Abstract
Prior work has shown the importance of TAM (Tyro3, Axl, Mer) receptor tyrosine kinases in GnRH neuronal development and reproductive function. It is unclear if TAM receptor actions are dependent on ligand activation for their functional effects; thus, we characterized reproductive phenotype of ligand Growth arrest specific gene (Gas6) null mice. Gas6 null mice showed delayed vaginal opening and delayed first estrus. Animals eventually attained normal estrous cycles as adults. The GnRH neuronal population was significantly decreased in Gas6 null adults and embryos, but the final positioning of cell bodies in the hypothalamus was normal. Vaginal tissue showed up-regulation of TAM receptor mRNAs in the absence of the ligand. These data confirm that Gas6 plays a role in early GnRH neuronal development and during vaginal opening. The phenotype of Gas6 KO mice suggests that TAMs function in a ligand-dependent and independent manner to control GnRH neuron development to modulate normal reproductive function.
Keywords: GnRH neurons, Gas6, TAM family
1. Introduction
The reproductive axis is primarily regulated by a small population of gonadotropin-releasing hormone (GnRH) neurons that originate near the olfactory placode and migrate across the cribriform plate into the basal forebrain (Schwanzel-Fukuda and Pfaff, 1989). Disruption of GnRH neuronal migration or survival, results in abnormal sexual maturation and infertility in mice and human (Cariboni et al., 2007; Tobet and Schwarting, 2006; Wierman et al., 2004).
Growth arrest specific gene (Gas6) is a vitamin K dependent secreted protein initially named because it was upregulated in growth arrested mammalian cells, was identified as a primary ligand for the TAM receptor kinase family that includes Tyro3, Axl and Mer (Schneider et al., 1988). Gas6 shares a 44% homology with another TAM family ligand, protein S, but has independent functions (Manfioletti et al., 1993). Gas6 has a Gla amino acid contain domain, with a loop of two disulfide bridges, followed by four EGF and two SHBG domain repeats. It activates TAM receptors through the SHBG domain and induces receptor phosphorylation. Axl has the highest binding affinity for Gas6 amongst all the TAM family members (Laurance et al., 2012). Both Gas6 and the TAM receptors are expressed in a tissue specific and developmental timed pattern (Linger et al., 2008). Higher expression of both Gas6 and TAM receptors in a cell specific fashion and their interaction in regulating downstream regulating pathways can play pivotal role in survival, proliferation and migration in a variety of cell types (Laurance et al., 2012). Gas6 plays a pivotal role in maintaining vascular homeostasis, platelet aggregation, atherosclerosis and genetic variants in Gas6 have been associated with vascular disease (Fernandez-Fernandez et al., 2008). In addition, Gas6 has been shown to rescue fibroblasts, vascular smooth muscle cells and cortical neurons from apoptosis triggered by serum starvation and amyloid beta protein (Goruppi et al., 1996; Nakano et al., 1996; Yagami et al., 2002). In the testes, Gas6 has been suggested to modulate the phagocytic ability of Sertoli cells working via Mer (Xiong et al., 2008). Gas6 expression in the testis, ovary and uterus has been reported, but its expression in the vagina and exact role in central reproductive function is yet to be investigated.
Relative to the reproductive axis, in vivo, TAMs are expressed in a variety of tissues, including the brain and ovary (Prieto et al., 2000; Wu et al., 2008). In models of GnRH neuron development, Axl and Tyro3 are expressed in early GnRH NLT neurons, Tyro3 and Mer are expressed in the post migratory GT1-7 neuronal cells, whereas Gas6 protein is not expressed (Pierce et al., 2008). TAMs contain an intracellular carboxy-terminus containing a tyrosine kinase domain as well as an N-terminal extracellular domain containing two immunoglobulin (Ig)-like domains and fibronectin type III (FNIII) repeats that are important for Gas6 binding and protein processing, respectively. Importantly, TAM receptors may signal either in response to ligand activation or by formation of homodimers or heterodimers with each other or other receptor tyrosine kinases like MET to signal independent of Gas6 (Pierce et al., 2008; Salian-Mehta et al., 2013) .
Our prior studies suggested that Gas6/Axl and Tyro3 interactions regulate chemotaxis, migration and survival in GnRH neuronal cells (Allen et al., 1999). In addition, mice null for Axl and Tyro3 exhibit a selective loss of GnRH neurons during embryogenesis associated with delayed puberty and permanently irregular estrous cycles (Pierce et al., 2011). The alterations in total number and distribution of GnRH neurons were hypothesized to be due to defects in the survival and migratory capabilities of GnRH neurons lacking both AXL and TYRO3 protein. Pituitary and ovarian function were normal, but ovariectomized Axl/Tyro3 null mice demonstrated an impaired ability to mount a sex steroid-induced LH surge, supporting a central defect due to early changes in the GnRH neuron population as responsible for the reproductive phenotype (Pierce et al., 2011).
To dissect the importance of the ligand dependence for TAM receptor functions, we initially studied Gas6 actions in GnRH neuronal cell models. In NLT GnRH neuronal cells, AXL and TYRO3 were shown to function both dependent and independent of ligand (Pierce et al., 2008). Gas6 activation of AXL/TYRO3 increased neuronal migration; whereas silencing of both AXL and TYRO3 reversed the response to Gas6, but had no effect on basal migration (Pierce et al., 2008). Additional studies suggested the importance of Gas6/Axl signaling in the protection of GnRH neurons from programmed cell death via both the ERK and PI3-K/AKT pathways (Allen et al., 2002; Allen et al., 1999). Although Gas6 modulated rates of cell death, untreated cells demonstrated higher rates of apoptosis when AXL and/or TYRO3 were silenced, suggesting the contribution of both ligand dependent and independent effects. In GnRH neuronal cell lines, Gas6 induced neuronal migration by activating Axl via p38 MAPK pathway. AXL/TYRO3 heterodimers were present in neuronal cells in the absence of ligand, and the addition of Gas6 caused no apparent changes in this molecular interaction (Pierce et al., 2008). Since migration and survival in GnRH neuronal cells were at least partially dependent on Gas6 activation of TAMs, we hypothesized that the loss of Gas6 in vivo would disrupt normal reproductive function i.e. timing of normal sexual maturation, estrous cyclicity and thus examined the reproductive phenotype of Gas6 KO mice.
