Abstract
The metabotropic glutamate (mGlu) receptors are a group of Class C Seven Transmembrane Spanning/G Protein Coupled Receptors (7TMRs/GPCRs). These receptors are activated by glutamate, one of the standard amino acids and the major excitatory neurotransmitter. By activating G protein-dependent and non G protein-dependent signaling pathways, mGlus modulate glutamatergic transmission in both the periphery and throughout the central nervous system. Since the discovery of the first mGlu receptor, especially the last decade, a great deal of progress has been made in understanding the signaling, structure, pharmacological manipulation and therapeutic indications of the 8 mGlu members.
Keywords: mGlu, 7TMR, pharmacology, allosteric modulation, heterodimer, drug discovery
1. Introduction
Glutamate is not only one of the 23 proteinogenic amino acids, but it is also the major excitatory neurotransmitter in the central nervous system (CNS). The glutamate receptors can be divided into two classes: the ionotropic glutamate receptors and the metabotropic glutamate receptors. While the ionotropic glutamate receptors (AMPA receptors, NMDA receptors and kainate receptors) mediate fast responses elicited by glutamate, the metabotropic glutamate (mGlu) receptors provide a mechanism by which glutamate can transduce environmental cues and modulate synaptic transmission via second messenger signaling pathways. Because of their widespread distribution, especially in the CNS, pharmacological manipulation of mGlus may represent ideal therapeutic interventions for a wide range of neurological and psychiatric disorders (Reviewed in (Niswender and Conn 2010, Gregory, Noetzel et al. 2013)).
2. Classification of mGlus
The 7 Transmembrane Spanning Receptors/G Protein Coupled Receptors (7TMRs/GPCRs) account for 4% of the entire protein-coding genome (Bjarnadottir, Gloriam et al. 2006) but represent the targets of approximately 40-50% of medicinal drugs on the market (Thomsen, Frazer et al. 2005). The core function of 7TMRs is to serve as a transducer of signals from the extracellular environment to the intracellular signaling machinery. The 7TMR superfamily can be classified into several classes: the Class A 7TMRs (or Rhodopsin-like receptors) account for almost 85% of the GPCR genes, the Class B 7TMRs (or secretin-like receptors) include 15 receptors and are regulated by peptide hormones, and the Class C 7TMRs, which are characterized by a large extracellular N-terminal domain and contain 22 distinct receptors. The Adhesion, Frizzled, Taste type-2 and other unclassified receptors comprise the rest of the superfamily (Bjarnadottir, Gloriam et al. 2006).
The mGlus belong to the Class C 7TMRs; this class also encompasses calcium sensing receptors, the GABAB receptor, taste receptors and other orphan Class C receptors. Since the cloning of rat mGlu1 in 1991, 8 mGlu subtypes have been cloned thus far, named mGlu1 through mGlu8. Within the family, the eight mGlu subtypes can be further classified into three groups, with an intragroup sequence homology of about 70% and an intergroup sequence homology of about 45% (reviewed in (Conn and Pin 1997)). The classification of mGlu receptors are summarized in Table 1: the group I mGlus include mGlu1 and mGlu5, the group II includes mGlu2 and mGlu3, whereas mGlu4, 6, 7 and 8 comprise the group III mGlus. While the group I mGlus are coupled to Gq, the group II and group III are coupled to Gi/o G proteins.
Table 1.
Classification, G protein coupling, splice variants and selective ligands for mGlu subtypes.
| Classification | G protein coupling | Receptor subtypes | Splice variants | Selective ligands |
|---|---|---|---|---|
| Group I | Gq/11 | mGlu1 | mGlu1a-h, mGlu1g393 mGlu1g620 Taste mGlu1 |
LY367385 (orthosteric agonist) Bay 36–7620 (NAM) Ro 67–7476 (PAM) Ro 67–4853 (PAM) VU71 (PAM) |
| mGlu5 | mGlu5a,b | CHPG (orthosteric agonist) MPEP (NAM) MTEP (NAM) CDPPB (PAM) CPPHA (PAM) VU0365396 (SAM) |
||
| Group II | Gi/o | mGlu2 | mGlu2 |
LY487379 (PAM) BINA (PAM) |
| mGlu3 | GRM3Δ2 GRM3 Δ 4 GRM3 Δ 2 Δ 3 |
ML337 (NAM) | ||
| Group III | Gi/o | mGlu4 | mGlu4a,b Taste mGlu4 |
LSP1-2111 (orthosteric agonist) LSP4-2022 (orthosteric agonist) PHCCC (PAM) VU0155041(PAM) VU0364770 (PAM) ADX88178 (PAM) Lu AF21934 (PAM) |
| mGlu6 | mGlu6a-c | |||
| mGlu7 | mGhu7a-e | AMN 082 (allosteric agonist) MMPIP (NAM) ADX71743 (NAM) |
||
| mGlu8 | mGlu8a-c | (S)-3,4-DCPG (orthosteric agonist) AZ12216052 (PAM) |
3. Structure of mGlus
3.1 General structural features of mGlus
As members of the Class C 7TMRs, the mGlus are characterized by a large N-terminal domain, commonly referred to as the Venus Flytrap Domain (VFD). Studies of the crystal structures of the mGlu1, 3 and 7 VFDs reveal that each VFD contains two lobes, which together form a clam shell-like structure, with the glutamate binding site found between the two lobes (Kunishima, Shimada et al. 2000, Tsuchiya, Kunishima et al. 2002, Acher and Bertrand 2005, Muto, Tsuchiya et al. 2007). Besides glutamate, the VFDs of some mGlus also bind other endogenous agonists of mGlus, such as cinnabarinic acid (Fazio, Lionetto et al. 2012) and L-serine-O-phosphate (Klunk, McClure et al. 1991) (Hampson, Huang et al. 1999), as well as magnesium and calcium, which can modulate receptor activity (Kubo, Miyashita et al. 1998, Kunishima, Shimada et al. 2000, Francesconi and Duvoisin 2004). Both structural (Kunishima, Shimada et al. 2000, Tsuchiya, Kunishima et al. 2002, Muto, Tsuchiya et al. 2007) and biochemical data (Romano, Yang et al. 1996) suggest that the VFDs from two distinct mGlu receptors sit back to back and dimerize together. Upon ligand binding, large conformational changes lead to closure of the two lobes. Closure of one or both VFDs within each mGlu dimer initiates receptor activation (Kniazeff, Bessis et al. 2004).
Connecting the VFD and the seven transmembrane spanning domain (7TMD) is the cysteine-rich domain (CRD). Based on structural studies with mGlu2, the CRD contains 9 cysteine residues; 8 of them form internal disulfide bonds to stabilize the structure of this domain. In addition, the ninth cysteine forms a disulfide bond linked to the VFD (Muto, Tsuchiya et al. 2007), such that the CRD senses the conformational changes induced by ligand binding and transmits it to the 7TMD. The 7TMD and intracellular loops play important roles in receptor-G protein coupling and receptor modulation. It has been shown that a single mutation in the 7TMD that disrupts the hydrogen-bonding network in TM6 and TM7 induces high constitutive activity of mGlu8 (Yanagawa, Yamashita et al. 2013), suggesting that TM6 and TM7 constrain the receptor in an inactive conformation and that rearrangement between these two helixes is critical for receptor activation. The 7TMD also provides an opportunity to modulate receptor activity at a site other than the traditional agonist-binding VFD. All mGlu small molecule allosteric modulators discovered to date are believed to bind to receptor 7TMDs.
The recently solved crystal structure of the mGlu1 7TMD bound to a negative allosteric modulator has provided us with a more detailed understanding on the structural characteristics and activation mechanisms of the receptors (Wu, Wang et al. 2014). This 2.8Å resolution structure has confirmed a parallel dimer conformation of the mGlu 7TMD, stabilized by cholesterol molecules. Although sharing few identical or even conserved residues with other 7TMRs, such as Class A receptors, the overall fold of the mGlu1 7TMD is highly consistent with those of other families. The discovered binding pocket for the negative allosteric modulator partially overlaps with the binding pocket for orthosteric ligands on Class A receptors. Interestingly, this pocket on mGlu1 is restricted by the second extracellular loop, which may explain the binding of native agonist at the level of the VFD instead of 7TMD.
The intracellular loops of mGlus are involved in G protein coupling and receptor phosphorylation. Specifically, the second intracellular loop is implicated in G protein coupling specificity of the mGlus (Pin, Joly et al. 1994, Gomeza, Joly et al. 1996, Havlickova, Blahos et al. 2003), which is usually the function of the third intracellular loop for Class A, rhodopsin-like receptors.
The C-terminal domain of the mGlus is within close proximity to the inner leaflet of the lipid bilayer. Although recent structural studies of the purified intracellular C-terminal domains from mGlu6, 7 and 8 suggest that the C-termini of unliganded mGlus are mediated by linear motifs rather than secondary/tertiary structures (Seebahn, Dinkel et al. 2011), these intracellular tails of mGlus interact with various intracellular proteins, and are subject to alternative splicing (see section 3.1 and 3.2.2), phosphorylation, and SUMOylation (reviewed in (Enz 2012)).
3.2 The dimeric complex of mGlus
As mentioned above, mGlus form stable, covalently linked dimers. Data suggesting constitutive conformation of mGlu homodimers emerged as early as 1996 (Romano, Yang et al. 1996), and was further supported by data obtained from mGlu VFD/CRD crystal structures (Kunishima, Shimada et al. 2000). Evidence from biochemical studies reveals that one or more cysteine residues on the N-terminal extracellular domain mediate the covalent and non-covalent interactions between two mGlu protomers (Ray and Hauschild 2000, Romano, Miller et al. 2001). In addition, several studies have shown that mGlus do not appear to form higher-order oligomers (Brock, Oueslati et al. 2007, Doumazane, Scholler et al. 2011), although this is the case for some other Class C 7TMRs, such as the GABAB receptor (Comps-Agrar, Kniazeff et al. 2012).
The activation machinery of mGlu homodimers has been studied in depth, particularly by Jean Philippe Pin's group, using a quality control system adapted from the GABAB receptor to generate mGlu dimers bearing specific mutations within one of the protomers. These data suggest that closure of one VFD per dimer is sufficient to activate the receptor, although closure of both VFDs is required to achieve full activity (Kniazeff, Bessis et al. 2004). When one or both VFDs are occupied, the 7TM domain of either protomer can be activated through intersubunit rearrangement (Brock, Oueslati et al. 2007). These findings are consistent with the hypothesis that only a single 7TM domain is turned on upon activation of each homodimeric receptor (Goudet, Kniazeff et al. 2005, Hlavackova, Goudet et al. 2005, Hlavackova, Zabel et al. 2012).
Examples exist for heterodimers of mGlus and Class A 7TMRs in the CNS. Gonzalez-Maeso et al. reported that mGlu2 receptors interact with 5-HT2A receptors through transmembrane helix domains and form functional complexes in brain cortex. Subsequent mutagenesis studies revealed that three residues within transmembrane domain 4 of mGlu2 are necessary to form the 5-HT2A-mGlu2 receptor heterocomplex (Moreno, Muguruza et al. 2012). Furthermore, hallucinogenic 5-HT2A agonists elicit unique responses at 5-HT2A/mGlu2 complexes, which are implicated in the pathogenesis of psychosis (Gonzalez-Maeso, Ang et al. 2008). However, it should be noted that, although the formation of mGlu2/5HT2A heterocomplexes has been validated by other groups, (Delille et al., 2012) the unique signal transduction pathways mediated by the hetereodimeric complex was not replicated. In addition, it was also shown that mGlu2 can interact with 5-HT2B, indicating that complex formation is not specific to the 5-HT2A-mGlu2 pair and challenging the biological relevance of the 5-HT2A-mGlu2 complex.
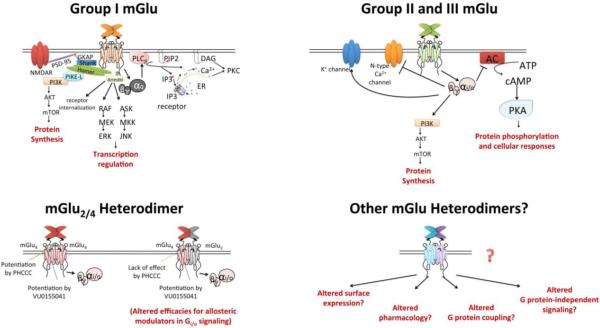
With regards to mGlu heterodimers, the VFD of mGlu1 can interact with full length mGlu5 and vice versa (Beqollari and Kammermeier 2010). In addition, a splice variant of mGlu1 that contains only the VFD functions as a dominant negative to potently block the signaling of full length mGlu1 or mGlu5 (Beqollari and Kammermeier 2010). Evidence for full length mGlu heterodimers initially emerged in in vitro expression systems (Doumazane, Scholler et al. 2011, Kammermeier 2012). By using a time-resolved FRET assay, Doumazane et al. demonstrated that group I mGlus can interact with each other, but do not associate with group II and group III mGlu subtypes; in contrast, group II and III mGlu receptors can co-assemble within and outside of the two groups. In addition, Kammermeier's study utilizing injected superior cervical ganglion cells suggests that heterodimerization may alter the pharmacology of mGlus and their modulators. Together, these findings indicate that the functions and signaling of mGlus could be much more diverse and complex than previous estimated.
Recently, the existence and pharmacology of mGlu2/4 heterodimers has been established in native tissues (Yin, Noetzel et al. 2014). Using biochemical and pharmacological approaches, this study confirms that mGlu2 and mGlu4 form a hetero-complex in vitro and extends the finding into rat and mouse brain tissues. In cell lines, as well as at corticostriatal synapses, the mGlu2/4 heterodimer exhibits a distinct pharmacological profile compared to mGlu2 or mGlu4 homodimer populations, with alterations in affinity and efficacy for both mGlu2 and mGlu4 allosteric modulators. Specifically, it has been shown that PHCCC and 4PAM-2, two positive allosteric modulators that bind to the same allosteric pocket, exhibit diminished efficacy at mGlu2/4 heterodimers. In contrast, the potentiation induced by VU0155041 and Lu AF21934, two PAMs that bind to a second allosteric pocket, remains similar at homomers or mGlu2/4 heteromers. This suggests that these allosteric binding pockets may encounter differential conformational changes upon hetero-interaction of the two receptor subunits, and that distinct classes of allosteric modulators can be differentially regulated by mGlu heterodimers. While dramatically shifting our understanding of the functional roles of the mGlu family, these findings also potentially explain the discordant pharmacological findings observed in native brain tissue (Ayala, Niswender et al. 2008, Niswender, Johnson et al. 2010) and provide important clues for rational development of therapeutic reagents that target specific mGlu receptor assemblies.
3.3 Alternative splicing of mGlus
All mGlu subtypes have been discovered to undergo alternative splicing, primarily at the C-terminus, and some of the better characterized splice variants are described below. In human, 8 different splice variants of mGlu1 exist, named mGlu1a, 1b, 1c, 1d, 1e, 1g, 1g-393, 1g-620 and 1h. Two newly identified exons in human GRM1 express a novel splice variant of metabotropic glutamate 1 receptor. mGlu1a is the longest variant and the others result from differential splice site usage, generating distinct isoforms with differing C-termini (reviewed in (Hermans and Challiss 2001)). The splice variant containing only the VFD has been shown to act as a dominant negative, preventing full length mGlu1 isoforms from signaling (Beqollari and Kammermeier 2010). Also within the group I mGlus, mGlu5a and mGlu5b are two splice variants for mGlu5 with similar pharmacological profiles (Minakami, Katsuki et al. 1994, Joly, Gomeza et al. 1995). Three splice variants of mGlu3 exist in human brain due to exon skipping events: GRM3Δ2 (lacking exon 2), GRM3Δ4 (lacking exon 4), and GRM3Δ2Δ3 (lacking exons 2 and 3). Among the three variants, GRM3Δ4 is most abundantly expressed and represents an mGlu3 receptor without a seven-transmembrane domain, which may have unique functions and relate to non-coding single nucleotide polymorphisms (SNPs) in patients with cognitive dysfunctions (Sartorius, Nagappan et al. 2006). As to the group III mGlus, the mGlu4 gene was described as undergoing alternative splicing to generate mGlu4a and mGlu4b (Thomsen, Pekhletski et al. 1997); however, this result has not been able to be replicated by other groups (Corti, Aldegheri et al. 2002). Three mGlu6 splice variants exists in human retina, with mGlu6b lacking 97 nucleotides from exon 6 and mGlu6c including 5 nucleotides from intron 5 (Valerio, Ferraboli et al. 2001). Both mGlu7 and mGlu8 can undergo alternative splicing at the C-terminus, resulting in at least 5 splice variants for mGlu7 and 2 variants formGlu8 (Corti, Restituito et al. 1998, Schulz, Stohr et al. 2002). In addition, another splice variant, mGlu8c, contains a 74 nucleotide insertion, resulting in a frame shift and termination of the polypeptide before the seven transmembrane domains (Malherbe, Kratzeisen et al. 1999). As the C-terminal intracellular domain plays important roles in protein-protein interactions and signal transduction, different splice variants may possess distinct profiles with regards to receptor activation, receptor modification and receptor internalization (Enz 2012). With the advances in sequencing technology, the diversity of mGlu splice variants is increasing rapidly. For example, 7 splice variants have been predicted for mGlu4 according to Ensembl genome database, although their existence still needs to be validated experimentally.
