake C. C., Oatley S. J. Protein-DNA and protein-hormone interactions in prealbumin: a model of the thyroid hormone nuclear receptor? Nature. 1977 Jul 14;268(5616):115–120. doi: 10.1038/268115a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Degroot L. J., Refetoff S., Strausser J., Barsano C. Nuclear triiodothyronine-binding protein: partial characterization and binding to chromatin. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4042–4046. doi: 10.1073/pnas.71.10.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt N. L., Ring J. C., Latham K. R., Baxter J. D. Thyroid hormone receptors. Alteration of hormone-binding specificity. J Biol Chem. 1979 Sep 10;254(17):8534–8539. [PubMed] [Google Scholar]
- Elgin S. C., Weintraub H. Chromosomal proteins and chromatin structure. Annu Rev Biochem. 1975;44:725–774. doi: 10.1146/annurev.bi.44.070175.003453. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Vidali G., Littau V. C., Allfrey V. G. Modulation by exogenous histones of phosphorylation of non-histone nuclear proteins in isolated rat liver nuclei. J Biol Chem. 1973 Nov 10;248(21):7595–7600. [PubMed] [Google Scholar]
- Koerner D., Schwartz H. L., Surks M. I., Oppenheimer J. H. Binding of selected iodothyronine analogues to receptor sites of isolated rat hepatic nuclei. High correlation between structural requirements for nuclear binding and biological activity. J Biol Chem. 1975 Aug 25;250(16):6417–6423. [PubMed] [Google Scholar]
- Kurtz D. T., Sippel A. E., Feigelson P. Effect of thyroid hormones on the level of the hepatic mRNA for alpha2u globulin. Biochemistry. 1976 Mar 9;15(5):1031–1036. doi: 10.1021/bi00650a013. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latham K. R., Ring J. C., Baxter J. D. Solubilized nuclear "receptors" for thyroid hormones. Physical characteristics and binding properties, evidence for multiple forms. J Biol Chem. 1976 Dec 10;251(23):7388–7397. [PubMed] [Google Scholar]
- MacLeod K. M., Baxter J. D. Chromatin receptors for thyroid hormones. Interactions of the solubilized proteins with DNA. J Biol Chem. 1976 Dec 10;251(23):7380–7387. [PubMed] [Google Scholar]
- MacLeod K. N., Baxter J. D. DNA binding of thyroid hormone receptors. Biochem Biophys Res Commun. 1975 Feb 3;62(3):577–583. doi: 10.1016/0006-291x(75)90437-4. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martial J. A., Seeburg P. H., Guenzi D., Goodman H. M., Baxter J. D. Regulation of growth hormone gene expression: synergistic effects of thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4293–4295. doi: 10.1073/pnas.74.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D., Sommer K. R., Panyim S., Spiker S., Chalkley R. A modified procedure for fractionating histones. Biochem J. 1972 Sep;129(2):349–353. doi: 10.1042/bj1290349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavasiliou S. S., Martial J. A., Latham K. R., Baxter J. D. Thyroid hormonelike actions of 3,3',5'-L-triiodothyronine nad 3,3'-diiodothyronine. J Clin Invest. 1977 Dec;60(6):1230–1239. doi: 10.1172/JCI108882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action in cell culture: domonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3488–3492. doi: 10.1073/pnas.70.12.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdon M. J., Isenberg I. Interactions between the subfractons of calf thymus H1 and nonhistone chromosomal proteins HMG1 and HMG2. Biochemistry. 1976 Sep 21;15(19):4242–4247. doi: 10.1021/bi00664a017. [DOI] [PubMed] [Google Scholar]
- Spaulding S. W., Davis P. J. Thyroxine binding to soluble proteins in rat liver and its sex dependence. Biochim Biophys Acta. 1971 Jan 19;229(1):279–283. doi: 10.1016/0005-2795(71)90345-x. [DOI] [PubMed] [Google Scholar]
- Spindler B. J., MacLeod K. M., Ring J., Baxter J. D. Thyroid hormone receptors. Binding characteristics and lack of hormonal dependency for nuclear localization. J Biol Chem. 1975 Jun 10;250(11):4113–4119. [PubMed] [Google Scholar]
- Stein G. S., Spelsberg T. C., Kleinsmith L. J. Nonhistone chromosomal proteins and gene regulation. Science. 1974 Mar 1;183(4127):817–824. doi: 10.1126/science.183.4127.817. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Koerner D., Dillman W., Oppenheimer J. H. Limited capacity binding sites for L-triiodothyronine in rat liver nuclei. Localization to the chromatin and partial characterization of the L-triiodothyronine-chromatin complex. J Biol Chem. 1973 Oct 25;248(20):7066–7072. [PubMed] [Google Scholar]
- Zweidler A. Resolution of histones by polyacrylamide gel electrophoresis in presence of nonionic detergents. Methods Cell Biol. 1978;17:223–233. [PubMed] [Google Scholar]