2. Methods
2.1 Reagents and Antibodies
Horseradish peroxidase (HRP)-conjugated secondary antibodies (Donkey anti-rabbit IgG and sheep anti-mouse IgG) were purchased from Biorad (Hercules, CA). Anti-GnRH was purchased from Affinity Bioreagents (Golden, CO) and biotinylated anti-rabbit secondary antibody from Calbiochem (San Diego, CA).
2.2 Mice
Gas6 KO mice established in a C57BL/6 N background were obtained from Dr. Peter Carmeliet of The Center for Transgene Technology and Gene Therapy, Flanders Interuniversity Institute of biotechnology, Leuven, Belgium. Animal care and experimental procedures were performed in accordance with the guidelines established by the Veterans Affairs Institutional Animal Care and Use Committee. Female mice were housed in microisolator cages in the same room as males (similarly housed) under a 12-h light cycle with food and water ad libitum. All mice were genotyped at time of weaning using primers: F 5′-GAGTGCCGTGATTCTGGTC-3′, R 5′-CCACTAAGGAAACAATAACTG-3′ for amplifying a Gas6 WT band at 500bp and F 5′-GAGTGCCGTGATTCTGGTC-3′ and R 5′-ATCTCTCGTGGGATCATT-3′ primers for amplifying a Gas6 KO band at 350bp.
2.3 RT-PCR
Vaginal tissues from adult mice in estrus phase of cyclicity were harvested and stored in RNA Later (Ambion, Foster City, CA) at −80°C. RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) and treated with DNase and cleaned up using RNeasy kit (Qiagen, Valencia, CA). 0.5 μg of RNA was reverse transcribed using iScript cDNA Synthesis Kit from Biorad (Hercules, CA) in PTC-200 thermal cycler (MJ Research, Waltham, MA). qPCR was performed in an Applied Biosystems real time PCR system using Power SYBR Green PCR master mix (Applied Biosystems, Foster city, CA) as described earlier (Salian-Mehta et al., 2013). The primer sequences used to amplify Axl were 5′-GGTCCCCCTGAGAACGTTAG-3′ and 5′-CATAAGTACCTCGGGGGTGT-3′. The primer sequences for amplifying Tyro3 were 5′-AGTGGAACGGTCTGATGCTG-3′ and 5′-AGAATGGCACACCTTCGACA-3′. The primer sequences for amplifying Mer were 5′-CTCTGGAGTGGAGGCACTG -3′ and 5′-ATCTTCCAGTCTGGGGTGGT-3′. Primer sequences for amplifying Gas6 were 5′-ATGGGTGCATGAGGAGTTGG-3′ and 5′-TGTTCGGGTGTAGTTGAGCC-3′. The quantity of Axl, Tyro3, Mer, and Gas6 mRNA was normalized to that of Gapdh mRNA. The primer sequences for amplifying Gapdh were 5′-GCACAGTCAAGGCCGAGAAT-3′ and 5′-GCCTTCTCCATGGTGGTGAA-3′. All amplification reactions for each sample were run in duplicates and a no template control was included in all experiments. The cycle threshold values (Ct) obtained for Axl, Tyro3 and Mer genes were normalized against Gapdh to calculate 2−ΔΔCt values from three separate experiments. Statistical differences between the means of WT and Gas6 KO groups were tested using unpaired t test.
2.4 Estrous studies
Female pups (21 to 28 days old) were checked daily for vaginal opening (VO) beginning from one week of weaning. Vaginal cytology was examined on daily vaginal smears that were stained with Toluidine Blue (Sigma-Aldrich, St. Louis, MO) until first estrus (FE) was observed. Smears were classified as diestrus, pro-estrus, estrus, or metestrus and cycle length was calculated as the number of days from the beginning of one estrous phase to the beginning of the next. Statistical differences between the means of two groups for evaluating VO, FE, interval between VO and FE were tested using unpaired t test and estrous cyclicity was tested using two-way ANOVA test.
2.5 Perfusion and Immunohistochemistry
Adult female mice in estrus phase of cyclicity were anesthetized with 10 mg/kg xylazine and 100 mg/kg ketamine. Each mouse was perfused intracardially with 2000 U heparin followed by 4% formaldehyde in 0.1 M phosphate buffer (PB) (pH 7.4). Brains were dissected out and immersed in 4% formaldehyde overnight and stored in 0.1 M PB at 4°C. Timed-pregnant mice were anesthetized as described; embryos were removed one at a time and perfused intracardially with 2 ml 4% formaldehyde in 0.1 M PB. Heads were removed and immersed in 4% formaldehyde overnight and stored in 0.1 M PB at 4°C. Perfused tissues were embedded in 5% agarose; adult brains were cut coronally into 55-μm sections on a Leica VT 1000S vibrating blade microtome; embryo heads were cut sagittally at 65-μm. Immunostaining for GnRH was performed as previously described using anti-GnRH antibody [1:10,000 (adult brains) or 1:1000 (embryo heads)] for 72 h followed by 1:1000 biotinylated anti-rabbit secondary antibody for 2 h (Pierce et al., 2008). Neurons positive for GnRH were counted manually using X200 (Zeiss Axiovert) microscope. In adults, GnRH-positive neurons were analyzed by slice; each slice comprised six 55-μm section and in E15 brains neurons were analyzed by compartment: nose, olfactory bulb, dorsal forebrain, and ventral forebrain as previously described (Pierce et al., 2008). Slice 0 was centered on the OVLT at the third ventricle. Negatively numbered slices corresponded to slices rostral to OVLT, whereas positively numbered slices corresponded to slices caudal to the OVLT.