Several isoforms of mGlus also exist due to alterations at the N-terminus; for example, Taste mGlu1 and Taste mGlu4 (Chaudhari, Landin et al. 2000), which play roles in detecting the taste of umami. These receptor variants, with approximately 50% of the N-terminus truncated, are expressed in taste buds. Compared to full-length receptors, these N-truncated variants lack much of the glutamate binding domain and thus exhibit lower potency when activated by glutamate (Chaudhari, Landin et al. 2000).
3.4 Signaling of mGlus
3.4.1 G protein-dependent signaling
Classically, group I mGlus are generally coupled to Gq/11 and activate phospholipase Cβ, which hydrolyses phosphotinositides into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol, a pathway leading to calcium mobilization and activation of protein kinase C (PKC). Other effectors downstream of Gq include phospholipase D, ion channels, c-Jun N-terminal kinase (JNK), mitogen-activated protein kinase/extracellular receptor kinase (MAPK/ERK), and the mammalian target of rapamycin (mTOR) pathway (Sayer 1998, Servitja, Masgrau et al. 1999, Page, Khidir et al. 2006, Li, Li et al. 2007) (Figure 1). In addition, evidence has emerged that group I mGlus can also activate Gs and Gi/o and their downstream pathways, and that distinct regions on the receptor are responsible for coupling of different G proteins (Francesconi and Duvoisin 1998, McCool, Pin et al. 1998).
Figure 1.
The group I, II and III mGlu receptors induce signal transduction through both G protein-dependent and independent pathways. mGlu2/4 heterodimerization differentially regulates the efficacies of mGlu4 PAMs binding to separate allosteric pockets, whereas the expression, signaling and pharmacology of other mGlu heteromers remain unexplored. GKAP, guanylate kinase-associated protein; PSD-95, postsynaptic density protein 95; NMDAR, N-methyl-D-aspartate receptor; PI3K, phosphatidylinositol 3-kinase; PIKE-L, phosphatidylinositol 3-kinase enhancer-long; AKT, protein kinase B; mTOR, mammalian target of rapamycin; ERK, extracellular signal-regulated kinase; ASK, apoptosis signal-regulating kinase; JNK, c-Jun N-terminal kinases; PIP2, Phosphatidylinositol 4,5-bisphosphate; DAG, diacyl-glycerol; IP3, inositol 1,4,5-trisphosphate; ER, endoplasmic reticulum; PKC, protein kinase C; AC, adenylate cyclase; PKA, protein kinase A.
As stated previously, the group II and group III mGlu receptors are coupled to Gi/o proteins and negatively regulate the activity of adenylyl cyclase. In addition, many ion channels have also been reported to be regulated by Gαi and the liberated Gβγ subunit (Guo and Ikeda 2005, Niswender, Johnson et al. 2008, Kammermeier 2012). Gi/o-mediated activation of MAPK and phosphatidyl inositol 3-kinase (PI3 kinase) pathways, as supported by the inhibitory effect of pertussis toxin (Iacovelli, Bruno et al. 2002), add another level of complexity to the G protein-mediated signaling of group II and III mGlus.
3.4.2 G protein-independent signaling
While 7TMRs transduce signals through various cellular pathways, their responsiveness may also be regulated by receptor desensitization. When a receptor is stimulated, activated G protein-coupled receptor kinase (GRK) then initiates a combination of events including receptor phosphorylation, arrestin binding, and receptor internalization (reviewed in (Krupnick and Benovic 1998)), providing a feedback mechanism that prevents receptor over-stimulation. For mGlus, such regulation is more thoroughly studied for group I mGlus than the other two groups. For example, internalization of mGlu1 has been shown to depend on GRK4 and β arrestin-1 (Dale, Bhattacharya et al. 2001, Iacovelli, Salvatore et al. 2003). Desensitization of mGlu5, however, seems to be dependent on GRK2 activity, suggesting different mGlu subtypes are regulated by distinct mechanisms (Sorensen and Conn 2003).
Besides regulating receptor desensitization, recruited β-arrestins are also well-known as scaffolding protein for signaling molecules. Reports have shown that active Src is recruited to activated 7TMRs by interaction with β-arrestin, which then results in phosphorylation of downstream molecules and consequently activation of the MAPK cascade (Luttrell, Ferguson et al. 1999). In addition, arrestins also directly facilitate the subcellular localization and activation of two MAPK cascades (the RAF→MEK→ extracellular signal-regulated kinases (RAF-MEK-ERK) cascade and the apoptosis signal-regulating kinase→MKK→c-Jun N-terminal kinases (ASK-MKK-JNK cascade)) (Pierce and Lefkowitz 2001), further expanding the dimension and complexity of signal transduction upon GPCR activation. This role of arrestins in meditating signal transduction events has been demonstrated for mGlus as well. For example, activation of mGlu7 significantly reduces N-methyl D-aspartate receptor (NMDAR)-mediated currents in prefrontal cortex pyramidal neurons in a β-arrestin/ERK signaling pathway-dependent manner (Gu, Liu et al. 2012).
In addition, mGlu receptors also demonstrate the ability to activate signaling cascades through protein-protein interactions. For instance, the C-terminal domains of mGlu1 and mGlu5 interact with Homer proteins, a group of scaffolding proteins for multiprotein complexes. Besides interacting with the receptor, Homer proteins demonstrate binding ability with inositol-1,4,5-triphosphate (IP3) receptors, ryanodine receptors, transient receptor channel-1 and 4 (TRPC1, TRPC4), P/Q-type Ca2+ channels, Shank, the phosphoinositide 3-kinase (PI3K)enhancer-long (PIKE-L) etc., coupling receptor activation to other signaling components within the cell (Rong, Ahn et al. 2003, Bockaert, Dumuis et al. 2004, Fagni, Ango et al. 2004). mGlu5 has been found to interact with the NMDA receptor via Homer and other scaffolding proteins and potentiate receptor activity (Tu, Xiao et al. 1999, Attucci, Carla et al. 2001, Pisani, Gubellini et al. 2001). In addition, it has been shown that disruption of mGlu5–Homer interactions selectively blocks mGlu activation of the PI3K-Akt-mTOR pathway (Ronesi and Huber 2008) and contribute to phenotypes of Fmr1 knockout mice, an animal model for Fragile X syndrome (Ronesi, Collins et al. 2012). Interestingly, Homer proteins include long Homer isoforms and short isoforms (Homer1a and Ania), which act as endogenous dominant-negatives and disrupt protein complexes containing the long Homer variant. The ratio of Homer 1a/long Homer bound to mGlu5 may associate with cognitive aging (Menard and Quirion 2012) and has been shown to be altered in Fmr1 knockout mice (Ronesi, Collins et al. 2012). In addition, genetic deletion of Homer1a rescues several phenotypes in Fmr1 knockout mice, suggesting the importance of Homer proteins in the mechanism of Fragile X syndrome and potential therapeutic intervention for this disease (Ronesi, Collins et al. 2012). Besides the PI3K-Akt-mTOR pathway, Homer also links mGlu5 to PIKE-L, which prevents cell apoptosis upon mGlu5 activation (Rong, Ahn et al. 2003). Interestingly, it has been shown that the disruption of Homer-mGlu5 interaction reduces astrocyte apoptosis (Paquet, Ribeiro et al. 2013), suggesting opposite functions of Homer in regulation of cell apoptosis in neurons and astrocytes. Besides the CNS, a point mutation of mGlu1 within the Homer binding region that has been discovered in the somatic cells of lung cancer patients (Esseltine, Willard et al. 2013), indicating important roles of Homer in the periphery.
Another well-studied protein-protein interaction is the mGlu7-protein interacting with C kinase 1 (PICK1) interaction. PICK1 was discovered as a peripheral membrane protein that interacts with protein kinase Cα (PKCα) (Staudinger, Zhou et al. 1995). Besides PICK1, the C-terminus of mGlu7 also interacts with Ca2+-calmodulin, G protein βγ subunits to modulate the activity of voltage-gated Ca2+ channel and negatively regulate neurotransmitter release (Dev, Nakanishi et al. 2001). Interestingly, phosphorylation of the receptor by PKC increases receptor binding to PICK1, which is required for stable surface expression of mGlu7 (Suh, Pelkey et al. 2008, Suh, Park et al. 2013), but, at the same time, inhibits the binding of Gβγ subunits and Ca2+-calmodulin, providing a delicate regulatory machinery. Disruption of the mGlu7-PICK1 interaction has been performed by genetic knock-in of an mGlu7 mutant that does not bind PICK1. The resulting animals exhibited significant defects in hippocampus-dependent spatial working memory and high susceptibility to convulsant drugs (Zhang, Bertaso et al. 2008). Additionally, injection of a competing peptide to rodents also resulted in behavioral symptoms and EEG discharges that are characteristic of absence epilepsy (Bertaso, Zhang et al. 2008). These data indicate that the mGlu7-PICK1 interaction is important for regulating mGlu7 signaling and may underlie certain disease mechanisms, including cognitive disorders and epilepsy.
4. Orthosteric modulation of mGlus
4.1 Non-selective ligands
Glutamate, the endogenous agonist of glutamate receptors (including ionotropic glutamate receptors), activates all mGlu subtypes, each with a distinct binding affinity and potency. Glutamate exhibits the lowest affinity for the group III mGlus and the highest for the group II receptors. As it pertains to synthetic ligands, (±) trans-ACPD (and its active isomer 1S,3R-ACPD) was the first identified agonist for mGlus with selectivity over ionotropic glutmate receptors and has served as a tool compound to assess the functional involvement of mGlu receptors in the regulation of signaling as a broad group (Palmer, Monaghan et al. 1989, Desai and Conn 1990, Manzoni, Fagni et al. 1990, Schoepp, Johnson et al. 1991, Schoepp, Johnson et al. 1991). Additionally, several amino acid analogs have been shown to be more selective for each of the three subgroups and this as will be discussed in the sections below.
4.2 Orthosteric ligands of group I mGlus
Quisqualic acid demonstrates high affinity and selectivity for mGlu1 and 5 (affinity~56 and 52 nM, respectively) (Ohashi, Maruyama et al. 2002), and was the first identified agonist for Group I mGlus. Mutagenesis studies revealed that the subtype selectivity of this compound results from a complex interplay of residues shaping the binding pocket of the receptor (Hermit, Greenwood et al. 2004). The use of this compound in native tissue, however, is limited by its activity at AMPA receptors and the subsequent complications that this brings to data interpretation (Watkins, Krogsgaard-Larsen et al. 1990).
3,5-DHPG selectively activates Group I mGlus, with its agonist activity residing exclusively in its S-isomer (Schoepp, Goldsworthy et al. 1994, Baker, Goldsworthy et al. 1995). Importantly, (S)-3,5 DHPG is devoid of activity on both group II and group III mGlus, as well as glutamate transporters (Schoepp, Jane et al. 1999), representing the most selective agonist for group I mGlus thus far, although it lacks the ability to differentiate mGlu1 from mGlu5. Within the group I mGlus, analogs of quisqualic acid and (S)-3,5-DHPG have been shown to be more selective for mGlu5 over mGlu1. A homolog of quisqualic acid, (S)-homoquisqualic acid, is a competitive antagonist at mGlu1 while being a full agonist at mGlu5, displaying some potential to differentiate between the two group I subtypes. However, its selectivity is jeopardized by agonist activity at mGlu2 (Brauner-Osborne and Krogsgaard-Larsen 1998). A DHPG derivative, CHPG, exhitibs high selectivity at mGlu5 over mGlu1, although the low potency on mGlu5 (~750 μM) limits the utility of this compound (Doherty, Palmer et al. 1997).
Multiple orthosteric antagonists for group I mGlus have been developed from a phenylglycine scaffold, including (S)-4CPG, (S)-4C3HPG and (S)-MCPG, although they also exhibit some activity at mGlu2 (Schoepp, Jane et al. 1999). Within the 4CPG scaffold, LY367385 is a highly selective antagonist of mGlu1 relative to mGlu5, representing a tool compound to discriminate between the two group I mGlus (Bruno, Battaglia et al. 1999).
4.3 Orthosteric ligands of group II mGlus
DCG IV is potent group II mGlu agonist with affinities of 110 and 150 nM at rat mGlu2 and mGlu3, respectively (Schweitzer, Kratzeisen et al. 2000). However, it also possesses NMDA receptor agonist activity (Ishida, Saitoh et al. 1993) and antagonist activity at group I and group III mGlu receptors at high concentrations (Brabet, Parmentier et al. 1998). Another compound, 2R,4R-APDC, has an EC50 value of ~400 nM on cloned human mGlu2 and mGlu3 and is devoid of activity at group I or group III mGlus up to 100μM (Schoepp, Jane et al. 1999).
Compounds with even higher potency come from the heterobicyclic amino acid scaffold, including LY379268, with an EC50 of 2.7 nM and 4.6 nM in cells expressing mGlu2 and mGlu3, respectively (Monn, Valli et al. 1999). While this compound shows no activity at ionotropic glutamate receptors, it does activate mGlu4, mGlu6 and mGlu8 at high concentrations (Monn, Valli et al. 1999). However, the nearly 1000 fold selectivity for mGlu2 and mGlu3 still makes LY379268 a commonly used tool compound for group II mGlus. Although currently no orthosteric agonist can completely differentiate between mGlu2 and mGlu3, an analog of LY354740 was found to exhibit mGlu2 agonist and mGlu3 antagonist activity (Dominguez, Prieto et al. 2005), providing an opportunity to tease apart the functional effects of mGlu2 from mGlu3.
EGLU is one of the first compounds that was found to antagonize mGlu2 and mGlu3 with little antagonism on group I or III mGlus (Jane, Thomas et al. 1996). LY341495 is an antagonist at mGlu2 and mGlu3 with high affinity (1.67 nM and 0.75 nM for human receptors, respectively) (Johnson, Wright et al. 1999). However, it also antagonizes group I and group III mGlus at submicromolar or micromolar concentration (Kingston, Ornstein et al. 1998).
4.4 Orthosteric ligands of group III mGlus
L-AP4 and L-SOP have been used as prototypical agonists for group III mGlus, with submicromolar to low micromolar potencies at mGlu4, 6, and 8, but around 200 μM potency and affinity at mGlu7 (Schoepp, Jane et al. 1999, Wright, Arnold et al. 2000). Although these two compounds are highly selective for group III receptors, they activate mGlu4 and mGlu8 with similar potencies. In contrast, (S)-3,4-DCPG exhibits 100-fold selectivity for mGlu8 over mGlu4 (Thomas, Wright et al. 2001) and has been used as a tool compound to study the function of mGlu8 in native systems (Zhai, Tian et al. 2002, Johnson, Jones et al. 2013). Recently, a virtual high throughput screening approach has been used to discover a new variable pocket and develop a group of mGlu4-preferring agonists, including LSP1-2111 and LSP4-2022 (Beurrier, Lopez et al. 2009, Goudet, Vilar et al. 2012). In particular, LSP4-2022 exhibits potencies of 0.1 μM at mGlu4 verses 29 μM at mGlu8, with no activity at the group I and II mGlus up to 100 μM (Goudet, Vilar et al. 2012), making this compound an important tool to selectively activate mGlu4.
MAP4 and MSOP, two analogs of L-AP4 and L-SOP, have been reported to be highly selective antagonists for group III mGlus (Schoepp, Jane et al. 1999). However, these two compound exhibited low potencies in a thallium flux assay where activation of G-protein regulated inwardly rectifying potassium channels (GIRK) was used as a readout of receptor activity (Niswender, Johnson et al. 2008). Besides these two compounds, CPPG antagonizes group III mGlus with at least 30-fold selectivity over other subtypes (Jane, Thomas et al. 1996).
5. Allosteric modulation of mGlus
5.1 Mechanism and advantages of allosteric modulation
Through years of research, orthosteric ligands useful in determining the physiological roles of mGlus have been developed which display group-selectivity. However, as all orthosteric ligands bind to the N-terminal VFD of mGlus, which is evolutionarily designed to bind glutamate, it is difficult to achieve subtype selectivity since the glutamate binding site is highly conserved across all eight subtypes. In addition, brain penetration and pharmacokinetics of these orthosteric ligands can be limited by their amino acid-like properties. Many of these hurdles have been overcome by targeting allosteric binding sites on the receptors. As indicated by the name, allosteric modulators bind to a site other than the endogenous agonist binding site, and provide modulatory effects on the affinity/efficacy of the orthosteric agonist, termed cooperativity. Indeed, the complex structure of mGlus offers a number of possibilities to develop novel allosteric modulators. Because of the greater sequence divergence demonstrated at allosteric sites, a series of subtype-selective allosteric agonists and positive, negative or silent allosteric modulators (PAM, NAM or SAMs) of mGlus have been identified through high through-put screening campaigns ((Varney, Cosford et al. 1999, Kinney, O'Brien et al. 2005, Mitsukawa, Yamamoto et al. 2005, Niswender, Johnson et al. 2008) and many others), which have greatly advanced studies on mGlu functions and accelerated the development of mGlu reagents as disease therapeutics.
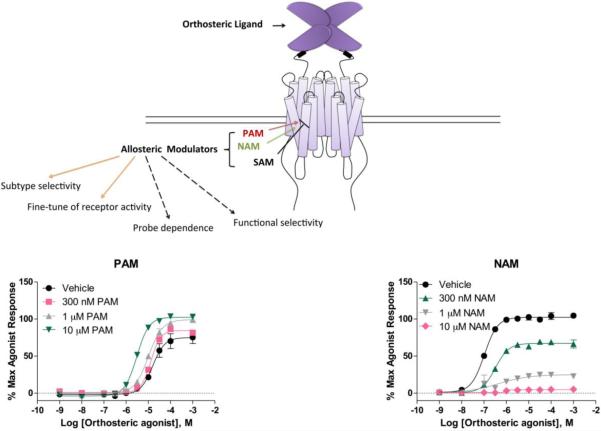
The mechanism of action of allosteric modulators is exemplified by the dataset shown in Figure 2. PAMs potentiate the response to orthosteric agonists by shifting the concentration-response curve of an orthosteric agonist to the left (with or without increasing the maxium response), indicating that the agonist response is being pharmacologically amplified. NAMs are molecules that antagonize the activity of agonists in a noncompetitive fashion through negative cooperativity. SAMs are neutral allosteric modulators that exhibit no apparent effect on agonist reponses on their own; however, such compounds can block the binding and subsequent receptor modulation induced by PAMs or NAMs.
Figure 2.
Mechanism of action of allosteric modulators. Instead of binding to the glutamate binding site on the N-terminal VFD, mGlu PAMs and NAMs bind to the 7TMD on the receptor and induce selective modulating effects, which can be blocked by SAMs. PAMs potentiate the response to orthosteric agonists by shifting the dose response curve of an orthosteric agonist to the left, whereas NAMs progressively antagonize the activity of agonists through negative cooperativity in a noncompetitive fashion.
Besides improved selectivity, PAMs and NAMs also provide some other advantages when compared to their orthosteric counterparts. First, the biological effects of PAMs and NAMs are dependent on the presence of the endogenous agonist; thus, they have the potential to preserve the spatial and temporal aspects of endogenous signaling. As PAMs which lack allosteric agonist activity will not constantly activate the receptor, they may also reduce the liability of receptor desensitization compared to the direct activation by orthosteric, or even allosteric, agonists. Allosteric modulators also bring a further advantage in that their modulating effect is saturable, thus providing a larger therapeutic window and potentially decreasing the risk of overdose. In addition, these allosteric molecules often possess drug-like properties and better pharmacokinetics and brain penetration compared to orthosteric ligands, an important feature considering the therapeutic indication of mGlus in CNS disorders.
Thusfar, the majority of identified mGlu allosteric modulators bind to the 7TMD region of the receptor. Results from mutagenesis studies indicate that the mGlu allosteric binding site likely corresponds to the orthosteric binding site in Class A 7TMRs (Litschig, Gasparini et al. 1999, Pagano, Ruegg et al. 2000, Malherbe, Kratochwil et al. 2003, Schaffhauser, Rowe et al. 2003, Chen, Goudet et al. 2008, Gregory, Noetzel et al. 2013). Indeed, recent structrual studies on mGlu1 suggest that the binding pocket for the NAM FITM is defined by residues on TM2, 3, 5, 6 and 7, which is analogous to the orthosteric site for many Class A receptors (Wu, Wang et al. 2014). In addition, multiple allosteric sites may exist on a given receptor, as examplified by mGlu4 (Drolet, Tugusheva et al. 2011) and mGlu5 (O'Brien, Lemaire et al. 2003, Chen, Nong et al. 2007, Chen, Goudet et al. 2008, Hammond, Rodriguez et al. 2010, Noetzel, Gregory et al. 2013).
The modulating effects induced by allosteric modulators can be quantified using operational models of allosterism (Leach, Sexton et al. 2007, Gregory, Noetzel et al. 2012):
where A and B are the molar concentration of the orthosteric agonist and the allosteric modulator, respectively; KA and KB are the equilibrium dissociation constant of the orthosteric agonist and the allosteric modulator, respectively; τA and τB quantify the efficacy of the orthosteric agonist and the allosteric modulator, respectively. Basal, Em and n represent the basal system response, maximal possible system response and the transducer function that links occupancy to response. Importantly, these models have introduced two parameters, α and β, to describe cooperativity of an allosteric ligand on the affinity and efficacy of orthosteric agonist. The operational models of allosterism not only allow quantitative estimation of modulator affinity and cooperativity values, which can be used to guide compound optimization processes, but can be used to derive reliable estimates of modulator affinities when radioligand is not available (Gregory, Noetzel et al. 2012).
Based on molecular pharmacology data, it has been hypothesized that binding of one PAM per mGlu dimer is sufficient to potentiate receptor activity (Goudet, Kniazeff et al. 2005). In contrast, binding of NAMs to both protomers appears to be necessary to inhibit receptor activation (Hlavackova, Goudet et al. 2005). Interestingly, PAMs can directly activate an N-terminal truncated mGlu (Goudet, Gaven et al. 2004, El Moustaine, Granier et al. 2012), suggesting that VFD-CRD region prevents PAMs from activating the receptor until glutamate is bound.
Despite the advantages mentioned above, many allosteric modulators are highly lipophilic, which diminishes their solubility, can affect their pharmacokinetic profile, and potentially increases off-target binding. The shallow structure-activity relationship among classes of allosteric ligands also represents a significant hurdle in the development of allosteric modulators. Additionally, because of the substantial activity alteration generated by minor structural changes, a number of mGlu allosteric modulator classes are susceptible to subtle “molecular switches” (Wood, Hopkins et al. 2011), by which compounds within a series can switch switch from NAM to PAM, PAM to NAM, become silent, or exhibit altered selectivity (Sharma, Kedrowski et al. 2009, Zhou, Manka et al. 2010, Lamb, Engers et al. 2011, Sheffler, Wenthur et al. 2012).
Allosteric compounds have also complicated our understanding of receptor pharmacology. As allosteric modulators potentiate/inhibit thereceptor through cooperativity with the orthosteric ligand being used, it is not surprising that “probe dependence” has been reported in some cases, in that modulators have differential effects depending upon the orthosteric ligand that is present (Suratman, Leach et al. 2011, Valant, Felder et al. 2012). Although examples still have yet to be discovered for mGlu receptors, cautions should be taken when choosing the orthosteric ligand for in vitro studies. In addition, similar to what has been described with some orthosteric ligands (Urban, Clarke et al. 2007), many allosteric compounds have been found to differentially stimulate multiple signaling cascades downstream of a receptor (Kenakin 2005), a phenomenon often termed “functional selectivity” “biased signaling”, or “ligand directed trafficking” (Mathiesen, Ulven et al. 2005, Zhang, Rodriguez et al. 2005, Urban, Clarke et al. 2007, Marlo, Niswender et al. 2009). Indeed, 7TMRs may adopt multiple structural conformations, and allosteric modulators may stabilize any of them, which can translate into the regulation of some signaling pathways but not others. Biased pharmacology of mGlu4 PAMs has also been observed through signal convergence when Gq-coupled receptors are co-activated (Yin, Zamorano et al. 2013). Although these findings highly complicate the application of allosteric modulators as disease therapeutics, it is conceivable that biased modulators may enhance the therapeutic outcome or avoid adverse effects when modulation/exclusion of a signal pathway is desired.
5.2 Allosteric modulators of group I mGlus
CPCCOEt is the first discovered NAM for mGlu1 and serves as a proof-of-concept example for the development of a selective mGlu ligand acting via an allosteric binding site (Litschig, Gasparini et al. 1999). A great number of structurally distinct compounds have now been identified as mGlu1-selective NAMs, including Bay 36–7620 (Carroll, Stolle et al. 2001), JNJ16259685 (Lavreysen, Wouters et al. 2004), YM-298198 (Kohara, Toya et al. 2005), FTIDC (Suzuki, Kimura et al. 2007), CFMMC (Fukuda, Suzuki et al. 2009), etc., many of which are potent and active in vivo. Ro 67–7476 and Ro 67–4853 represent two chemical series of selective mGlu1 PAMs (Knoflach, Mutel et al. 2001). In addition, VU71, an analog of the mGlu5 PAM CDPPB, has also been shown to specifically potentiate mGlu1. Interestingly, none of the three mGlu1 PAMs bind to the traditional NAM site (Hemstapat, de Paulis et al. 2006). More recently, a group of 9H-xanthene-9-carboxylic acid oxazol-2-yl-amides were reported as potent mGlu1 PAMs with improved pharmacokinetic profiles (Vieira, Huwyler et al. 2009).
Selective allosteric modulators have also been developed for mGlu5. SIB-1757 and SIB-1893 were the first mGlu5 selective allosteric antagonists to be discovered. Further structural modification of the same scaffold led to the discovery of the now widely used compounds MPEP and MTEP, two mGlu5 NAMs with improved potency, selectivity, and pharmacokinetic profile (Gasparini, Lingenhohl et al. 1999, Cosford, Tehrani et al. 2003, Lea and Faden 2006). Ongoing discovery and development work lead to a number of novel mGlu5 NAMs, many of which are in clinical development for multiple indications. An early mGlu5 NAM, fenobam, was evaluated in a small clinical trial in Fragile X syndrome (FXS) patients and exhibited efficacies in half of the patients (Berry-Kravis, Hessl et al. 2009). Currently, the mGlu5 NAMs ADX48621 (Dipraglurant) from Addex and AFQ056 (Mavoglurant) from Novartis are under Phase II/III clinical trials and demonstrate efficacy in Parkinson's disease levodopa-induced dyskinesia (PD-LID) and FXS (AddexPharmaceuticalsPressRelease , Berg, Godau et al. 2011, Jacquemont, Curie et al. 2011). Besides FXS, RO4917523 (RG7090), an mGlu5 NAM from Roche is being evaluated in Phase II clinical trial for treatment-resistant depression. Preclinically, novel mGlu5 NAMs from the (3-cyano-5-fluorophenyl)biaryl series demonstrated efficacious in operant sensation seeking test, an mouse model of addiction (Lindsley, Bates et al. 2011), further expanding potential indications and encouraging continued research for mGlu5 NAMs.
Several mGlu5 PAMs have been derived from multiple chemical series and more than one binding site exists for mGlu5 PAMs. VU-29, CDPPB and DFB and other compounds from those series interact with the common “MPEP” site (O'Brien, Lemaire et al. 2003, Chen, Nong et al. 2007), whereas CPPHA and the subsequently discovered NCFP interact with the receptor at a distinct allosteric site (Chen, Goudet et al. 2008) (Noetzel, Gregory et al. 2013). A benzamide scaffold, as exemplified by VU0357121, has been suggested to bind to a non-MPEP, non-CPPHA site on mGlu5 (Hammond, Rodriguez et al. 2010), raising the possibility of the existence of at least three PAM binding sites. Along with the discovery of PAMs, DCB (a DFB analog) and VU0365396 (a benzamide series analog) appear to be SAMs, with neutral activity at MPEP and non-MPEP sites (O'Brien, Lemaire et al. 2003, Hammond, Rodriguez et al. 2010), representing useful tool compounds to further investigate the allosteric binding sites on mGlu5.
5.3 Allosteric modulators of group II mGlus
Several NAMs from a dihydrobenzo[1,4]diazepin-2-one series have been reported to antagonize both mGlu2 and mGlu3 in a non-competitive fashion (Hemstapat, Da Costa et al. 2007,Woltering, Adam et al. 2008, Woltering, Wichmann et al. 2008). Recently, selective NAMs for mGlu3, ML289 and ML337, have also been discovered via a “molecular switch” from an mGlu5 PAM scaffold, where small modification to the structure changes its mode of pharmacology (Sheffler, Wenthur et al. 2010, Sheffler, Wenthur et al. 2012, Wenthur, Morrison et al. 2013).
BINA, LY487379 (along with CBiPES) and THIIC represent three chemical scaffolds of highly selective mGlu2 PAMs that lacks activity at mGlu3 (Johnson, Barda et al. 2005, Galici, Jones et al. 2006, Fell, Witkin et al. 2011). Additionally, a group of mGlu2 SAMs have been identified through FRET-based binding assays and slight modification of these SAMs yields three mGlu2 NAMs with mGlu3 PAM activity, indicating that identification of SAMs is a useful approach to discover novel mGlu allosteric modulators (Schann, Mayer et al. 2010). Mutagenesis studies have revealed that amino acids that are essential for mGlu2 PAM activity are dispensible for NAMs, and the converse has also been reported (Schaffhauser, Rowe et al. 2003, Rowe, Schaffhauser et al. 2008, Lundstrom, Bissantz et al. 2011). These data suggest that mGlu2 PAMs and NAMs may bind to different allosteric pockets, although binding studies using appropriate allosteric radioligands are required to validate this hypothesis.
5.4 Allosteric modulators of group III mGlus
Allosteric modulation has also been demonstrated to be a successful approach to selectively modulate mGlu4, 7 and 8. An mGlu1 partial antagonist, PHCCC, was the first identified PAM for mGlu4 with no activity on 6 of the other mGlu subtypes except for mGlu6 (Maj, Bruno et al. 2003, Marino, Williams et al. 2003, Beqollari and Kammermeier 2008). In in vitro mGlu4 assays, PHCCC exhibits no agonist activity by itself but increases the potency of glutamate at mGlu4 and acts as a proof-of-concept compound for targeting mGlu4 as a potential therapeutic strategy for disorders such as Parkinson's disease (Marino, Williams et al. 2003). Further optimization of the PHCCC scaffold has been challenging (Niswender et al., 2008, Williams et al 2010); however, a high-throughput screening campaign led to the identification of VU0155041 and VU0080421 as examples of non-PHCCC scaffold mGlu4 PAMs (Niswender, Johnson et al. 2008, Niswender, Lebois et al. 2008). Recently, many other mGlu4 PAMs, including 4PAM-2, VU0364770, ADX88178, LuAF21934 and others (Drolet, Tugusheva et al. 2011, Jones, Bubser et al. 2012, Le Poul, Bolea et al. 2012, Bennouar, Uberti et al. 2013), have emerged with significant improvements in potency, selectivity and pharmacokinetic profile compared to PHCCC. Particularly, VU0364770, ADX88178 and LuAF21934 exhibit high potency with good pharmacokinetic profiles for use as tool compounds; each of these ligands shows in vivo efficacy in animal models of PD and other disorders. Interestingly, VU0155041 also exhibits allosteric agonist activity at mGlu4 when tested in vitro (Niswender, Johnson et al. 2008), suggesting an alternative mechanism of action compared to PHCCC. Indeed, data obtained from radioligand binding assays has demonstrated that VU0155041 binds to a unique allosteric site on mGlu4 which is different from the binding site of PHCCC and 4PAM-2 (Drolet, Tugusheva et al. 2011); such differences may underlie the diverse performance of mGlu4 PAMs in mGlu2/4-expressing tissues, such as the corticostriatal synapses (Yin, Noetzel et al. 2014).
Allosteric compounds for mGlu7 suffer from pharmacological complications. AMN082 was discovered in a high through-put screen and is an orally active, brain-penetrant allosteric agonist of mGlu7 that directly activate the receptor by binding to the transmembrane domain (Mitsukawa, Yamamoto et al. 2005). Although AMN082 inhibits cAMP accumulation and stimulates GTPγS binding in cells expressing mGlu7, it was subsequently shown to have no effect on mGlu7 in activating a promiscuous G protein or the GIRK potassium channels (Suzuki, Tsukamoto et al. 2007, Ayala, Niswender et al. 2008). Furthermore, it does not activate mGlu7 at the Shaffer collateral-CA1 synapse in hippocampal slices (Ayala, Niswender et al. 2008). In addition, AMN082 and its major metabolite exhibit physiologically relevant binding affinity at several transporters, such as the serotonin transporter, the dopamine transporter and the norepinephrine transporter (Sukoff Rizzo, Leonard et al. 2011). With these caveats, the physiological and pharmacological effects of AMN082 in vivo should ideally be confirmed using mGlu7 knockout mice versus wildtype mice. When administered orally, AMN082 has been shown to induce a robust increase in stress hormone levels in wildtype mice, but not in mGlu7 knockout animals (Mitsukawa, Yamamoto et al. 2005), supporting a role of mGlu7 in conditions involving chronic stress. In addition, intraperitoneal injection of AMN082 induced antidepresant-like behavior in wildtype animals but not mGlu7 knockout littermates when they were examined in a tail suspension test (Palucha, Klak et al. 2007). However, it should be noted that AMN082 significantly decreased spontaneous locomotor activity and induced body tremors in both mGlu7 knockout mice and wildtype animals, suggesting phenotypes that are mediated by an off-target effect(s). Similar to AMN082, a selective mGlu7 NAM, termed MMPIP, also exhibits pathway dependence in different assays. It has been shown that MMPIP inhibits mGlu7-mediated calcium mobilization through Gα15 (Suzuki, Tsukamoto et al. 2007, Niswender, Johnson et al. 2010)); however, its potency is much lower in affecting mGlu7-mediated inhibition of cAMP accumulation or activation of GIRK channels (Suzuki, Tsukamoto et al. 2007, Niswender, Johnson et al. 2010). In addition, it appears to be unable to block mGlu7-modulated neurotransmission-at the Shaffer collateral-CA1 synapse (Niswender, Johnson et al. 2010). Therefore, caution should be taken in terms of the pathway dependence and selectivity when utilizing these two compounds. More recently, ADX71743 has been discovered to be a potent, selective and brain-penetrant NAM for mGlu7. Administration of ADX71743 in vivo resulted in an anxiolytic-like effect in the marble burying and elevated plus maze tasks, suggesting that inhibition of mGlu7 is a promising approach for the treatment of anxiety disorders (Kalinichev, Rouillier et al. 2013).