Statistical differences for evaluating difference in total GnRH neuronal counts between adult and embryonic WT and Gas6 KO groups were tested using the non-parametric Mann-Whitney U test. In adult and embryonic brains, to analyze differences between groups for neurons in different locations, two-way ANOVA test was used considering location as repeated measure. Post-hoc significance at any one location was evaluated by examining the 95% confidence interval for the means at each location.
2.6 Statistical Analysis
All data are expressed as mean ± SEM for each group. Statistical methods that were used are described under each experimental approach. P-values were reported as <0.05 or <0.01. Statistical analyses were performed using GraphPad Software QuickCalcs Online Calculators for Scientists (www.graphpad.com; GraphPad Software, Inc., La Jolla, CA), Microsoft Office Excel 2003 version 11 (Microsoft Corp., Remond, WA), and SPSS software (SPSS Inc., Chicago, IL).
3. Results
3.1 Loss of Gas6 delays the onset of vaginal opening in female mice
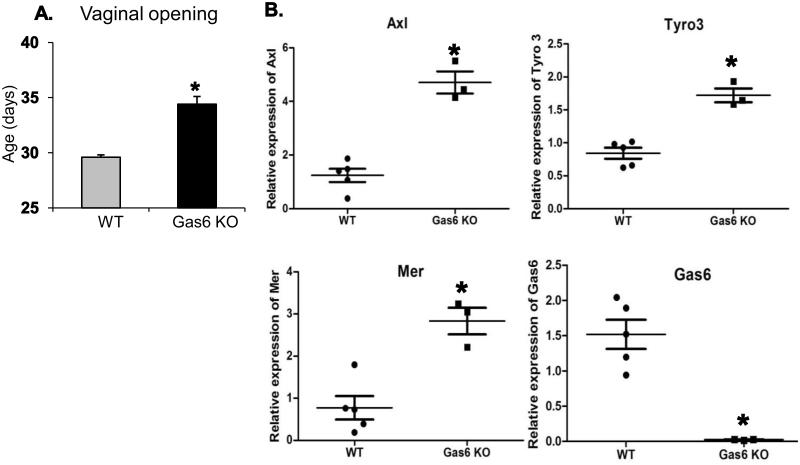
To determine the requirements for the TAM ligand, Gas6, in normal reproductive function, Gas6 KO and WT mice were bred and their age at vaginal opening was assessed. Female Gas6 KO and WT pups were evaluated daily within one week of weaning (21 to 28 days of age). Vaginal opening occurred in WT controls at 29.6 ± 0.2 d (n = 8), whereas Gas6 KO mice showed a significant delay in vaginal opening at 34.4 ± 0.7 d (n = 8, p = 0.0001) (Fig. 1A). Since prior work had suggested that Mer null mice have disorders of vaginal opening, but not Axl or Tyro3 null mice (Wu et al., 2008), and our prior studies of mice lacking both Axl and Tyro3, had not shown delayed vaginal opening, we asked if there were alteration in TAM mRNA expression in vaginal tissues of Gas6 KO compared to WT littermates. Levels of mRNA for Axl, Tyro3, Mer and Gas6 were measured in vaginas from WT and Gas6 KO mice using qRT-PCR. Gas6 mRNA expression was detected in vaginal tissues obtained from WT mice and was absent in Gas6 KO mice as expected (Fig. 1B).
Figure 1. Puberty is delayed in Gas6 KO females.
Panel A: Gas6 KO mice have delayed vaginal opening compared with WT mice (n = 8, p < 0.001, unpaired t-test).Panel B: Semi-quantitative real time polymerase chain reaction (qRT-PCR) of TAM family member and Gas6 mRNA levels in vagina obtained from WT and Gas6 KO mice (n = 5 for WT and n = 3 for Gas6 KO,* p < 0.05, unpaired t-test).
All TAM family members including Axl (3.7 fold), Tyro3 (2.0 fold) and Mer (3.6-fold) mRNA levels were up-regulated in Gas6 KO vaginal tissues compared to WT controls (Fig.1B). Protein lysates from vaginal tissues obtained from Gas6 KO and WT mice were variably intact, but overall the TYRO3, MER and GAS6 protein levels were correlated to mRNA levels (Supplementary Fig. S1). In contrast, in the uterine tissues (Supplementary Fig. S2) even though TAM (Axl, Tyro3, Mer) mRNA levels were low but they were detectable and no significant changes in mRNA were observed in Gas6 KO mice compared to WT mice uterine tissues. Gas6 mRNA levels were undetectable in Gas6 KO uterine tissues as expected. These results support that effects observed in the vaginal were different from other reproductive tract tissues. Thus in the absence of the ligand, Gas6, TAM receptor mRNAs are upregulated, yet a defect in vaginal opening was detected suggesting the importance of Gas6 signaling in the timing of this process.