AZ12216052 is the best characterized selective mGlu8 PAM with an EC50 of 1 μM (Duvoisin, Pfankuch et al. 2010). This compound is systemically available upon intraperitoneal administration, and has been used in animal models for CNS diseases, such as anxiety (Duvoisin, Pfankuch et al. 2010, Duvoisin, Villasana et al. 2011). However, activity of AZ12216052 was retained in mGlu8 knockout animals (Duvoisin, Villasana et al. 2011), suggesting that the compound may have off target effects that mediate some of its anxiolytic profile.
6. Therapeutic indications of mGlus
6.1 CNS-related diseases
6.1.1 Schizophrenia and Alzheimer's disease
Schizophrenia is a debilitating psychiatric illness that affects approximately 1% of the population. Three clusters of symptoms exist for schizophrenia patients, these are classified as positive symptoms (hallucinations, delusions, paranoia), negative symptoms (social withdrawal and anhedonia) and cognitive impairments (attention, memory and problem solving deficits) (AmericanPsychiatricAssociation 2000, Nuechterlein, Barch et al. 2004). A number of neurotransmission systems are disturbed in schizophrenia patients; the most well studied is the dopaminergic system. A dopamine hypothesis has been proposed to link altered brain function to the misfiring of dopaminergic neurons based on the fact that amphetamines, which have been shown to exacerbate the psychotic symptoms in schizophrenia, increase dopamine release (Laruelle, Abi-Dargham et al. 1996), and drugs that block dopamine receptor function reduce psychotic symptoms. Although current therapies targeting the dopamine D2 receptor (typical antipsychotics) are available, multiple adverse drug effects can occur with these treatments and the therapeutic effects on the negative symptoms or cognitive deficits are limited (Liberman and Glick 2004, Liberman 2005, Pramyothin and Khaodhiar 2010). Atypical antipsychotics are as effective as the typical antipsychotics, although their affinity for dopamine D2 receptor is much lower (Jones and Pilowsky 2002), suggesting that the dopamine hypothesis may not be sufficient to explain the mechanism of schizophrenia. On the other hand, modulation of the glutamatergic pathway appears to be an attractive alternative approach for schizophrenia treatment (reviewed in (Herman, Bubser et al. 2012)). For example, the NMDA receptor antagonists phencyclidine (PCP), ketamine and MK-801 have been reported to induce psychotomimetic effects in healthy human subjects and exacerbate symptoms in schizophrenia patients (Lahti, Koffel et al. 1995, Adler, Malhotra et al. 1999). In contrast, agonists at the glycine site of the NMDA receptor which enhance receptor function have been shown to exhibit beneficial effects in schizophrenia patients (Heresco-Levy, Ermilov et al. 2004, Heresco-Levy and Javitt 2004).
As development of direct-acting NMDA receptor agonists has been limited by the potential risk of side effects such as excitotoxicity and seizures, mGlu5 has been found to interact with the NMDA receptor via scaffolding proteins and to potentiate receptor activity, providing an alternative approach to indirectly modulate NMDA receptor activity (Attucci, Carla et al. 2001, Pisani, Gubellini et al. 2001). Consistent with this, a number of selective mGlu5 PAMs have been demonstrated to potentiate mGlu5 as well as NMDA receptor activity in brain slices (O'Brien, Lemaire et al. 2004, Rodriguez, Nong et al. 2005, Chen, Nong et al. 2007) and, as such, represent promising therapeutic agents for schizophrenia patients. Furthermore, mGlu5 PAMs, such as CDPPB, ADX47273, VU0360172 and LSN2463359, exhibit antipsychotic-like and procognitive effects in rodent models of schizophrenia (Kinney, O'Brien et al. 2005, Liu, Grauer et al. 2008, Rodriguez, Grier et al. 2010, Gastambide, Cotel et al. 2012), making allosteric potentiation of mGlu5 a promising avenue to pursue. However, cautions should be taken regarding side effects induced by mGlu5 potentiation, which may limit the development of mGlu5 PAMs as treatment for schizophrenia. For example, administration of 4 structually distinct mGlu5 PAMs developed at Merck and Co. demonstrated significant neurotoxicity in wildtype animals but not mGlu5 knockout mice, indicating a mechanism-based side effect induced by mGlu5 potentiation (Parmentier-Batteur, Hutson et al. 2013). Similarly, VU0422465, an allosteric agonist-PAM of mGlu5, was shown to induce epileptiform activity and behavioral convulsions in rodents. In contrast, VU0361747, an mGlu5 PAM that does not show allosteric agonist activity in vitro, demonstrated robust antipsychotic efficacy without inducing adverse behavioral effects (Rook, Noetzel et al. 2013). These data suggest that development of mGlu5 PAMs without agonist activity may be required to limit toxicity. Additionally, it has been proposed that mGlu5 PAMs with lower levels of cooperativity may be preferred to limit neurotoxic side effects.
Beyond mGlu5, the group II mGlus are also expressed in regions potentially involved in schizophrenia and cognitive function, such as the prefrontal cortex and hippocampus (Wright, Johnson et al. 2013). Group II mGlu agonists (or their prodrugs) including LY379268, LY354740 and LY2140023, have shown to exhibit antipsychotic-like effects in both animal models and human subjects of schizophrenia in the positive and negative symptom domains of the disease (Cartmell, Monn et al. 1999, Patil, Zhang et al. 2007). Studies using knockout animals suggest that the therapeutic effect of group II mGlu agonists may rely on their activity at mGlu2 instead of mGlu3 (Spooren, Gasparini et al. 2000, Fell, Svensson et al. 2008), and selective mGlu2 PAMs have been shown to have robust antipsychotic-like efficacy in animal models (Galici, Echemendia et al. 2005, Galici, Jones et al. 2006, Benneyworth, Xiang et al. 2007). It has been recently discovered that mGlu2 can heterodimerize with the serotonin 5-HT2A receptor (Gonzalez-Maeso, Ang et al. 2008) and reduce serotonin-stimulated glutamate release at the thalamocortical synapse, a synapse that is implicated in schizophrenia (Marek, Wright et al. 2001, Benneyworth, Xiang et al. 2007), indicating that the interaction between mGlu2 and serotonin 5-HT2A receptor might play a role in mediating the antipsychotic effects of mGlu2 activation.
Besides schizophrenia, psychosis has also been associated with Alzheimer's disease, the most common form of dementia that is characterized by deposition of toxic β-amyloid protein. Interestingly, activation of the group II mGlus using DCG IV triggers production and release of Alzheimer's β-amyloid (1-42) from isolated intact nerve terminals (Kim, Fraser et al. 2010). In addition, LY566332, an mGlu2 PAM, amplifies Aβ-induced neurodegeneration, which can be blocked by the mGlu2/3 receptor antagonist LY341495 (Caraci, Molinaro et al. 2011). Based on results of these preclinical studies, selective mGlu2 NAMs are now being pursued for cognition enhancement and antipsychotic activity in Alzheimer's disease.
6.1.2 Fragile X syndrome
Fragile X Syndrome (FXS) is the most common monogenic form of autism (Garber, Visootsak et al. 2008). FXS is caused by the expansion of CGG repeats and subsequent methylation and silencing of the gene encoding fragile X mental retardation protein (FMRP) (Fu, Kuhl et al. 1991, Pieretti, Zhang et al. 1991). FMRP act as a translational repressor in neuronal dendrites that regulates translation of the proteins that promote long-term depression (LTD). Therefore, group I mGlu-dependent LTD is excessive in mouse models of FXS (Huber, Gallagher et al. 2002, Bear, Huber et al. 2004). Specifically, mGlu5 has been suggested to play a role in the cognitive impairments observed in patients with FXS (Dolen, Osterweil et al. 2007) and antagonists of mGlu5 have been shown to have beneficial effects in FXS models. Specifically, acute treatment with the mGlu5 NAM CTEP corrects elevated hippocampal LTD, protein synthesis, and audiogenic seizures in mouse model (Michalon, Sidorov et al. 2012). In addition, chronic treatment using CTEP is able to rescue cognitive deficits in the Fmr1 knockout mouse, suggesting that pharmacological intervention could correct FXS symptoms even after the disease phenotype is established (Michalon, Sidorov et al. 2012). Several selective mGlu5 NAMs are now in clinical trials for FXS patients (www.ClinicalTrials.gov) and have shown promising therapeutic effects (Berry-Kravis, Hessl et al. 2009). Interestingly, in a recent clinical trial which evaluated the effect of mGlu5 NAM AFQ056 in male FXS patients, heterogeneity was discovered in terms of patient response to AFQ056 treatment: within 25 patients, 7 patients with full FMR1 promoter methylation and no detectable FMR1 messenger RNA exhibited significant improvement whereas no effect was detected in the remaining 18 patients who exhibited partial promoter methylation (Jacquemont, Curie et al. 2011). This study, if confirmed with a larger patient group, would suggest a beneficial effect of blocking the mGlu5 receptor in FXS patients with full promoter methylation and provide important criteria in patient selection.
6.1.3 Anxiety
In addition to potential efficacy in treating FXS, mGlu5 NAMs also represent potential therapeutics for anxiety disorders. As mentioned previously, mGlu5 activation potentiates NMDA receptor activity and an increase in NMDA receptor function has been associated with anxiety (Riaza Bermudo-Soriano, Perez-Rodriguez et al. 2012). Therefore, antagonists of mGlu5 may possess anxiolytic activity and have been tested in both preclinical and clinical studies. The early mGlu5 NAM MPEP exhibited anxiolytic efficacy in several rodent models of anxiety (Spooren, Vassout et al. 2000, Tatarczynska, Klodzinska et al. 2001). Another potent and selective mGlu5 NAM, fenobam, demonstrated anxiolytic efficacy in clinical trials (Pecknold, McClure et al. 1982, Porter, Jaeschke et al. 2005), providing further validation for targeting mGlu5 for anxiety disorders.
In addition to mGlu5 antagonism, activation of group II mGlus has potential as a novel approach for the treatment of anxiety disorders through modulation of glutamatergic transmission. Several orthosteric agonists, including LY354740 and LY404039, demonstrate anxiolytic efficacy in rodent models and/or human subjects (Schoepp, Wright et al. 2003, Rorick-Kehn, Johnson et al. 2007, Dunayevich, Erickson et al. 2008). While studies using mGlu3-selective compounds are quite limited, BINA and CBiPES, two selective mGlu2 PAMs, also exhibit anxiolytic effects in rodent behavioral models, such as stress-induced hyperthermia and elevated plus maze tasks (Johnson, Barda et al. 2005, Galici, Jones et al. 2006).
6.1.4 Parkinson's disease
Parkinson's disease (PD) is a debilitating neurodegenerative disorder characterized by movement symptoms including tremor, rigidity, bradykinesia and postural instability, as well as disturbances in sleep, depression and cognitive impairments (Jankovic 2008, Johnson, Conn et al. 2009). The pathology of PD stems from severe degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc), a brain structure that plays important roles in the basal ganglia to control motor function (Surmeier and Sulzer 2013). Within the basal ganglia, dopamine released from SNc neurons delicately controls the balance of between the “direct pathway” and the “indirect pathway”, which oppose each other in controlling motor output in the basal ganglia. In PD patients, however, the loss of dopaminergic neurons leads to an overall increase of activity in the indirect pathway, which ultimately inhibits motor function in PD patients (reviewed in (Johnson, Conn et al. 2009)). Thus, according to this model, a rebalancing of the basal ganglia circuitry is predicted to alleviate disease symptoms.
The current gold standard treatment for PD is dopamine replacement therapy using L-DOPA, the precursor of dopamine. However, long-term treatment with L-DOPA results in “wearing-off” of efficacy and development of side effects, such as dyskinesias and psychiatric complications (Chen and Swope 2007). In addition, no treatment is available to delay the progression of the disease. The mGlu4 receptor is highly expressed presynaptically at the first synapse in the indirect pathway (the striatopallidal synapse) (Bradley, Standaert et al. 1999), thus providing an exciting alternative approach to rebalance the basal ganglia circuitry for PD treatment. Administration of the group III mGlu agonists L-AP4 or L-SOP, and recently the more mGlu4-selective agonists LSP1-2111 and LSP4-2022, has been shown to reduce GABAergic transmission at the striatopallidal synapse and demonstrate efficacy in several rodent PD models, including haloperidol-induced catalepsy and 6-OHDA-induced motor deficits (Wittmann, Marino et al. 2001, Matsui and Kita 2003, Valenti, Marino et al. 2003, MacInnes, Messenger et al. 2004, Macinnes and Duty 2008, Beurrier, Lopez et al. 2009, Goudet, Vilar et al. 2012). Recently, numerous highly selective mGlu4 PAMs have been developed from different chemical series and exhibit robust efficacy in preclinical rodent models. Administration of either PHCCC or VU0155041, two mGlu4 PAMs that bind to distinct binding sites on the receptor, reversed parkinsonian behavior in PD animal models, such as reserpine-induced akinesia as well as haloperidol-induced catalepsy (Marino, Williams et al. 2003, Niswender, Johnson et al. 2008). In another example, VU0364770, a systemically active mGlu4 PAM, produced a reversal of forelimb asymmetry induced by unilateral 6-hydroxydopamine (6-OHDA) lesion of the median forebrain bundle either alone or in combination with L-DOPA (Jones, Bubser et al. 2012). In contrast, Lu AF21934 alone exhibited no effect in the akinesia induced by unilateral 6-OHDA lesion of the SNc unless a sub-threshold dose of L-DOPA was co-administered. Similarly, ADX88178 alone had no impact on forelimb akinesia induced by a bilateral 6-OHDA lesion. However, coadministration of ADX88178 with a low dose of L-DOPA enabled a robust reversal of the forelimb akinesia deficit. The difference between PAMs in 6-OHDA lesion models could result from distinctions in the lesion protocols used by the different research groups. It is also possible that the discrepancy is mediated by different pharmacological profiles of mGlu4 PAMs. Detailed studies of PAMs using the same lesion procedure will be required to elucidate the mechanism for these differences.
Besides a symptom-alleviating effect, mGlu4 PAMs also possess other potential benefits, such as potential disease-modifying efficacy and a lack of L-DOPA-mediated side effects, such as dyskinesia. PHCCC and VU0155041 has been shown to reduce dopaminergic cell death (Battaglia, Busceti et al. 2006, Betts, O'Neill et al. 2012), possibly due to reduced excessive excitatory drive onto dopamine neurons, and provide the potential to slow disease progression. In addition, LuAF21934, a selective mGlu4 PAM related to the VU0155041 series, has been shown to decrease the incidence of L-DOPA-induced dyskinesia (Bennouar, Uberti et al. 2013), further making mGlu4 an attractive target for PD treatment.
Despite the similar anti-parkinsonian effect observed with PHCCC and VU0155041, their differential responses at the corticostriatal synapses, presumably due to the presence of mGlu2/4 heteromers, are worth investigation with regard to additional benefits and adverse effects. Corticostriatal synapses have been shown to be overactive in dopamine-depleted animals (Picconi, Centonze et al. 2004, Centonze, Gubellini et al. 2005), contributing to the loss of spines in striatal medium spiny neurons in PD (Garcia, Neely et al. 2010). In addition, the deregulated plasticity (such as long-term depression and depotentiation) at corticostriatal synapses may underlie the mechanism of L-DOPA-induced dyskinesias (Picconi, Centonze et al. 2003, Picconi, Bagetta et al. 2011). Therefore, VU0155041-like mGlu4 PAMs that potentiate mGlu4-containing heteromers may potentially provide additional therapeutic effects, such as restoring morphology of striatal neurons and reversing L-DOPA-induced dyskinesias. In contrast, PHCCC-like PAMs with selectivity for homodimers might be preferable if potentiating mGlu2/4 signaling proves to engender side effects.
6.1.5 Other CNS disorders
Besides the disorders mentioned above, targeting mGlus also represents potential therapeutic approaches for other CNS diseases. For example, activation of mGlu4 inhibits the release of neuroinflammatory chemokines and increases the recovery from experimental autoimmune encephalomyelitis (Besong, Battaglia et al. 2002), a model for multiple sclerosis. This anti-inflammatory effect of mGlu4 has been postulated to be mediated by its activity in dendritic cells and T cells (Fallarino, Volpi et al. 2010). Furthermore, activation of mGlu2,3 or inhibition of mGlu5 has demonstrated efficacy in decreasing drug seeking behavior in rodents, providing therapeutic potential in drug abuse (Laruelle, Abi-Dargham et al. 1996, Bossert, Gray et al. 2006, Peters and Kalivas 2006, Liechti, Lhuillier et al. 2007, Lu, Uejima et al. 2007). Compounds targeting mGlus are also under preclinical and clinical development for depression, gastroesophogeal reflux disorder and migraine, among others (reviewed in (Gregory, Noetzel et al. 2013)).
6.2 Peripheral diseases
6.2.1 Pain sensation
Besides their synaptic and extrasynaptic expression in the CNS, mGlus also have a widespread distribution in the periphery and non-neural tissues and regulate a variety of physiological and pathological processes (reviewed in (Jones and Pilowsky 2002)). Particularly, several mGlu subtypes are involved in the pain sensation process ((Varney and Gereau 2002, Parmentier-Batteur, Hutson et al. 2013)). mGlu5 is expressed on the peripheral terminals of sensory neurons and the selective mGlu5 NAM, MPEP, demonstrates an inhibitory effect on inflammatory hyperalgesia (Rook, Noetzel et al. 2013). The group I mGlus also play a role in the generation of mechanical hyperalgesia following peripheral nerve injury. In a rodent spinal nerve lesion model, the group I mGlu receptor antagonist DL-amino-3-phosphonopropionic acid significantly delayed the onset of pain-induced paw withdrawal threshold reduction (Paquet, Ribeiro et al. 2013).
The group II and group III mGlus, on the other hand, negatively modulate nociceptive behavior. In the same model mentioned above, Jang et al. demonstrated that intraplantar injection of the group II mGlu receptor agonist APDC also delayed the onset of mechanical hyperalgesia (Paquet, Ribeiro et al. 2013), suggesting that peripheral group II mGlu receptors inhibit the induction of neuropathic pain. In addition, the injection of either APDC (a group II mGlu agonist) or L-AP4 (a group III mGlu agonist) into the arthritic rodents negatively modulates nociceptive behavior during both the induction and maintenance phases of carrageenan-induced arthritic pain (Lee, Park et al. 2013).
6.2.2 Congenital stationary night blindness
The mGlu6 subtype is expressed exclusively in the ON-bipolar cells in the retina and several mutations of mGlu6 lead to congenital stationary night blindness, a disorder characterized by myopia and impairment of night vision (Mathiesen, Ulven et al. 2005, Zeitz, Forster et al. 2007). These mutations have been found to interfere with proper protein trafficking to the cell surface (Zeitz, Forster et al. 2007), or disturb Go-mediated signaling downstream of mGlu6 activation (Mathiesen, Ulven et al. 2005), suggesting that Go-coupling of mGlu6 is essential for its function in retinal ON bipolar cells.
7. Concluding Remarks
The important functions of mGlus are becoming increasingly appreciated with a focus in the CNS, but also in the peripheral organs and tissues. During the last decade, a number of selective ligands, both orthosteric and allosteric, have been discovered that have expanded our understanding of receptor function and can be applied to clinical studies. In addition, novel findings in receptor biology, such as the crystal structure of the mGlu 7TMD and the heterodimerization of receptors, opens new avenues for the development of mGlu-based therapeutics.
HIGHLIGHTS.
Detailed review of the biology of mGlu receptors
Summary of the pharmacological tools and therapeutic merits of mGlu receptors
Review of mGlu structural features and evidence for heteromeric conformations
Acknowledgement
This work was supported by grant NS078262 and a Basic Research Grant from the International Rett Syndrome Foundation.
Glossary
- 2R,4R-APDC
(2R,4R)-4-Aminopyrrolidine-2,4-dicarboxylate
- 6-OHDA
6-hydroxydopamine
- 7TMR/GPCR
Seven Transmembrane Spanning/G Protein Coupled Receptor
- ADX88178
5-methyl-N-(4-methylpyrimidin-2-yl)-4-(1H-pyrazol-4-yl)thiazol-2-amine
- AFQ056
(3aS,5S,7aR)-methyl 5-hydroxy-5-(m-tolylethynyl)octahydro-1H-indole-1-carboxylate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMN082
N1,N2-dibenzhydrylethane-1,2-diamine
- BINA
biphenyl-indanone A
- cAMP
cyclic adenosine monophosphate
- CBiPES
N-[4’-cyano-biphenyl-3-yl]-N-(3-pyridinylmethyl)-ethanesulfonamide hydrochloride
- CDPPB
3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide
- CHPG
(RS)-2-chloro-5-hydroxyphenylglycine
- CNS
central nervous system
- CPCCOEt
7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester
- CRD
cysteine-rich domain
- CPPG
(RS)-α-Cyclopropyl-4-phosphonophenylglycine
- CPPHA
N-[4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl]-2-hydroxybenzamide
- DCG-IV
(2S,1’R,2’R,3’R)-2-(2,3-dicarboxycyclopropyl)glycine
- DCPG
(S)-3,4-dicarboxyphenylglycine
- DFB
[(3-fluorophenyl)methylene]hydrazone-3-fluorobenzaldehyde
- DHPG
(S)-3,5-dihydroxyphenylglycine
- FITM EGLU
(2S)-α-Ethylglutamic acid
- ERK
extracellular signal-regulated kinase
- FITM
4-fluoro-N-[4-[6-(isopropylamino)pyrimidin-4-yl]thiazol-2-yl]-N-methylbenzamide
- FMRP
fragile X mental retardation protein
- FXS
Fragile X syndrome
- GABA
γ-aminobutyric acid
- GIRK
G protein coupled inwardly rectifying potassium channel
- JNJ16259685
(3,4-dihydro-2H-pyrano[2,3]b quinolin-7-yl) (cis-4-methoxycyclohexyl) methanone
- L-AP4
L-(+)-2-Amino-4-phosphonobutyric acid
- L-DOPA
L-3,4-dihydroxyphenylalanine (levodopa)
- L-SOP
L-serine-O-phosphate
- LSP1-2111
((2S)-2-amino-4-[hydroxy[hydroxy(4-hydroxy-3-methoxy-5-nitro-phenyl)methyl] phosphoryl]butanoic acid)
- LSP4-2022
(2S)-2-amino-4-(((4-(carboxymethoxy)phenyl)(hydroxy)methyl)(hydroxy)phosphoryl)butanoic acid Lu AF21934 (1S,2S)-N1-(3,4-dichlorophenyl)cyclohexane-1,2-dicarboxamide
- LY2140023
(1R,4S,5S,6S)-2-thiabicyclo[3.1.0]-hexane-4,6-dicarboxylic acid,4-[(2S)-2-amino-4-(methylthio)-1-oxobutyl]amino-,2,2-dioxide monohydrate
- LY341495
2S-2-amino-2-(1S,2S-2-carboxycyclopropyl-1-yl)-3-(xanth-9-y l)propanoic acid LY354740 2-aminobicyclo[3.1.0]hexane 2,6-dicarboxylate
- LY379268
(1R,4R,5S,6R)-4-Amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid
- LY404039
(–)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid
- LY487379
2,2,2-trifluoro-N-[4-(2-methoxyphenoxy) phenyl]-N-(3-pyridinylmethyl)ethanesulfonamide
- LY566332
N-4’-cyano-biphenyl-3-yl)-N-(3-pyridinylmethyl)-ethanesulfonamide hydrochloride
- MAPK
mitogen activated protein kinase
- MCPG
(S)-a-methyl-4-carboxyphenylglycine
- mGlu
metabotropic glutamate receptor
- MMPIP
6-(4-methoxyphenyl)-5-methyl-3-(4-pyridinyl)-isoxazolo [4,5-c]pyridine-4(5H)-one hydrochloride
- MPEP
2-methyl-6-(phenylethynyl)-pyridine
- MTEP
3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine
- mTOR
mammalian target of rapamycin
- NAM
negative allosteric modulator
- NMDA
N-methyl-D-aspartate
- PAM
positive allosteric modulator
- PD
Parkinson's disease
- PHCCC
N-phenyl-7-(hydroxylimino)cyclopropa[b]chromen-1a-carboxamide
- PI3K
phosphatidylinositol 3-kinase
- SAM
silent allosteric modulator
- SIB-1757
6-methyl-2-(phenylazo)-3-pyridinol
- SIB-1893
2-methyl-6-(2-phenylethenyl)pyridine
- THIIC
N-(4-((2-(trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1-methyl-1H-imidazole-4-carboxamide
- VFD
Venus flytrap domain
- VU0155041
(±)-cis-2-(3,5-Dicholorphenylcarbamoyl)cyclohexanecarboxylic acid
- VU0364770
N-(3-chlorophenyl)picolinamide
- VU29
4-nitro-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acher FC, Bertrand HO. Amino acid recognition by Venus flytrap domains is encoded in an 8-residue motif. Biopolymers. 2005;80(2-3):357–366. doi: 10.1002/bip.20229. [DOI] [PubMed] [Google Scholar]
- AddexPharmaceuticalsPressRelease Addex reports positive top line phase IIa data for dipraglurant in Parkinson's disease levodopa-Induced dyskinedia (PD-LID) http://www.addextherapeutics.com/investors/press-releases/
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156(10):1646–1649. doi: 10.1176/ajp.156.10.1646. [DOI] [PubMed] [Google Scholar]
- AmericanPsychiatricAssociation Diagnostic and statistical manual of mental disorders. 4th edition 2000.
- Attucci S, Carla V, Mannaioni G, Moroni F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. Br J Pharmacol. 2001;132(4):799–806. doi: 10.1038/sj.bjp.0703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Niswender CM, Luo Q, Banko JL, Conn PJ. Group III mGluR regulation of synaptic transmission at the SC-CA1 synapse is developmentally regulated. Neuropharmacology. 2008;54(5):804–814. doi: 10.1016/j.neuropharm.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SR, Goldsworthy J, Harden RC, Salhoff CR, Schoepp DD. Enzymatic resolution and pharmacological activity of the enantiomers of 3,5-dihydroxyphenylglycine, a metabotropic glutamate receptor agonist. Bioorganic & Medicinal Chemistry Letters. 1995;5(3):223–228. [Google Scholar]
- Battaglia G, Busceti CL, Molinaro G, Biagioni F, Traficante A, Nicoletti F, Bruno V. Pharmacological activation of mGlu4 metabotropic glutamate receptors reduces nigrostriatal degeneration in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurosci. 2006;26(27):7222–7229. doi: 10.1523/JNEUROSCI.1595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol. 2007;72(2):477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- Bennouar KE, Uberti MA, Melon C, Bacolod MD, Jimenez HN, Cajina M, Kerkerian-Le Goff L, Doller D, Gubellini P. Synergy between L-DOPA and a novel positive allosteric modulator of metabotropic glutamate receptor 4: implications for Parkinson's disease treatment and dyskinesia. Neuropharmacology. 2013;66:158–169. doi: 10.1016/j.neuropharm.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Beqollari D, Kammermeier PJ. The mGlu(4) receptor allosteric modulator N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide acts as a direct agonist at mGlu(6) receptors. Eur J Pharmacol. 2008;589(1-3):49–52. doi: 10.1016/j.ejphar.2008.06.054. [DOI] [PubMed] [Google Scholar]
- Beqollari D, Kammermeier PJ. Venus fly trap domain of mGluR1 functions as a dominant negative against group I mGluR signaling. J Neurophysiol. 2010;104(1):439–448. doi: 10.1152/jn.00799.2009. [DOI] [PubMed] [Google Scholar]
- Berg D, Godau J, Trenkwalder C, Eggert K, Csoti I, Storch A, Huber H, Morelli-Canelo M, Stamelou M, Ries V, Wolz M, Schneider C, Di Paolo T, Gasparini F, Hariry S, Vandemeulebroecke M, Abi-Saab W, Cooke K, Johns D, Gomez-Mancilla B. AFQ056 treatment of levodopa-induced dyskinesias: results of 2 randomized controlled trials. Mov Disord. 2011;26(7):1243–1250. doi: 10.1002/mds.23616. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009;46(4):266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaso F, Zhang C, Scheschonka A, de Bock F, Fontanaud P, Marin P, Huganir RL, Betz H, Bockaert J, Fagni L, Lerner-Natoli M. PICK1 uncoupling from mGluR7a causes absence-like seizures. Nat Neurosci. 2008;11(8):940–948. doi: 10.1038/nn.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besong G, Battaglia G, D'Onofrio M, Di Marco R, Ngomba RT, Storto M, Castiglione M, Mangano K, Busceti CL, Nicoletti FR, Bacon K, Tusche M, Valenti O, Conn PJ, Bruno V, Nicoletti F. Activation of group III metabotropic glutamate receptors inhibits the production of RANTES in glial cell cultures. J Neurosci. 2002;22(13):5403–5411. doi: 10.1523/JNEUROSCI.22-13-05403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MJ, O'Neill MJ, Duty S. Allosteric modulation of the group III mGlu(4) receptor provides functional neuroprotection in the 6-hydroxydopamine rat model of Parkinson's disease. Br J Pharmacol. 2012;166(8):2317–2330. doi: 10.1111/j.1476-5381.2012.01943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurrier C, Lopez S, Revy D, Selvam C, Goudet C, Lherondel M, Gubellini P, Kerkerian-LeGoff L, Acher F, Pin JP, Amalric M. Electrophysiological and behavioral evidence that modulation of metabotropic glutamate receptor 4 with a new agonist reverses experimental parkinsonism. FASEB J. 2009;23(10):3619–3628. doi: 10.1096/fj.09-131789. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, Schioth HB. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. 2006;88(3):263–273. doi: 10.1016/j.ygeno.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Dumuis A, Fagni L, Marin P. GPCR-GIP networks: a first step in the discovery of new therapeutic drugs? Curr Opin Drug Discov Devel. 2004;7(5):649–657. [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31(10):2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabet I, Parmentier ML, De Colle C, Bockaert J, Acher F, Pin JP. Comparative effect of L-CCG-I, DCG-IV and gamma-carboxy-L-glutamate on all cloned metabotropic glutamate receptor subtypes. Neuropharmacology. 1998;37(8):1043–1051. doi: 10.1016/s0028-3908(98)00091-4. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Standaert DG, Rhodes KJ, Rees HD, Testa CM, Levey AI, Conn PJ. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J Comp Neurol. 1999;407(1):33–46. [PubMed] [Google Scholar]
- Brauner-Osborne H, Krogsgaard-Larsen P. Pharmacology of (S)-homoquisqualic acid and (S)-2-amino-5-phosphonopentanoic acid [(S)-AP5] at cloned metabotropic glutamate receptors. Br J Pharmacol. 1998;123(2):269–274. doi: 10.1038/sj.bjp.0701616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C, Oueslati N, Soler S, Boudier L, Rondard P, Pin JP. Activation of a dimeric metabotropic glutamate receptor by intersubunit rearrangement. J Biol Chem. 2007;282(45):33000–33008. doi: 10.1074/jbc.M702542200. [DOI] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Kingston A, O'Neill MJ, Catania MV, Di Grezia R, Nicoletti F. Neuroprotective activity of the potent and selective mGlu1a metabotropic glutamate receptor antagonist, (+)-2-methyl-4 carboxyphenylglycine (LY367385): comparison with LY357366, a broader spectrum antagonist with equal affinity for mGlu1a and mGlu5 receptors. Neuropharmacology. 1999;38(2):199–207. doi: 10.1016/s0028-3908(98)00159-2. [DOI] [PubMed] [Google Scholar]
- Caraci F, Molinaro G, Battaglia G, Giuffrida ML, Riozzi B, Traficante A, Bruno V, Cannella M, Merlo S, Wang X, Heinz BA, Nisenbaum ES, Britton TC, Drago F, Sortino MA, Copani A, Nicoletti F. Targeting group II metabotropic glutamate (mGlu) receptors for the treatment of psychosis associated with Alzheimer's disease: selective activation of mGlu2 receptors amplifies beta-amyloid toxicity in cultured neurons, whereas dual activation of mGlu2 and mGlu3 receptors is neuroprotective. Mol Pharmacol. 2011;79(3):618–626. doi: 10.1124/mol.110.067488. [DOI] [PubMed] [Google Scholar]
- Carroll FY, Stolle A, Beart PM, Voerste A, Brabet I, Mauler F, Joly C, Antonicek H, Bockaert J, Muller T, Pin JP, Prezeau L. BAY36-7620: a potent non-competitive mGlu1 receptor antagonist with inverse agonist activity. Mol Pharmacol. 2001;59(5):965–973. [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther. 1999;291(1):161–170. [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Rossi S, Picconi B, Pisani A, Bernardi G, Calabresi P, Baunez C. Subthalamic nucleus lesion reverses motor abnormalities and striatal glutamatergic overactivity in experimental parkinsonism. Neuroscience. 2005;133(3):831–840. doi: 10.1016/j.neuroscience.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci. 2000;3(2):113–119. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Swope DM. Pharmacotherapy for Parkinson's disease. Pharmacotherapy. 2007;27(12 Pt 2):161S–173S. doi: 10.1592/phco.27.12part2.161S. [DOI] [PubMed] [Google Scholar]
- Chen Y, Goudet C, Pin JP, Conn PJ. N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybe nzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol Pharmacol. 2008;73(3):909–918. doi: 10.1124/mol.107.040097. [DOI] [PubMed] [Google Scholar]
- Chen Y, Nong Y, Goudet C, Hemstapat K, de Paulis T, Pin JP, Conn PJ. Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses. Mol Pharmacol. 2007;71(5):1389–1398. doi: 10.1124/mol.106.032425. [DOI] [PubMed] [Google Scholar]
- Comps-Agrar L, Kniazeff J, Brock C, Trinquet E, Pin JP. Stability of GABAB receptor oligomers revealed by dual TR-FRET and drug-induced cell surface targeting. FASEB J. 2012;26(8):3430–3439. doi: 10.1096/fj.12-203646. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110(3):403–420. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- Corti C, Restituito S, Rimland JM, Brabet I, Corsi M, Pin JP, Ferraguti F. Cloning and characterization of alternative mRNA forms for the rat metabotropic glutamate receptors mGluR7 and mGluR8. Eur J Neurosci. 1998;10(12):3629–3641. doi: 10.1046/j.1460-9568.1998.00371.x. [DOI] [PubMed] [Google Scholar]