3.2 Loss of Gas6 delays the onset of sexual maturation in female mice
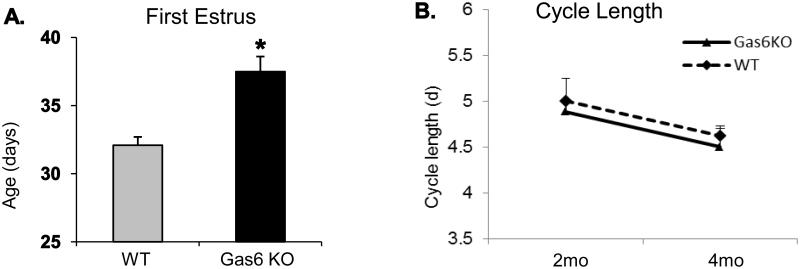
We then examined the influence of Gas6 on the age at first estrus (FE) and estrous cyclicity. Once vaginal opening was observed, vaginal cytology was determined by collecting vaginal smears daily until estrus, marked by the day when approximately 90 to 100% cornified epithelial cells, was observed. First estrus occurred in WT controls at 32.1 ± 0.6 d (n = 16); in comparison, sexual maturation was significantly delayed in Gas6 KO mice: at 37.5 d ± 1.1 d (n = 8, p = 0.0041) (Fig. 2A). No body weight measurements were done during pubertal onset, which could determine if the observed delayed VO seen in the Gas6 KO mice was associated with a reduced body weight. However, at 2-4 months, the weights of WT (24 ± 1.1 g, n = 6) and Gas6 KO (25.1 ± 0.9 g, n = 4) mice were similar (p = 0.5) and no obvious differences in their body weights were seen. The interval between average age at VO and average age at FE was not increased in Gas6 KO mice (3.1 ± 0.6 d vs. 2.5 ± 0.4 d in WT, p = 0.44), in contrast to our prior observations showing that loss of TAM receptors in Axl/Tyro3 null mice (5.6 d ± 1.5 vs. 1.5 d ± 0.7 in WT, p = 0.02) (Pierce et al., 2008). These data supported both ligand-dependent and independent functions of TAM receptors in reproductive development.
Figure 2. Delayed sexual maturation in Gas6 KO females.
Panel A: First estrus was delayed in Gas6 KO mice, compared with WT mice (n=8, p < 0.001, unpaired t-test). Panel B: Gas6 KO and WT mice have similar estrous cycle length at 2 and 4 months (n = 5 for WT and Gas6 KO time points, unpaired t-test).
3.3 Adult Estrous cyclicity is normal in Gas6 KO female mice
Reproductive success depends on the development and maintenance of regular estrous cycles in mice. To ask if Gas6 KO mice had altered estrous cyclicity, vaginal smears were collected from Gas6 KO and WT mice at 2 and 4 months of age and the vaginal cytology of groups of animals examined for at least 12 consecutive days. The average cycle length in Gas6 KO mice did not differ from that in the WT controls: at 2 months, Gas6 KO 5.0 ± 0.3 d (n = 5) and WT 4.9 ± 0.1 d (n = 13, p = 0.64). Similarly, at 4 months, WT cycle length was 4.5 ± 0.2 d (n = 5) compared with 4.6 ± 0.1 d (n = 5, p = 0.66) in the Gas6 KO mice (Fig. 2B). Both 2 and 4 month old Gas6 KO mice showed comparable estrous cyclicity (%) across all phases (Diestrus, Proestrus, Estrus and Metestrus) to that of WT controls (Supplementary Fig.S3 and S4). In both 2 month and 4 month old groups, no significant differences were seen between the genotypes (WT and Gas6 KO) in the overall estrus cycle length. However, in both 2 month (F = 49.56, p < 0.0001) and 4-month (F = 174.4, p < 0.0001) old groups significant interaction between genotypes and cyclicity was observed. In the 2 month group, Gas6 KO mice displayed a significantly longer time in diestrus and shorter time in estrus and post-estrus phases (p < 0.001) with no change in time spent in the proestrus phase (p > 0.05). At 4 months, Gas6 KO spent a longer interval in proestrus and less in diestrus and post-estrus (p <0.001), with no changes were seen in estrus (p > 0.05). Together these data suggest that although the estrus cycle lengths are normal Gas6 KO mice exhibit subtle but significant variability in cycle stages that may impact on reproductive function. However, Gas6 KO females (aged 2 to 4 months) were able to conceive under routine breeding conditions and successfully delivered average-sized litters of 6.3 ± 0.97 pups/liter compared to 9 ± 0.93 pups/liter from WT controls (p = NS) and showed no obvious deficit in maternal behavior. No maternal problems were observed in Gas6 KO mothers during delivery and nursing of pups. Gas6 KO pups showed normal survival and development. The delay of puberty in the Gas6 KO females suggested a defect in the ability of the hypothalamic/pituitary/gonadal axis to initiate reproductive function. To determine a potential central deficit, the number and distribution of GnRH-positive neurons in Gas6 KOs were assessed in adult mice and during embryogenesis.
3.4 Decreased total number of GnRH neurons but normal distribution in adult Gas6 KO brains
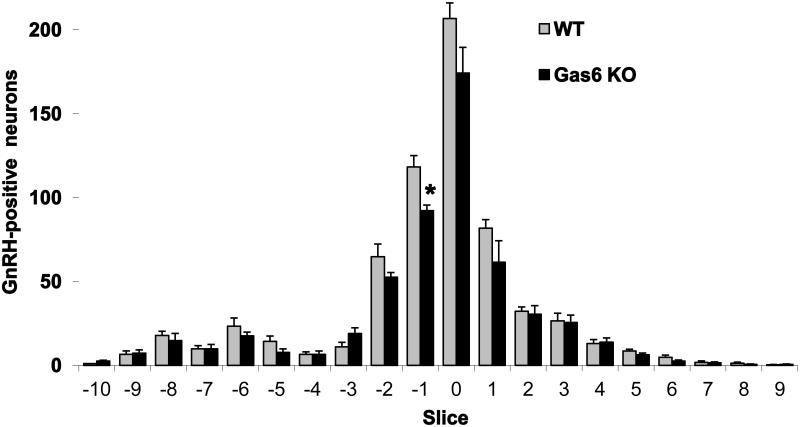
To examine the GnRH neuronal distribution in adult mice, brains from perfused adult Gas6 KO and WT female mice (n = 4 each), aged 2 to 6 months, were sectioned coronally (from the rostral diagonal band of Broca region to the caudal hypothalamus) and examined for immunoreactive GnRH as indicated by nickel intensified 3, 3’-diaminobenzidine reaction product and bright-field microscopy (Fig. 3). Neurons containing immunoreactive GnRH were counted in each section by an investigator blinded to genotype. Brains from Gas6 KO mice had a 16% decrease in the total number of GnRH neurons compared with WT (545 ± 19 vs. 649 ± 13, respectively, p = 0.004).
Figure 3. Gas6 KO adult mice have reduced GnRH neurons compared to WT.
Loss of GnRH neurons in Gas6 KO adult mice were seen in the regions surrounding the OVLT (−2 to +1) compared to WT (n = 4, *p < 0.01, aged 2-4 months, unpaired t-test).
The GnRH neurons were mapped in relation to their positions in the forebrain, with position zero located in the coronal plane of the organum vasculosum of the lamina terminalis (OVLT) at the opening of the third ventricle. The loss of GnRH neurons in Gas6 KO was most evident in the region surrounding the OVLT (−2 to +1), with slice −1 showing a significant 22% reduction in Gas6 KO mice compared to WT brains. However, the pattern of distribution was similar to that in WT brains. In KO mice, 69.8% of the total GnRH neuron population was located adjacent to the OVLT, compared with 72.7% in WT mice, p = NS (Fig.3). These data suggested that there was a decrease in the total GnRH neurons across development and this was most notable for cells in the OVLT region. Nonetheless, some GnRH neurons were found in all locations of the population in the forebrain, perhaps allowing for compensation and normal reproductive function in the adult.
3.5 GnRH-positive neuron distribution is disrupted in Gas6 null E15 embryos
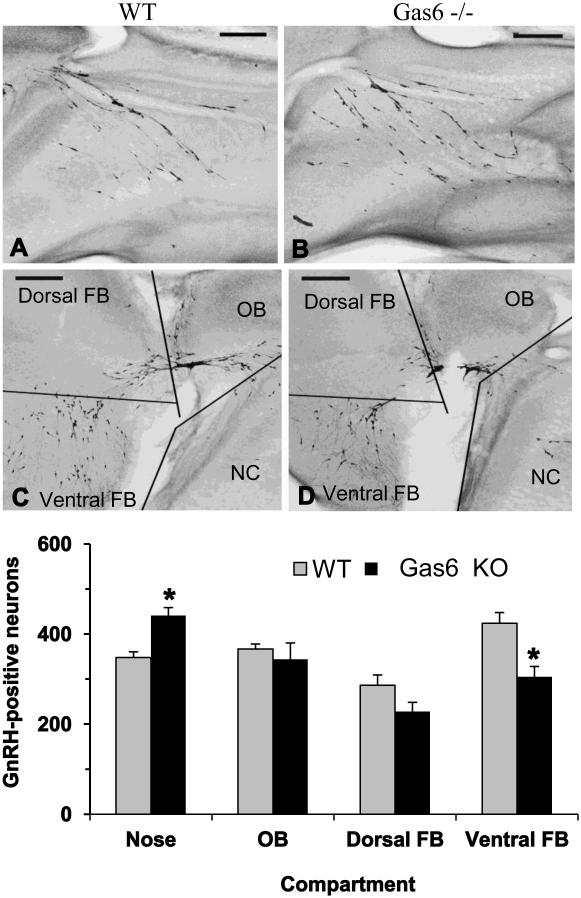
To determine if early GnRH neuron development was abnormal in Gas6 KO mice, the total number and distribution of GnRH-positive neurons in WT and Gas6 KO brains was evaluated at E15, a time when the GnRH neuron population typically crosses the cribriform plate en route to the forebrain (Livne et al., 1993). Embryo heads were sectioned parasagittally, and an investigator blind to group counted GnRH-positive neurons and assigned them to a compartment corresponding to the migratory path (nose, olfactory bulb, dorsal forebrain, and ventral forebrain) (Fig.4). The total number of GnRH-positive neurons in Gas6 KO embryo brains was 1322 ± 25 (n = 5), a small but significant decrease of 7% compared with WT embryos (WT neurons 1425 ± 27, n = 5, p = 0.02). With our n = 5 (power = 0.80), we estimate conservatively that we were able to assess for changes on the order of 112 cells or more with a SD of 56. 59 (α probability = 0.05).
Figure 4. Gas6 KO embryos demonstrate disruption in GnRH neuronal number and position.
Inset shows low magnification images of E15 Gas6 KO embryos with an increase in GnRH-positive neurons in the Nasal Compartment (B) and a decrease in the Forebrain (D) regions compared with nasal compartment and Forebrain regions of WT embryos (A, B). Bar graph depicts fewer GnRH neurons in the ventral forebrain and subsequent increase in their numbers in the nasal compartment in Gas6 KO compared to WT. (n = 5,* p < 0.05, two-way ANOVA considering location as a repeated measure)
Distribution analysis of immunoreactive GnRH neurons in Gas6 KO embryonic heads revealed that the nasal compartment, corresponding to the beginning of the migratory process, contained approximately 27% more GnRH neurons than in WT heads (348 ± 13 vs. 441 ± 18, respectively, p = 0.002). The neuron counts in the olfactory bulb and dorsal forebrain areas were unchanged, while the ventral forebrain numbers were decreased significantly by 28% (p = 0.006) (Fig.4). Together these data suggested that the early retention of GnRH neurons in the nasal compartment of Gas6 KO mice may contribute to the reproductive phenotype.
4. Discussion
In this report, we examined the phenotype of mice deleted for Gas6 to delineate its significance in the ligand dependent activation of TAM receptors to influence GnRH neuronal migration, survival and impact reproductive development. Gas6 KO mice have earlier been reported to be fertile (Yanagita et al., 2002) ; however, in-depth studies on reproductive development had not been examined. We demonstrate an early loss of GnRH neurons during embryonic development that persists in adulthood and a concomitant impairment in the early stages of sexual maturation in Gas6 KO mice.