- Cosford ND, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, Bristow L, Brodkin J, Jiang X, McDonald I, Rao S, Washburn M, Varney MA. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J Med Chem. 2003;46(2):204–206. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- Dale LB, Bhattacharya M, Seachrist JL, Anborgh PH, Ferguson SS. Agonist-stimulated and tonic internalization of metabotropic glutamate receptor 1a in human embryonic kidney 293 cells: agonist-stimulated endocytosis is beta-arrestin1 isoform-specific. Mol Pharmacol. 2001;60(6):1243–1253. doi: 10.1124/mol.60.6.1243. [DOI] [PubMed] [Google Scholar]
- Desai MA, Conn PJ. Selective activation of phosphoinositide hydrolysis by a rigid analogue of glutamate. Neurosci Lett. 1990;109(1-2):157–162. doi: 10.1016/0304-3940(90)90555-n. [DOI] [PubMed] [Google Scholar]
- Dev KK, Nakanishi S, Henley JM. Regulation of mglu(7) receptors by proteins that interact with the intracellular C-terminus. Trends Pharmacol Sci. 2001;22(7):355–361. doi: 10.1016/s0165-6147(00)01684-9. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Henley JM, Collingridge GL, Jane DE. (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but no mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997;36(2):265–267. doi: 10.1016/s0028-3908(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56(6):955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez C, Prieto L, Valli MJ, Massey SM, Bures M, Wright RA, Johnson BG, Andis SL, Kingston A, Schoepp DD, Monn JA. Methyl substitution of 2-aminobicyclo[3.1.0]hexane 2,6-dicarboxylate (LY354740) determines functional activity at metabotropic glutamate receptors: identification of a subtype selective mGlu2 receptor agonist. J Med Chem. 2005;48(10):3605–3612. doi: 10.1021/jm040222y. [DOI] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Eric T, Rondard P, Pin JP. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 2011;25(1):66–77. doi: 10.1096/fj.10-163147. [DOI] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin JP. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 2011;25(1):66–77. doi: 10.1096/fj.10-163147. [DOI] [PubMed] [Google Scholar]
- Drolet R, Tugusheva K, Liverton N, Vogel R, Reynolds IJ, Hess FJ, Renger JJ, Kern JT, Celanire S, Tang L, Poli S, Campo B, Bortoli J, D'Addona D. Binding property characterization of a novel mGluR4 positive allosteric modulator.. Neuroscience Meeting Planner; Society for Neuroscience, Washington, DC. 2011.2011. [Google Scholar]
- Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology. 2008;33(7):1603–1610. doi: 10.1038/sj.npp.1301531. [DOI] [PubMed] [Google Scholar]
- Duvoisin RM, Pfankuch T, Wilson JM, Grabell J, Chhajlani V, Brown DG, Johnson E, Raber J. Acute pharmacological modulation of mGluR8 reduces measures of anxiety. Behav Brain Res. 2010;212(2):168–173. doi: 10.1016/j.bbr.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin RM, Villasana L, Davis MJ, Winder DG, Raber J. Opposing roles of mGluR8 in measures of anxiety involving non-social and social challenges. Behav Brain Res. 2011;221(1):50–54. doi: 10.1016/j.bbr.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Moustaine D, Granier S, Doumazane E, Scholler P, Rahmeh R, Bron P, Mouillac B, Baneres JL, Rondard P, Pin JP. Distinct roles of metabotropic glutamate receptor dimerization in agonist activation and G-protein coupling. Proc Natl Acad Sci U S A. 2012;109(40):16342–16347. doi: 10.1073/pnas.1205838109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz R. Structure of metabotropic glutamate receptor C-terminal domains in contact with interacting proteins. Front Mol Neurosci. 2012;5:52. doi: 10.3389/fnmol.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esseltine JL, Willard MD, Wulur IH, Lajiness ME, Barber TD, Ferguson SS. Somatic mutations in GRM1 in cancer alter metabotropic glutamate receptor 1 intracellular localization and signaling. Mol Pharmacol. 2013;83(4):770–780. doi: 10.1124/mol.112.081695. [DOI] [PubMed] [Google Scholar]
- Fagni L, Ango F, Perroy J, Bockaert J. Identification and functional roles of metabotropic glutamate receptor-interacting proteins. Semin Cell Dev Biol. 2004;15(3):289–298. doi: 10.1016/j.semcdb.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Volpi C, Fazio F, Notartomaso S, Vacca C, Busceti C, Bicciato S, Battaglia G, Bruno V, Puccetti P, Fioretti MC, Nicoletti F, Grohmann U, Di Marco R. Metabotropic glutamate receptor-4 modulates adaptive immunity and restrains neuroinflammation. Nat Med. 2010;16(8):897–902. doi: 10.1038/nm.2183. [DOI] [PubMed] [Google Scholar]
- Fazio F, Lionetto L, Molinaro G, Bertrand HO, Acher F, Ngomba RT, Notartomaso S, Curini M, Rosati O, Scarselli P, Di Marco R, Battaglia G, Bruno V, Simmaco M, Pin JP, Nicoletti F, Goudet C. Cinnabarinic acid, an endogenous metabolite of the kynurenine pathway, activates type 4 metabotropic glutamate receptors. Mol Pharmacol. 2012;81(5):643–656. doi: 10.1124/mol.111.074765. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039). J Pharmacol Exp Ther. 2008;326(1):209–217. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Witkin JM, Falcone JF, Katner JS, Perry KW, Hart J, Rorick-Kehn L, Overshiner CD, Rasmussen K, Chaney SF, Benvenga MJ, Li X, Marlow DL, Thompson LK, Luecke SK, Wafford KA, Seidel WF, Edgar DM, Quets AT, Felder CC, Wang X, Heinz BA, Nikolayev A, Kuo MS, Mayhugh D, Khilevich A, Zhang D, Ebert PJ, Eckstein JA, Ackermann BL, Swanson SP, Catlow JT, Dean RA, Jackson K, Tauscher-Wisniewski S, Marek GJ, Schkeryantz JM, Svensson KA. N-(4-((2-(trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1-methyl-1H-imidazole-4-carboxamide (THIIC), a novel metabotropic glutamate 2 potentiator with potential anxiolytic/antidepressant properties: in vivo profiling suggests a link between behavioral and central nervous system neurochemical changes. J Pharmacol Exp Ther. 2011;336(1):165–177. doi: 10.1124/jpet.110.172957. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Duvoisin RM. Role of the second and third intracellular loops of metabotropic glutamate receptors in mediating dual signal transduction activation. J Biol Chem. 1998;273(10):5615–5624. doi: 10.1074/jbc.273.10.5615. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Duvoisin RM. Divalent cations modulate the activity of metabotropic glutamate receptors. J Neurosci Res. 2004;75(4):472–479. doi: 10.1002/jnr.10853. [DOI] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Jr., Warren ST, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Fukuda J, Suzuki G, Kimura T, Nagatomi Y, Ito S, Kawamoto H, Ozaki S, Ohta H. Identification of a novel transmembrane domain involved in the negative modulation of mGluR1 using a newly discovered allosteric mGluR1 antagonist, 3-cyclohexyl-5-fluoro-6-methyl-7-(2-morpholin-4-ylethoxy)-4H-chromen-4-one. Neuropharmacology. 2009;57(4):438–445. doi: 10.1016/j.neuropharm.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Galici R, Echemendia NG, Rodriguez AL, Conn PJ. A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther. 2005;315(3):1181–1187. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]
- Galici R, Jones CK, Hemstapat K, Nong Y, Echemendia NG, Williams LC, de Paulis T, Conn PJ. Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J Pharmacol Exp Ther. 2006;318(1):173–185. doi: 10.1124/jpet.106.102046. [DOI] [PubMed] [Google Scholar]
- Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur J Hum Genet. 2008;16(6):666–672. doi: 10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia BG, Neely MD, Deutch AY. Cortical regulation of striatal medium spiny neuron dendritic remodeling in parkinsonism: modulation of glutamate release reverses dopamine depletion-induced dendritic spine loss. Cereb Cortex. 2010;20(10):2423–2432. doi: 10.1093/cercor/bhp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Velicelebi G, Kuhn R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38(10):1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Gastambide F, Cotel MC, Gilmour G, O'Neill MJ, Robbins TW, Tricklebank MD. Selective remediation of reversal learning deficits in the neurodevelopmental MAM model of schizophrenia by a novel mGlu5 positive allosteric modulator. Neuropsychopharmacology. 2012;37(4):1057–1066. doi: 10.1038/npp.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeza J, Joly C, Kuhn R, Knopfel T, Bockaert J, Pin JP. The second intracellular loop of metabotropic glutamate receptor 1 cooperates with the other intracellular domains to control coupling to G-proteins. J Biol Chem. 1996;271(4):2199–2205. doi: 10.1074/jbc.271.4.2199. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452(7183):93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudet C, Gaven F, Kniazeff J, Vol C, Liu J, Cohen-Gonsaud M, Acher F, Prezeau L, Pin JP. Heptahelical domain of metabotropic glutamate receptor 5 behaves like rhodopsin-like receptors. Proc Natl Acad Sci U S A. 2004;101(1):378–383. doi: 10.1073/pnas.0304699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudet C, Kniazeff J, Hlavackova V, Malhaire F, Maurel D, Acher F, Blahos J, Prezeau L, Pin JP. Asymmetric functioning of dimeric metabotropic glutamate receptors disclosed by positive allosteric modulators. J Biol Chem. 2005;280(26):24380–24385. doi: 10.1074/jbc.M502642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudet C, Vilar B, Courtiol T, Deltheil T, Bessiron T, Brabet I, Oueslati N, Rigault D, Bertrand HO, McLean H, Daniel H, Amalric M, Acher F, Pin JP. A novel selective metabotropic glutamate receptor 4 agonist reveals new possibilities for developing subtype selective ligands with therapeutic potential. FASEB J. 2012;26(4):1682–1693. doi: 10.1096/fj.11-195941. [DOI] [PubMed] [Google Scholar]
- Gregory KJ, Noetzel MJ, Niswender CM. Pharmacology of metabotropic glutamate receptor allosteric modulators: structural basis and therapeutic potential for CNS disorders. Prog Mol Biol Transl Sci. 2013;115:61–121. doi: 10.1016/B978-0-12-394587-7.00002-6. [DOI] [PubMed] [Google Scholar]
- Gregory KJ, Noetzel MJ, Rook JM, Vinson PN, Stauffer SR, Rodriguez AL, Emmitte KA, Zhou Y, Chun AC, Felts AS, Chauder BA, Lindsley CW, Niswender CM, Conn PJ. Investigating metabotropic glutamate receptor 5 allosteric modulator cooperativity, affinity, and agonism: enriching structure-function studies and structure-activity relationships. Mol Pharmacol. 2012;82(5):860–875. doi: 10.1124/mol.112.080531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Liu W, Wei J, Yan Z. Regulation of N-methyl-D-aspartic acid (NMDA) receptors by metabotropic glutamate receptor 7. J Biol Chem. 2012;287(13):10265–10275. doi: 10.1074/jbc.M111.325175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Ikeda SR. Coupling of metabotropic glutamate receptor 8 to N-type Ca2+ channels in rat sympathetic neurons. Mol Pharmacol. 2005;67(6):1840–1851. doi: 10.1124/mol.105.010975. [DOI] [PubMed] [Google Scholar]
- Hammond AS, Rodriguez AL, Townsend SD, Niswender CM, Gregory KJ, Lindsley CW, Conn PJ. Discovery of a Novel Chemical Class of mGlu(5) Allosteric Ligands with Distinct Modes of Pharmacology. ACS Chem Neurosci. 2010;1(10):702–716. doi: 10.1021/cn100051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson DR, Huang XP, Pekhletski R, Peltekova V, Hornby G, Thomsen C, Thogersen H. Probing the ligand-binding domain of the mGluR4 subtype of metabotropic glutamate receptor. J Biol Chem. 1999;274(47):33488–33495. doi: 10.1074/jbc.274.47.33488. [DOI] [PubMed] [Google Scholar]
- Havlickova M, Blahos J, Brabet I, Liu J, Hruskova B, Prezeau L, Pin JP. The second intracellular loop of metabotropic glutamate receptors recognizes C termini of G-protein alpha-subunits. J Biol Chem. 2003;278(37):35063–35070. doi: 10.1074/jbc.M306555200. [DOI] [PubMed] [Google Scholar]
- Hemstapat K, Da Costa H, Nong Y, Brady AE, Luo Q, Niswender CM, Tamagnan GD, Conn PJ. A novel family of potent negative allosteric modulators of group II metabotropic glutamate receptors. J Pharmacol Exp Ther. 2007;322(1):254–264. doi: 10.1124/jpet.106.117093. [DOI] [PubMed] [Google Scholar]
- Hemstapat K, de Paulis T, Chen Y, Brady AE, Grover VK, Alagille D, Tamagnan GD, Conn PJ. A novel class of positive allosteric modulators of metabotropic glutamate receptor subtype 1 interact with a site distinct from that of negative allosteric modulators. Mol Pharmacol. 2006;70(2):616–626. doi: 10.1124/mol.105.021857. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Ermilov M, Lichtenberg P, Bar G, Javitt DC. High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biol Psychiatry. 2004;55(2):165–171. doi: 10.1016/s0006-3223(03)00707-8. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC. Comparative effects of glycine and D-cycloserine on persistent negative symptoms in schizophrenia: a retrospective analysis. Schizophr Res. 2004;66(2-3):89–96. doi: 10.1016/S0920-9964(03)00129-4. [DOI] [PubMed] [Google Scholar]
- Herman EJ, Bubser M, Conn PJ, Jones CK. Metabotropic glutamate receptors for new treatments in schizophrenia. Handb Exp Pharmacol. 2012;(213):297–365. doi: 10.1007/978-3-642-25758-2_11. [DOI] [PubMed] [Google Scholar]
- Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J. 2001;359(Pt 3):465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermit MB, Greenwood JR, Brauner-Osborne H. Mutation-induced quisqualic acid and ibotenic acid affinity at the metabotropic glutamate receptor subtype 4: ligand selectivity results from a synergy of several amino acid residues. J Biol Chem. 2004;279(33):34811–34817. doi: 10.1074/jbc.M404109200. [DOI] [PubMed] [Google Scholar]
- Hlavackova V, Goudet C, Kniazeff J, Zikova A, Maurel D, Vol C, Trojanova J, Prezeau L, Pin JP, Blahos J. Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. EMBO J. 2005;24(3):499–509. doi: 10.1038/sj.emboj.7600557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlavackova V, Zabel U, Frankova D, Batz J, Hoffmann C, Prezeau L, Pin JP, Blahos J, Lohse MJ. Sequential inter- and intrasubunit rearrangements during activation of dimeric metabotropic glutamate receptor 1. Sci Signal. 2012;5(237):ra59. doi: 10.1126/scisignal.2002720. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99(11):7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli L, Bruno V, Salvatore L, Melchiorri D, Gradini R, Caricasole A, Barletta E, De Blasi A, Nicoletti F. Native group-III metabotropic glutamate receptors are coupled to the mitogen-activated protein kinase/phosphatidylinositol-3-kinase pathways. J Neurochem. 2002;82(2):216–223. doi: 10.1046/j.1471-4159.2002.00929.x. [DOI] [PubMed] [Google Scholar]
- Iacovelli L, Salvatore L, Capobianco L, Picascia A, Barletta E, Storto M, Mariggio S, Sallese M, Porcellini A, Nicoletti F, De Blasi A. Role of G protein-coupled receptor kinase 4 and beta-arrestin 1 in agonist-stimulated metabotropic glutamate receptor 1 internalization and activation of mitogen-activated protein kinases. J Biol Chem. 2003;278(14):12433–12442. doi: 10.1074/jbc.M203992200. [DOI] [PubMed] [Google Scholar]
- Ishida M, Saitoh T, Shimamoto K, Ohfune Y, Shinozaki H. A novel metabotropic glutamate receptor agonist: marked depression of monosynaptic excitation in the newborn rat isolated spinal cord. Br J Pharmacol. 1993;109(4):1169–1177. doi: 10.1111/j.1476-5381.1993.tb13745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, Ramos FJ, Cornish K, He Y, Paulding C, Neri G, Chen F, Hadjikhani N, Martinet D, Meyer J, Beckmann JS, Delange K, Brun A, Bussy G, Gasparini F, Hilse T, Floesser A, Branson J, Bilbe G, Johns D, Gomez-Mancilla B. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med. 2011;3(64):64ra61. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- Jane DE, Thomas NK, Tse HW, Watkins JC. Potent antagonists at the L-AP4- and (1S,3S)-ACPD-sensitive presynaptic metabotropic glutamate receptors in the neonatal rat spinal cord. Neuropharmacology. 1996;35(8):1029–1035. doi: 10.1016/s0028-3908(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Johnson BG, Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD. [3H]-LY341495 as a novel antagonist radioligand for group II metabotropic glutamate (mGlu) receptors: characterization of binding to membranes of mGlu receptor subtype expressing cells. Neuropharmacology. 1999;38(10):1519–1529. doi: 10.1016/s0028-3908(99)00053-2. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Conn PJ, Niswender CM. Glutamate receptors as therapeutic targets for Parkinson's disease. CNS Neurol Disord Drug Targets. 2009;8(6):475–491. doi: 10.2174/187152709789824606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Jones CK, Tantawy MN, Bubser M, Marvanova M, Ansari MS, Baldwin RM, Conn PJ, Niswender CM. The metabotropic glutamate receptor 8 agonist (S)-3,4-DCPG reverses motor deficits in prolonged but not acute models of Parkinson's disease. Neuropharmacology. 2013;66:187–195. doi: 10.1016/j.neuropharm.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE, McKinzie DL, Nisenbaum ES, Tizzano JP, Schoepp DD. Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s). Psychopharmacology (Berl) 2005;179(1):271–283. doi: 10.1007/s00213-004-2099-9. [DOI] [PubMed] [Google Scholar]