Gas6 KO mice ultimately are fertile and produce healthy litters of average size. A closer examination, however, indicated that the onset of sexual maturation was significantly delayed in Gas6 KO female mice. The pubertal defect, however, differed from those we observed in Axl/Tyro3 KO mice. Whereas vaginal opening was not affected and first estrus was delayed (by 4 d) in Axl/Tyro3 KO mice, both vaginal opening and first estrus were delayed about 5 d in Gas6 KO mice. First estrus (and estrous cyclicity) are dependent on a normal GnRH-induced LH surge mechanism, and the mice without Axl and Tyro3 were shown to have persistently prolonged estrous cycles and an impaired LH surge response (Pierce et al., 2008; Pierce et al., 2011). In contrast, adult mice without the major ligand, Gas6, had regular normal estrous cycles, suggesting recovery or compensation in the hypothalamic/pituitary/gonadal axis by adulthood by TAM mediated ligand-independent processes.
Expression of Gas6 across brain development has only been studied in limited fashion in the rat where it was detected in total brain samples by northern blot analysis in rat E17 (corresponding to mouse E15.5) and remains high throughout adulthood (Hill, 2014; Prieto et al., 1999). No further data on Gas6 expression in the time of GnRH gene expression during development has been reported. Gas6 mRNA was detected in post-migratory GnRH neuronal cell lines, but its protein was not detected in either NLT and GT1-7 cells, suggesting in vivo it may be secreted from adjacent neurons or glia (Pierce et al., 2008). Together these data support the hypothesis that Gas6 may influence late GnRH neuronal migration. Gas6 protects NIHT3 and vascular smooth muscle cells from serum withdrawal induced apoptosis (Goruppi et al., 1996). In GnRH neurons, Gas6/Axl signaling is critical for neuronal survival (Pierce et al., 2008). However, the process of GnRH neuronal migration is only partially dependent on Gas6 dependence to activate Axl downstream signaling pathway.
To determine if the loss of neuronal migration observed in the mice lacking Axl/Tyro3 was dependent on Gas6 induced TAM activation, further studies using Gas6 KO were necessary. Gas6 KO mice displayed a reduced GnRH neuron population, albeit not as severe (16% reduction in total number of GnRH-positive neurons) versus 24% in Axl/Tyro3 null mice (Pierce et al., 2008). The TAM null mice showed an altered distribution of neurons that are shifted rostrally and far fewer GnRH positive neurons (24.2% reduction in GnRH positive neurons) in ventral forebrain reaching their ultimate destination, suggestive of a migratory defect (Pierce et al., 2008). The GnRH neuron distribution in Gas6 KO mice was also defective during embryogenesis, suggesting that the migratory path may have been altered or obstructed, leading to retention of neurons in the nasal compartment. However, the GnRH neurons in Gas6 KO adult mice, although decreased in number showed a normal distribution, suggesting early loss of Gas6 action can be compensated across later development. Reports have suggested that the minimum GnRH neuron requirement for pulsatile GnRH secretion and reproductive function is quite low (<70 GnRH neurons. males need 12% and females need 12-34% of the population) (Herbison et al., 2008). This implies that several other factors including the organization of the neuron network as well as receptivity of the GnRH neuron to estradiol positive feedback are critical for normal sexual development and reproductive competence (Lederman et al., 2010).
In contrast to prior reports suggesting a lack of TAM expression in vaginal tissues (Wu et al., 2008), both WT and Gas6 KO tissues expressed variable, but detectable amounts of each of the TAM family mRNAs. In Wu et al (2008), the stage of estrous cyclicity was not reported which may explain the discrepancy in the results. Normal onset of vaginal opening is thought to be dependent on GnRH activation of the pituitary gonadotropins, luteinizing hormone and follicle stimulating hormone secretion to mediate upregulation of ovarian LH receptors and subsequent estrogen production (Grosdemouge et al., 2003). Because estrous cyclicity was unaffected and cycle length did not differ from WT controls, delayed vaginal opening in Gas6 KO mice may be due to a combined defect in vaginal development and disrupted central control. Interestingly, mice lacking all 3 TAM members (triple knockouts, or TKO) exhibit a high incidence of distal vaginal atresia which has been attributed to a loss of MER, since the single Mer KO but not the Axl or Tyro3 KO have delayed vaginal opening (Wu et al., 2008). Together these data suggest that Gas6/Mer signaling plays a pivotal role in vaginal development, while Tyro3 and Axl play a supportive role.
Mer has been shown to mediate ingestion of apoptotic cells by phagocytes (Prasad et al., 2006; Scott et al., 2001) suggesting that the vaginal atresia observed in some Mer single, double with loss of either Axl or Tyro3, and triple (missing all TAM receptors) knockout mice may have resulted from impaired phagocytosis (Wu et al., 2008). Other researchers have shown that the tissue remodeling required for maturation of the female mouse reproductive tract is an apoptosis-dependent process and that vaginal opening does not occur in transgenic mice that express Bcl2 protein in vaginal mucosa (Bless et al., 2006). Furthermore, Bax−/−Bak−/− transgenic mice have imperforate vaginal canal. A functional study of mouse macrophages revealed that compared with wild type, phagocytosis was reduced when macrophages lacked either Gas6 or Mer (Schwarting et al., 2006). The fact that the Gas6 KO had delayed VO despite up-regulation of all 3 TAM receptor expressions further supports the hypothesis that the timing of vaginal opening is Gas6 dependent. We propose that the early defect in the Gas6 KO mice is secondary to the loss of Gas6 activation of Mer in the vagina. The delayed estrous and cycling defects are in turn related to the Gas6 dependent and independent effects on early survival of GnRH neurons during migration. The overall phenotype is most likely due to a combination of central and peripheral defects.
Gas6 is homologous to Protein S (ProS), a minor TAM ligand that has a role in the coagulation cascade and has been shown to signal to TAM family members in diverse cells (Lemke and Rothlin, 2008). ProS expression was detected in both NLT and GT1-7 GnRH neuronal cell lines; however in our hands, experiments where ProS was either silenced or overexpressed in the presence or absence of Gas6 did not alter TAM signaling in NLT GnRH neuronal cells (unpublished observations), suggesting it is not a relevant ligand in TAM mediated effects in reproductive development and sexual maturation. In the absence of ProS null mice, we cannot exclude whether it or other factors may act as relevant ligands in mediating the reproductive compensation in Gas6 null mice during adulthood.
Ligand dependent activation of TAM family receptors induces receptor dimerization and is very crucial in mediating diverse processes including phagocytosis, aggregation and cell differentiation in various cell types (Vendel et al., 2006). In GnRH neurons, TAM mediated functions like neuronal movement and survival; protein processing and downstream signaling pathways are dependent on ligand activation. However, autophosphorylation and chemotactic responses like cellular aggregation may be ligand independent. Evidence of ligand independent homophilic interactions amongst TAM family members or heterotypic crosstalk of Gas6/TAM receptors with other heparan sulfate ligand binding receptors like Met (Bisenius et al., 2006; Salian-Mehta et al., 2013) and G–protein coupled receptors like Prokineticin receptor 2 (ProkR2) (Matsumoto et al., 2006) suggest their possible role in impacting the normal function of the TAM receptors. In the absence of Gas6, these ligand independent TAM activations may potentially overlap in regulating GnRH neuronal development and maintaining fertility. Taken together our data suggest that Axl and Tyro3 use both Gas6 ligand dependent and independent mechanisms to contribute to normal GnRH neuronal development and function that ultimately leads to an optimal reproductive competence.
Supplementary Material
Figure S1: TYRO3, MER and GAS6 protein expression in vaginal tissue lysates of WT and Gas6 KO mice.
Figure S2: Semi-quantitative real time polymerase chain reaction (qRT-PCR) of TAM family member and Gas6 mRNA levels in uterus obtained from WT and Gas6 KO mice (n = 5 for WT and n=3 for Gas6 KO,* p < 0.05).
Figure S3: Gas6 KO mice show similar phases of % of estrous cyclicity compared to 2 month old WT mice (n = 8 for WT and n = 5 for Gas6 KO, two-way ANOVA with Bonferroni posttest).
Figure S4: 4 month old Gas6 KO mice show similar phases of % of estrous cyclicity compared to their respective WT mice (n = 5 for WT and n = 4 for Gas6 KO, two-way ANOVA with Bonferroni posttest).
Highlights.
Reproductive phenotype of Growth arrest specific gene 6(Gas6) null was characterized.
GnRH neuronal population was significantly decreased in Gas6 KO embryos and adults.
Gas6 plays a critical role in the timing of vaginal opening
Axl/Tyro3 use ligand-dependent and independent mechanism for GnRH neuronal function.
Acknowledgements
We thank Dr. Greg Lemke from Salk Institute (La Jolla, CA) for kindly providing the Gas6 knockouts that have been used in these studies. This work was supported by National Institute of Health Grant (HD31191) to M.E.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen MP, Zeng C, Schneider K, Xiong X, Meintzer MK, Bellosta P, Basilico C, Varnum B, Heidenreich KA, Wierman ME. Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt. Mol Endocrinol. 1999;13:191–201. doi: 10.1210/mend.13.2.0230. [DOI] [PubMed] [Google Scholar]
- Allen MP, Xu M, Linseman DA, Pawlowski JE, Bokoch GM, Heidenreich KA, Wierman ME. Adhesion-related kinase repression of gonadotropin-releasing hormone gene expression requires Rac activation of the extracellular signal-regulated kinase pathway. J Biol Chem. 2002;277:38133–38140. doi: 10.1074/jbc.M200826200. [DOI] [PubMed] [Google Scholar]
- Bisenius ES, Veeramachaneni DN, Sammonds GE, Tobet S. Sex differences and the development of the rabbit brain: effects of vinclozolin. Biol Reprod. 2006;75:469–476. doi: 10.1095/biolreprod.106.052795. [DOI] [PubMed] [Google Scholar]
- Bless E, Raitcheva D, Henion TR, Tobet S, Schwarting GA. Lactosamine modulates the rate of migration of GnRH neurons during mouse development. Eur J Neurosci. 2006;24:654–660. doi: 10.1111/j.1460-9568.2006.04955.x. [DOI] [PubMed] [Google Scholar]
- Cariboni A, Maggi R, Parnavelas JG. From nose to fertility: the long migratory journey of gonadotropin-releasing hormone neurons. Trends in neurosciences. 2007;30:638–644. doi: 10.1016/j.tins.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez L, Bellido-Martin L, Garcia de Frutos P. Growth arrest-specific gene 6 (GAS6) An outline of its role in haemostasis and inflammation. Thrombosis and haemostasis. 2008;100:604–610. doi: 10.1160/th08-04-0253. [DOI] [PubMed] [Google Scholar]
- Goruppi S, Ruaro E, Schneider C. Gas6, the ligand of Axl tyrosine kinase receptor, has mitogenic and survival activities for serum starved NIH3T3 fibroblasts. Oncogene. 1996;12:471–480. [PubMed] [Google Scholar]
- Grosdemouge I, Bachelot A, Lucas A, Baran N, Kelly PA, Binart N. Effects of deletion of the prolactin receptor on ovarian gene expression. Reproductive biology and endocrinology : RB&E. 2003;1:12. doi: 10.1186/1477-7827-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149:597–604. doi: 10.1210/en.2007-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MA. Embryology Carnegie Stage Comparison. 2014 http://embryology.med.unsw.edu.au/embryology/index.php?title=Carnegie_Stage_Comparison.