- Joly C, Gomeza J, Brabet I, Curry K, Bockaert J, Pin JP. Molecular, functional, and pharmacological characterization of the metabotropic glutamate receptor type 5 splice variants: comparison with mGluR1. J Neurosci. 1995;15(5 Pt 2):3970–3981. doi: 10.1523/JNEUROSCI.15-05-03970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Bubser M, Thompson AD, Dickerson JW, Turle-Lorenzo N, Amalric M, Blobaum AL, Bridges TM, Morrison RD, Jadhav S, Engers DW, Italiano K, Bode J, Daniels JS, Lindsley CW, Hopkins CR, Conn PJ, Niswender CM. The metabotropic glutamate receptor 4-positive allosteric modulator VU0364770 produces efficacy alone and in combination with L-DOPA or an adenosine 2A antagonist in preclinical rodent models of Parkinson's disease. J Pharmacol Exp Ther. 2012;340(2):404–421. doi: 10.1124/jpet.111.187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HM, Pilowsky LS. Dopamine and antipsychotic drug action revisited. Br J Psychiatry. 2002;181:271–275. doi: 10.1192/bjp.181.4.271. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Rouillier M, Girard F, Royer-Urios I, Bournique B, Finn T, Charvin D, Campo B, Le Poul E, Mutel V, Poli S, Neale SA, Salt TE, Lutjens R. ADX71743, a potent and selective negative allosteric modulator of metabotropic glutamate receptor 7: in vitro and in vivo characterization. J Pharmacol Exp Ther. 2013;344(3):624–636. doi: 10.1124/jpet.112.200915. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ. Functional and pharmacological characteristics of metabotropic glutamate receptors 2/4 heterodimers. Mol Pharmacol. 2012;82(3):438–447. doi: 10.1124/mol.112.078501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat Rev Drug Discov. 2005;4(11):919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- Kim SH, Fraser PE, Westaway D, St George-Hyslop PH, Ehrlich ME, Gandy S. Group II metabotropic glutamate receptor stimulation triggers production and release of Alzheimer's amyloid(beta)42 from isolated intact nerve terminals. J Neurosci. 2010;30(11):3870–3875. doi: 10.1523/JNEUROSCI.4717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37(1):1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Kinney GG, O'Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL., Jr. A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther. 2005;313(1):199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- Klunk WE, McClure RJ, Pettegrew JW. Possible roles of L-phosphoserine in the pathogenesis of Alzheimer's disease. Mol Chem Neuropathol. 1991;15(1):51–73. doi: 10.1007/BF03161056. [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prezeau L, Pin JP. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Struct Mol Biol. 2004;11(8):706–713. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Mutel V, Jolidon S, Kew JN, Malherbe P, Vieira E, Wichmann J, Kemp JA. Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site. Proc Natl Acad Sci U S A. 2001;98(23):13402–13407. doi: 10.1073/pnas.231358298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara A, Toya T, Tamura S, Watabiki T, Nagakura Y, Shitaka Y, Hayashibe S, Kawabata S, Okada M. Radioligand binding properties and pharmacological characterization of 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimidazole-2-carboxamide (YM-298198), a high-affinity, selective, and noncompetitive antagonist of metabotropic glutamate receptor type 1. J Pharmacol Exp Ther. 2005;315(1):163–169. doi: 10.1124/jpet.105.087171. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Miyashita T, Murata Y. Structural basis for a Ca2+-sensing function of the metabotropic glutamate receptors. Science. 1998;279(5357):1722–1725. doi: 10.1126/science.279.5357.1722. [DOI] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407(6807):971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13(1):9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Lamb JP, Engers DW, Niswender CM, Rodriguez AL, Venable DF, Conn PJ, Lindsley CW. Discovery of molecular switches within the ADX-47273 mGlu5 PAM scaffold that modulate modes of pharmacology to afford potent mGlu5 NAMs, PAMs and partial antagonists. Bioorg Med Chem Lett. 2011;21(9):2711–2714. doi: 10.1016/j.bmcl.2010.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93(17):9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavreysen H, Wouters R, Bischoff F, Nobrega Pereira S, Langlois X, Blokland S, Somers M, Dillen L, Lesage AS. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004;47(7):961–972. doi: 10.1016/j.neuropharm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Bolea C, Girard F, Poli S, Charvin D, Campo B, Bortoli J, Bessif A, Luo B, Koser AJ, Hodge LM, Smith KM, DiLella AG, Liverton N, Hess F, Browne SE, Reynolds IJ. A potent and selective metabotropic glutamate receptor 4 positive allosteric modulator improves movement in rodent models of Parkinson's disease. J Pharmacol Exp Ther. 2012;343(1):167–177. doi: 10.1124/jpet.112.196063. [DOI] [PubMed] [Google Scholar]
- Lea P. M. t., Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12(2):149–166. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K, Sexton PM, Christopoulos A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci. 2007;28(8):382–389. doi: 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Lee KS, Park EH, Cho HY, Kim YI, Han HC. Peripheral group II and III metabotropic glutamate receptors in the knee joint attenuate carrageenan-induced nociceptive behavior in rats. Neurosci Lett. 2013;542:21–25. doi: 10.1016/j.neulet.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Li XM, Li CC, Yu SS, Chen JT, Sabapathy K, Ruan DY. JNK1 contributes to metabotropic glutamate receptor-dependent long-term depression and short-term synaptic plasticity in the mice area hippocampal CA1. Eur J Neurosci. 2007;25(2):391–396. doi: 10.1111/j.1460-9568.2006.05300.x. [DOI] [PubMed] [Google Scholar]
- Liberman RP. Drug and psychosocial curricula for psychiatry residents for treatment of schizophrenia: part II. Psychiatr Serv. 2005;56(1):28–30. doi: 10.1176/appi.ps.56.1.28. [DOI] [PubMed] [Google Scholar]
- Liberman RP, Glick ID. Drug and psychosocial curricula for psychiatry residents for treatment of schizophrenia: part I. Psychiatr Serv. 2004;55(11):1217–1219. doi: 10.1176/appi.ps.55.11.1217. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27(34):9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley CW, Bates BS, Menon UN, Jadhav SB, Kane AS, Jones CK, Rodriguez AL, Conn PJ, Olsen CM, Winder DG, Emmitte KA. (3-Cyano-5-fluorophenyl)biaryl negative allosteric modulators of mGlu(5): Discovery of a new tool compound with activity in the OSS mouse model of addiction. ACS Chem Neurosci. 2011;2(8):471–482. doi: 10.1021/cn100099n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litschig S, Gasparini F, Rueegg D, Stoehr N, Flor PJ, Vranesic I, Prezeau L, Pin JP, Thomsen C, Kuhn R. CPCCOEt, a noncompetitive metabotropic glutamate receptor 1 antagonist, inhibits receptor signaling without affecting glutamate binding. Mol Pharmacol. 1999;55(3):453–461. [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, Smith D, Olsen M, Kouranova E, Lai M, Pruthi F, Pulicicchio C, Day M, Gilbert A, Pausch MH, Brandon NJ, Beyer CE, Comery TA, Logue S, Rosenzweig-Lipson S, Marquis KL. ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1- yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther. 2008;327(3):827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61(5):591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lundstrom L, Bissantz C, Beck J, Wettstein JG, Woltering TJ, Wichmann J, Gatti S. Structural determinants of allosteric antagonism at metabotropic glutamate receptor 2: mechanistic studies with new potent negative allosteric modulators. Br J Pharmacol. 2011;164(2b):521–537. doi: 10.1111/j.1476-5381.2011.01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283(5402):655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Macinnes N, Duty S. Group III metabotropic glutamate receptors act as hetero-receptors modulating evoked GABA release in the globus pallidus in vivo. Eur J Pharmacol. 2008;580(1-2):95–99. doi: 10.1016/j.ejphar.2007.10.030. [DOI] [PubMed] [Google Scholar]
- MacInnes N, Messenger MJ, Duty S. Activation of group III metabotropic glutamate receptors in selected regions of the basal ganglia alleviates akinesia in the reserpine-treated rat. Br J Pharmacol. 2004;141(1):15–22. doi: 10.1038/sj.bjp.0705566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj M, Bruno V, Dragic Z, Yamamoto R, Battaglia G, Inderbitzin W, Stoehr N, Stein T, Gasparini F, Vranesic I, Kuhn R, Nicoletti F, Flor PJ. (−)-PHCCC, a positive allosteric modulator of mGluR4: characterization, mechanism of action, and neuroprotection. Neuropharmacology. 2003;45(7):895–906. doi: 10.1016/s0028-3908(03)00271-5. [DOI] [PubMed] [Google Scholar]
- Malherbe P, Kratochwil N, Knoflach F, Zenner MT, Kew JN, Kratzeisen C, Maerki HP, Adam G, Mutel V. Mutational analysis and molecular modeling of the allosteric binding site of a novel, selective, noncompetitive antagonist of the metabotropic glutamate 1 receptor. J Biol Chem. 2003;278(10):8340–8347. doi: 10.1074/jbc.M211759200. [DOI] [PubMed] [Google Scholar]
- Malherbe P, Kratzeisen C, Lundstrom K, Richards JG, Faull RL, Mutel V. Cloning and functional expression of alternative spliced variants of the human metabotropic glutamate receptor 8. Brain Res Mol Brain Res. 1999;67(2):201–210. doi: 10.1016/s0169-328x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Manzoni O, Fagni L, Pin JP, Rassendren F, Poulat F, Sladeczek F, Bockaert J. (trans)-1-amino-cyclopentyl-1,3-dicarboxylate stimulates quisqualate phosphoinositide-coupled receptors but not ionotropic glutamate receptors in striatal neurons and Xenopus oocytes. Mol Pharmacol. 1990;38(1):1–6. [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience. 2001;105(2):379–392. doi: 10.1016/s0306-4522(01)00199-3. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Williams DL, Jr., O'Brien JA, Valenti O, McDonald TP, Clements MK, Wang R, DiLella AG, Hess JF, Kinney GG, Conn PJ. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson's disease treatment. Proc Natl Acad Sci U S A. 2003;100(23):13668–13673. doi: 10.1073/pnas.1835724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlo JE, Niswender CM, Days EL, Bridges TM, Xiang Y, Rodriguez AL, Shirey JK, Brady AE, Nalywajko T, Luo Q, Austin CA, Williams MB, Kim K, Williams R, Orton D, Brown HA, Lindsley CW, Weaver CD, Conn PJ. Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol Pharmacol. 2009;75(3):577–588. doi: 10.1124/mol.108.052886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen JM, Ulven T, Martini L, Gerlach LO, Heinemann A, Kostenis E. Identification of indole derivatives exclusively interfering with a G protein-independent signaling pathway of the prostaglandin D2 receptor CRTH2. Mol Pharmacol. 2005;68(2):393–402. doi: 10.1124/mol.104.010520. [DOI] [PubMed] [Google Scholar]
- Matsui T, Kita H. Activation of group III metabotropic glutamate receptors presynaptically reduces both GABAergic and glutamatergic transmission in the rat globus pallidus. Neuroscience. 2003;122(3):727–737. doi: 10.1016/j.neuroscience.2003.08.032. [DOI] [PubMed] [Google Scholar]
- McCool BA, Pin JP, Harpold MM, Brust PF, Stauderman KA, Lovinger DM. Rat group I metabotropic glutamate receptors inhibit neuronal Ca2+ channels via multiple signal transduction pathways in HEK 293 cells. J Neurophysiol. 1998;79(1):379–391. doi: 10.1152/jn.1998.79.1.379. [DOI] [PubMed] [Google Scholar]
- Menard C, Quirion R. Successful cognitive aging in rats: a role for mGluR5 glutamate receptors, homer 1 proteins and downstream signaling pathways. PLoS One. 2012;7(1):e28666. doi: 10.1371/journal.pone.0028666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, Jaeschke G, Bear MF, Lindemann L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74(1):49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakami R, Katsuki F, Yamamoto T, Nakamura K, Sugiyama H. Molecular cloning and the functional expression of two isoforms of human metabotropic glutamate receptor subtype 5. Biochem Biophys Res Commun. 1994;199(3):1136–1143. doi: 10.1006/bbrc.1994.1349. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Yamamoto R, Ofner S, Nozulak J, Pescott O, Lukic S, Stoehr N, Mombereau C, Kuhn R, McAllister KH, van der Putten H, Cryan JF, Flor PJ. A selective metabotropic glutamate receptor 7 agonist: activation of receptor signaling via an allosteric site modulates stress parameters in vivo. Proc Natl Acad Sci U S A. 2005;102(51):18712–18717. doi: 10.1073/pnas.0508063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, Harkness AR, Grutsch JL, Jr., Wright RA, Johnson BG, Andis SL, Kingston A, Tomlinson R, Lewis R, Griffey KR, Tizzano JP, Schoepp DD. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem. 1999;42(6):1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuellar F, Mocci G, Seto J, Callado LF, Neve RL, Milligan G, Sealfon SC, Lopez-Gimenez JF, Meana JJ, Benson DL, Gonzalez-Maeso J. Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A.mGlu2) receptor heteromerization and its psychoactive behavioral function. J Biol Chem. 2012;287(53):44301–44319. doi: 10.1074/jbc.M112.413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto T, Tsuchiya D, Morikawa K, Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 2007;104(10):3759–3764. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Luo Q, Ayala JE, Kim C, Conn PJ, Weaver CD. A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors. Mol Pharmacol. 2008;73(4):1213–1224. doi: 10.1124/mol.107.041053. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Miller NR, Ayala JE, Luo Q, Williams R, Saleh S, Orton D, Weaver CD, Conn PJ. Context-dependent pharmacology exhibited by negative allosteric modulators of metabotropic glutamate receptor 7. Mol Pharmacol. 2010;77(3):459–468. doi: 10.1124/mol.109.058768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Weaver CD, Jones CK, Xiang Z, Luo Q, Rodriguez AL, Marlo JE, de Paulis T, Thompson AD, Days EL, Nalywajko T, Austin CA, Williams MB, Ayala JE, Williams R, Lindsley CW, Conn PJ. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol Pharmacol. 2008;74(5):1345–1358. doi: 10.1124/mol.108.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Lebois EP, Luo Q, Kim K, Muchalski H, Yin H, Conn PJ, Lindsley CW. Positive allosteric modulators of the metabotropic glutamate receptor subtype 4 (mGluR4): Part I. Discovery of pyrazolo[3,4-d]pyrimidines as novel mGluR4 positive allosteric modulators. Bioorg Med Chem Lett. 2008;18(20):5626–5630. doi: 10.1016/j.bmcl.2008.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzel MJ, Gregory KJ, Vinson PN, Manka JT, Stauffer SR, Lindsley CW, Niswender CM, Xiang Z, Conn PJ. A novel metabotropic glutamate receptor 5 positive allosteric modulator acts at a unique site and confers stimulus bias to mGlu5 signaling. Mol Pharmacol. 2013;83(4):835–847. doi: 10.1124/mol.112.082891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72(1):29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Lemaire W, Chen TB, Chang RS, Jacobson MA, Ha SN, Lindsley CW, Schaffhauser HJ, Sur C, Pettibone DJ, Conn PJ, Williams DL., Jr. A family of highly selective allosteric modulators of the metabotropic glutamate receptor subtype 5. Mol Pharmacol. 2003;64(3):731–740. doi: 10.1124/mol.64.3.731. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Lemaire W, Wittmann M, Jacobson MA, Ha SN, Wisnoski DD, Lindsley CW, Schaffhauser HJ, Rowe B, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL., Jr. A novel selective allosteric modulator potentiates the activity of native metabotropic glutamate receptor subtype 5 in rat forebrain. J Pharmacol Exp Ther. 2004;309(2):568–577. doi: 10.1124/jpet.103.061747. [DOI] [PubMed] [Google Scholar]