- Laurance S, Lemarie CA, Blostein MD. Growth arrest-specific gene 6 (gas6) and vascular hemostasis. Advances in nutrition. 2012;3:196–203. doi: 10.3945/an.111.001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman MA, Lebesgue D, Gonzalez VV, Shu J, Merhi ZO, Etgen AM, Neal-Perry G. Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacology. 2010;58:314–320. doi: 10.1016/j.neuropharm.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nature reviews. Immunology. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Advances in cancer research. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livne I, Gibson MJ, Silverman AJ. Biochemical differentiation and intercellular interactions of migratory gonadotropin-releasing hormone (GnRH) cells in the mouse. Dev Biol. 1993;159:643–656. doi: 10.1006/dbio.1993.1271. [DOI] [PubMed] [Google Scholar]
- Manfioletti G, Brancolini C, Avanzi G, Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol. 1993;13:4976–4985. doi: 10.1128/mcb.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuichi K, Shigeyoshi Y. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci U S A. 2006;103:4140–4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Kawamoto K, Higashino K, Arita H. Prevention of growth arrest-induced cell death of vascular smooth muscle cells by a product of growth arrest-specific gene, gas6. FEBS letters. 1996;387:78–80. doi: 10.1016/0014-5793(96)00395-x. [DOI] [PubMed] [Google Scholar]
- Pierce A, Bliesner B, Xu M, Nielsen-Preiss S, Lemke G, Tobet S, Wierman ME. Axl and Tyro3 modulate female reproduction by influencing gonadotropin-releasing hormone neuron survival and migration. Mol Endocrinol. 2008;22:2481–2495. doi: 10.1210/me.2008-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A, Xu M, Bliesner B, Liu Z, Richards J, Tobet S, Wierman ME. Hypothalamic but not pituitary or ovarian defects underlie the reproductive abnormalities in Axl/Tyro3 null mice. Mol Cell Endocrinol. 2011;339:151–158. doi: 10.1016/j.mce.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P, Lemke G. TAM receptor function in the retinal pigment epithelium. Molecular and cellular neurosciences. 2006;33:96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Prieto AL, Weber JL, Tracy S, Heeb MJ, Lai C. Gas6, a ligand for the receptor protein-tyrosine kinase Tyro-3, is widely expressed in the central nervous system. Brain research. 1999;816:646–661. doi: 10.1016/s0006-8993(98)01159-7. [DOI] [PubMed] [Google Scholar]
- Prieto AL, Weber JL, Lai C. Expression of the receptor protein-tyrosine kinases Tyro-3, Axl, and mer in the developing rat central nervous system. The Journal of comparative neurology. 2000;425:295–314. [PubMed] [Google Scholar]
- Salian-Mehta S, Xu M, Wierman ME. AXL and MET crosstalk to promote gonadotropin releasing hormone (GnRH) neuronal cell migration and survival. Molecular and cellular endocrinology. 2013;374:92–100. doi: 10.1016/j.mce.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Schwarting GA, Henion TR, Nugent JD, Caplan B, Tobet S. Stromal cell-derived factor-1 (chemokine C-X-C motif ligand 12) and chemokine C-X-C motif receptor 4 are required for migration of gonadotropin-releasing hormone neurons to the forebrain. J Neurosci. 2006;26:6834–6840. doi: 10.1523/JNEUROSCI.1728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:201–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Schwarting GA. Minireview: recent progress in gonadotropin-releasing hormone neuronal migration. Endocrinology. 2006;147:1159–1165. doi: 10.1210/en.2005-1275. [DOI] [PubMed] [Google Scholar]
- Vendel AC, Terry MD, Striegel AR, Iverson NM, Leuranguer V, Rithner CD, Lyons BA, Pickard GE, Tobet SA, Horne WA. Alternative splicing of the voltage-gated Ca2+ channel beta4 subunit creates a uniquely folded N-terminal protein binding domain with cell-specific expression in the cerebellar cortex. J Neurosci. 2006;26:2635–2644. doi: 10.1523/JNEUROSCI.0067-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierman ME, Pawlowski JE, Allen MP, Xu M, Linseman DA, Nielsen-Preiss S. Molecular mechanisms of gonadotropin-releasing hormone neuronal migration. Trends Endocrinol Metab. 2004;15:96–102. doi: 10.1016/j.tem.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Wu H, Tang H, Chen Y, Wang H, Han D. High incidence of distal vaginal atresia in mice lacking Tyro3 RTK subfamily. Molecular reproduction and development. 2008;75:1775–1782. doi: 10.1002/mrd.20917. [DOI] [PubMed] [Google Scholar]
- Xiong W, Chen Y, Wang H, Wu H, Lu Q, Han D. Gas6 and the Tyro 3 receptor tyrosine kinase subfamily regulate the phagocytic function of Sertoli cells. Reproduction. 2008;135:77–87. doi: 10.1530/REP-07-0287. [DOI] [PubMed] [Google Scholar]
- Yagami T, Ueda K, Asakura K, Sakaeda T, Nakazato H, Kuroda T, Hata S, Sakaguchi G, Itoh N, Nakano T, Kambayashi Y, Tsuzuki H. Gas6 rescues cortical neurons from amyloid beta protein-induced apoptosis. Neuropharmacology. 2002;43:1289–1296. doi: 10.1016/s0028-3908(02)00333-7. [DOI] [PubMed] [Google Scholar]
- Yanagita M, Ishimoto Y, Arai H, Nagai K, Ito T, Nakano T, Salant DJ, Fukatsu A, Doi T, Kita T. Essential role of Gas6 for glomerular injury in nephrotoxic nephritis. The Journal of clinical investigation. 2002;110:239–246. doi: 10.1172/JCI14861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: TYRO3, MER and GAS6 protein expression in vaginal tissue lysates of WT and Gas6 KO mice.
Figure S2: Semi-quantitative real time polymerase chain reaction (qRT-PCR) of TAM family member and Gas6 mRNA levels in uterus obtained from WT and Gas6 KO mice (n = 5 for WT and n=3 for Gas6 KO,* p < 0.05).
Figure S3: Gas6 KO mice show similar phases of % of estrous cyclicity compared to 2 month old WT mice (n = 8 for WT and n = 5 for Gas6 KO, two-way ANOVA with Bonferroni posttest).
Figure S4: 4 month old Gas6 KO mice show similar phases of % of estrous cyclicity compared to their respective WT mice (n = 5 for WT and n = 4 for Gas6 KO, two-way ANOVA with Bonferroni posttest).