- Ohashi H, Maruyama T, Higashi-Matsumoto H, Nomoto T, Nishimura S, Takeuchi Y. A novel binding assay for metabotropic glutamate receptors using [3H] L-quisqualic acid and recombinant receptors. Z Naturforsch C. 2002;57(c):345–355. doi: 10.1515/znc-2002-3-425. [DOI] [PubMed] [Google Scholar]
- Pagano A, Ruegg D, Litschig S, Stoehr N, Stierlin C, Heinrich M, Floersheim P, Prezeau L, Carroll F, Pin JP, Cambria A, Vranesic I, Flor PJ, Gasparini F, Kuhn R. The non-competitive antagonists 2-methyl-6-(phenylethynyl)pyridine and 7-hydroxyiminocyclopropan[b]chromen-1a-carboxylic acid ethyl ester interact with overlapping binding pockets in the transmembrane region of group I metabotropic glutamate receptors. J Biol Chem. 2000;275(43):33750–33758. doi: 10.1074/jbc.M006230200. [DOI] [PubMed] [Google Scholar]
- Page G, Khidir FA, Pain S, Barrier L, Fauconneau B, Guillard O, Piriou A, Hugon J. Group I metabotropic glutamate receptors activate the p70S6 kinase via both mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase (ERK 1/2) signaling pathways in rat striatal and hippocampal synaptoneurosomes. Neurochem Int. 2006;49(4):413–421. doi: 10.1016/j.neuint.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Palmer E, Monaghan DT, Cotman CW. Trans-ACPD, a selective agonist of the phosphoinositide-coupled excitatory amino acid receptor. Eur J Pharmacol. 1989;166(3):585–587. doi: 10.1016/0014-2999(89)90383-x. [DOI] [PubMed] [Google Scholar]
- Palucha A, Klak K, Branski P, van der Putten H, Flor PJ, Pilc A. Activation of the mGlu7 receptor elicits antidepressant-like effects in mice. Psychopharmacology (Berl) 2007;194(4):555–562. doi: 10.1007/s00213-007-0856-2. [DOI] [PubMed] [Google Scholar]
- Paquet M, Ribeiro FM, Guadagno J, Esseltine JL, Ferguson SS, Cregan SP. Role of metabotropic glutamate receptor 5 signaling and homer in oxygen glucose deprivation-mediated astrocyte apoptosis. Mol Brain. 2013;6:9. doi: 10.1186/1756-6606-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier-Batteur S, Hutson PH, Menzel K, Uslaner JM, Mattson BA, O'Brien JA, Magliaro BC, Forest T, Stump CA, Tynebor RM, Anthony NJ, Tucker TJ, Zhang XF, Gomez R, Huszar SL, Lambeng N, Faure H, Le Poul E, Poli S, Rosahl TW, Rocher JP, Hargreaves R, Williams TM. Mechanism based neurotoxicity of mGlu5 positive allosteric modulators - Development challenges for a promising novel antipsychotic target. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13(9):1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T. Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J Clin Psychopharmacol. 1982;2(2):129–133. [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186(2):143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Picconi B, Bagetta V, Ghiglieri V, Paille V, Di Filippo M, Pendolino V, Tozzi A, Giampa C, Fusco FR, Sgobio C, Calabresi P. Inhibition of phosphodiesterases rescues striatal long-term depression and reduces levodopa-induced dyskinesia. Brain. 2011;134(Pt 2):375–387. doi: 10.1093/brain/awq342. [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6(5):501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Rossi S, Bernardi G, Calabresi P. Therapeutic doses of L-dopa reverse hypersensitivity of corticostriatal D2-dopamine receptors and glutamatergic overactivity in experimental parkinsonism. Brain. 2004;127(Pt 7):1661–1669. doi: 10.1093/brain/awh190. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci. 2001;2(10):727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Pin JP, Joly C, Heinemann SF, Bockaert J. Domains involved in the specificity of G protein activation in phospholipase C-coupled metabotropic glutamate receptors. EMBO J. 1994;13(2):342–348. doi: 10.1002/j.1460-2075.1994.tb06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106(3):579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard TM, Buttelmann B, Kolczewski S, Peters JU, Prinssen E, Wichmann J, Vieira E, Muhlemann A, Gatti S, Mutel V, Malherbe P. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther. 2005;315(2):711–721. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- Pramyothin P, Khaodhiar L. Metabolic syndrome with the atypical antipsychotics. Curr Opin Endocrinol Diabetes Obes. 2010;17(5):460–466. doi: 10.1097/MED.0b013e32833de61c. [DOI] [PubMed] [Google Scholar]
- Ray K, Hauschild BC. Cys-140 is critical for metabotropic glutamate receptor-1 dimerization. J Biol Chem. 2000;275(44):34245–34251. doi: 10.1074/jbc.M005581200. [DOI] [PubMed] [Google Scholar]
- Riaza Bermudo-Soriano C, Perez-Rodriguez MM, Vaquero-Lorenzo C, Baca-Garcia E. New perspectives in glutamate and anxiety. Pharmacol Biochem Behav. 2012;100(4):752–774. doi: 10.1016/j.pbb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Rodriguez AL, Grier MD, Jones CK, Herman EJ, Kane AS, Smith RL, Williams R, Zhou Y, Marlo JE, Days EL, Blatt TN, Jadhav S, Menon UN, Vinson PN, Rook JM, Stauffer SR, Niswender CM, Lindsley CW, Weaver CD, Conn PJ. Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Mol Pharmacol. 2010;78(6):1105–1123. doi: 10.1124/mol.110.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AL, Nong Y, Sekaran NK, Alagille D, Tamagnan GD, Conn PJ. A close structural analog of 2-methyl-6-(phenylethynyl)-pyridine acts as a neutral allosteric site ligand on metabotropic glutamate receptor subtype 5 and blocks the effects of multiple allosteric modulators. Mol Pharmacol. 2005;68(6):1793–1802. doi: 10.1124/mol.105.016139. [DOI] [PubMed] [Google Scholar]
- Romano C, Miller JK, Hyrc K, Dikranian S, Mennerick S, Takeuchi Y, Goldberg MP, O'Malley KL. Covalent and noncovalent interactions mediate metabotropic glutamate receptor mGlu5 dimerization. Mol Pharmacol. 2001;59(1):46–53. [PubMed] [Google Scholar]
- Romano C, Yang WL, O'Malley KL. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J Biol Chem. 1996;271(45):28612–28616. doi: 10.1074/jbc.271.45.28612. [DOI] [PubMed] [Google Scholar]
- Ronesi JA, Collins KA, Hays SA, Tsai NP, Guo W, Birnbaum SG, Hu JH, Worley PF, Gibson JR, Huber KM. Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nat Neurosci. 2012;15(3):431–440. S431. doi: 10.1038/nn.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28(2):543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6(11):1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Rook JM, Noetzel MJ, Pouliot WA, Bridges TM, Vinson PN, Cho HP, Zhou Y, Gogliotti RD, Manka JT, Gregory KJ, Stauffer SR, Dudek FE, Xiang Z, Niswender CM, Daniels JS, Jones CK, Lindsley CW, Conn PJ. Unique signaling profiles of positive allosteric modulators of metabotropic glutamate receptor subtype 5 determine differences in in vivo activity. Biol Psychiatry. 2013;73(6):501–509. doi: 10.1016/j.biopsych.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Johnson BG, Knitowski KM, Salhoff CR, Witkin JM, Perry KW, Griffey KI, Tizzano JP, Monn JA, McKinzie DL, Schoepp DD. In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology (Berl) 2007;193(1):121–136. doi: 10.1007/s00213-007-0758-3. [DOI] [PubMed] [Google Scholar]
- Rowe BA, Schaffhauser H, Morales S, Lubbers LS, Bonnefous C, Kamenecka TM, McQuiston J, Daggett LP. Transposition of three amino acids transforms the human metabotropic glutamate receptor (mGluR)-3-positive allosteric modulation site to mGluR2, and additional characterization of the mGluR2-positive allosteric modulation site. J Pharmacol Exp Ther. 2008;326(1):240–251. doi: 10.1124/jpet.108.138271. [DOI] [PubMed] [Google Scholar]
- Sartorius LJ, Nagappan G, Lipska BK, Lu B, Sei Y, Ren-Patterson R, Li Z, Weinberger DR, Harrison PJ. Alternative splicing of human metabotropic glutamate receptor 3. J Neurochem. 2006;96(4):1139–1148. doi: 10.1111/j.1471-4159.2005.03609.x. [DOI] [PubMed] [Google Scholar]
- Sayer RJ. Group I metabotropic glutamate receptors mediate slow inhibition of calcium current in neocortical neurons. J Neurophysiol. 1998;80(4):1981–1988. doi: 10.1152/jn.1998.80.4.1981. [DOI] [PubMed] [Google Scholar]
- Schaffhauser H, Rowe BA, Morales S, Chavez-Noriega LE, Yin R, Jachec C, Rao SP, Bain G, Pinkerton AB, Vernier JM, Bristow LJ, Varney MA, Daggett LP. Pharmacological characterization and identification of amino acids involved in the positive modulation of metabotropic glutamate receptor subtype 2. Mol Pharmacol. 2003;64(4):798–810. doi: 10.1124/mol.64.4.798. [DOI] [PubMed] [Google Scholar]
- Schann S, Mayer S, Franchet C, Frauli M, Steinberg E, Thomas M, Baron L, Neuville P. Chemical switch of a metabotropic glutamate receptor 2 silent allosteric modulator into dual metabotropic glutamate receptor 2/3 negative/positive allosteric modulators. J Med Chem. 2010;53(24):8775–8779. doi: 10.1021/jm101069m. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Goldsworthy J, Johnson BG, Salhoff CR, Baker SR. 3,5-dihydroxyphenylglycine is a highly selective agonist for phosphoinositide-linked metabotropic glutamate receptors in the rat hippocampus. J Neurochem. 1994;63(2):769–772. doi: 10.1046/j.1471-4159.1994.63020769.x. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38(10):1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Johnson BG, Salhoff CR, McDonald JW, Johnston MV. In vitro and in vivo pharmacology of trans- and cis-(+−)-1-amino-1,3-cyclopentanedicarboxylic acid: dissociation of metabotropic and ionotropic excitatory amino acid receptor effects. J Neurochem. 1991;56(5):1789–1796. doi: 10.1111/j.1471-4159.1991.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Johnson BG, True RA, Monn JA. Comparison of (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD)- and 1R,3S-ACPD-stimulated brain phosphoinositide hydrolysis. Eur J Pharmacol. 1991;207(4):351–353. doi: 10.1016/0922-4106(91)90010-f. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress. 2003;6(3):189–197. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- Schulz HL, Stohr H, Weber BH. Characterization of three novel isoforms of the metabotrobic glutamate receptor 7 (GRM7). Neurosci Lett. 2002;326(1):37–40. doi: 10.1016/s0304-3940(02)00306-3. [DOI] [PubMed] [Google Scholar]
- Schweitzer C, Kratzeisen C, Adam G, Lundstrom K, Malherbe P, Ohresser S, Stadler H, Wichmann J, Woltering T, Mutel V. Characterization of [(3)H]-LY354740 binding to rat mGlu2 and mGlu3 receptors expressed in CHO cells using semliki forest virus vectors. Neuropharmacology. 2000;39(10):1700–1706. doi: 10.1016/s0028-3908(99)00265-8. [DOI] [PubMed] [Google Scholar]
- Seebahn A, Dinkel H, Mohrluder J, Hartmann R, Vogel N, Becker CM, Sticht H, Enz R. Structural characterization of intracellular C-terminal domains of group III metabotropic glutamate receptors. FEBS Lett. 2011;585(3):511–516. doi: 10.1016/j.febslet.2010.12.042. [DOI] [PubMed] [Google Scholar]
- Servitja JM, Masgrau R, Sarri E, Picatoste F. Group I metabotropic glutamate receptors mediate phospholipase D stimulation in rat cultured astrocytes. J Neurochem. 1999;72(4):1441–1447. doi: 10.1046/j.1471-4159.1999.721441.x. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kedrowski J, Rook JM, Smith RL, Jones CK, Rodriguez AL, Conn PJ, Lindsley CW. Discovery of molecular switches that modulate modes of metabotropic glutamate receptor subtype 5 (mGlu5) pharmacology in vitro and in vivo within a series of functionalized, regioisomeric 2- and 5-(phenylethynyl)pyrimidines. J Med Chem. 2009;52(14):4103–4106. doi: 10.1021/jm900654c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler DJ, Wenthur CJ, Bruner JA, Carrington SJ, Vinson PN, Gogi KK, Blobaum AL, Morrison RD, Vamos M, Cosford ND, Stauffer SR, Daniels JS, Niswender CM, Conn PJ, Lindsley CW. Development of a novel, CNS-penetrant, metabotropic glutamate receptor 3 (mGlu3) NAM probe (ML289) derived from a closely related mGlu5 PAM. Bioorg Med Chem Lett. 2012;22(12):3921–3925. doi: 10.1016/j.bmcl.2012.04.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler DJ, Wenthur CJ, Brunner JA, Daniels JS, Morrison RD, Blobaum AL, Dawson ES, Engers JL, Niswender CM, Conn PJ, Lindsley CW. Development of the First Selective mGlu3 NAM from an mGlu5 PAM Hit. 2010 [PubMed] [Google Scholar]
- Sorensen SD, Conn PJ. G protein-coupled receptor kinases regulate metabotropic glutamate receptor 5 function and expression. Neuropharmacology. 2003;44(6):699–706. doi: 10.1016/s0028-3908(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Gasparini F, van der Putten H, Koller M, Nakanishi S, Kuhn R. Lack of effect of LY314582 (a group 2 metabotropic glutamate receptor agonist) on phencyclidine-induced locomotor activity in metabotropic glutamate receptor 2 knockout mice. Eur J Pharmacol. 2000;397(1):R1–2. doi: 10.1016/s0014-2999(00)00269-7. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Vassout A, Neijt HC, Kuhn R, Gasparini F, Roux S, Porsolt RD, Gentsch C. Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine in rodents. J Pharmacol Exp Ther. 2000;295(3):1267–1275. [PubMed] [Google Scholar]
- Staudinger J, Zhou J, Burgess R, Elledge SJ, Olson EN. PICK1: a perinuclear binding protein and substrate for protein kinase C isolated by the yeast two-hybrid system. J Cell Biol. 1995;128(3):263–271. doi: 10.1083/jcb.128.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Park JY, Park S, Jou I, Roche PA, Roche KW. Regulation of metabotropic glutamate receptor 7 (mGluR7) internalization and surface expression by Ser/Thr protein phosphatase 1. J Biol Chem. 2013;288(24):17544–17551. doi: 10.1074/jbc.M112.439513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Pelkey KA, Lavezzari G, Roche PA, Huganir RL, McBain CJ, Roche KW. Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron. 2008;58(5):736–748. doi: 10.1016/j.neuron.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukoff Rizzo SJ, Leonard SK, Gilbert A, Dollings P, Smith DL, Zhang MY, Di L, Platt BJ, Neal S, Dwyer JM, Bender CN, Zhang J, Lock T, Kowal D, Kramer A, Randall A, Huselton C, Vishwanathan K, Tse SY, Butera J, Ring RH, Rosenzweig-Lipson S, Hughes ZA, Dunlop J. The metabotropic glutamate receptor 7 allosteric modulator AMN082: a monoaminergic agent in disguise? J Pharmacol Exp Ther. 2011;338(1):345–352. doi: 10.1124/jpet.110.177378. [DOI] [PubMed] [Google Scholar]
- Suratman S, Leach K, Sexton P, Felder C, Loiacono R, Christopoulos A. Impact of species variability and ‘probe-dependence’ on the detection and in vivo validation of allosteric modulation at the M4 muscarinic acetylcholine receptor. Br J Pharmacol. 2011;162(7):1659–1670. doi: 10.1111/j.1476-5381.2010.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Sulzer D. The pathology roadmap in Parkinson disease. Prion. 2013;7(1):85–91. doi: 10.4161/pri.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki G, Kimura T, Satow A, Kaneko N, Fukuda J, Hikichi H, Sakai N, Maehara S, Kawagoe-Takaki H, Hata M, Azuma T, Ito S, Kawamoto H, Ohta H. Pharmacological characterization of a new, orally active and potent allosteric metabotropic glutamate receptor 1 antagonist, 4-[1-(2-fluoropyridin-3-yl)-5-methyl-1H-1,2,3-triazol-4-yl]-N-isopropyl-N-methyl-3,6-dihydropyridine-1(2H)-carboxamide (FTIDC). J Pharmacol Exp Ther. 2007;321(3):1144–1153. doi: 10.1124/jpet.106.116574. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Tsukamoto N, Fushiki H, Kawagishi A, Nakamura M, Kurihara H, Mitsuya M, Ohkubo M, Ohta H. In vitro pharmacological characterization of novel isoxazolopyridone derivatives as allosteric metabotropic glutamate receptor 7 antagonists. J Pharmacol Exp Ther. 2007;323(1):147–156. doi: 10.1124/jpet.107.124701. [DOI] [PubMed] [Google Scholar]
- Tatarczynska E, Klodzinska A, Chojnacka-Wojcik E, Palucha A, Gasparini F, Kuhn R, Pilc A. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol. 2001;132(7):1423–1430. doi: 10.1038/sj.bjp.0703923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NK, Wright RA, Howson PA, Kingston AE, Schoepp DD, Jane DE. (S)-3,4-DCPG, a potent and selective mGlu8a receptor agonist, activates metabotropic glutamate receptors on primary afferent terminals in the neonatal rat spinal cord. Neuropharmacology. 2001;40(3):311–318. doi: 10.1016/s0028-3908(00)00169-6. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Pekhletski R, Haldeman B, Gilbert TA, O'Hara P, Hampson DR. Cloning and characterization of a metabotropic glutamate receptor, mGluR4b. Neuropharmacology. 1997;36(1):21–30. doi: 10.1016/s0028-3908(96)00153-0. [DOI] [PubMed] [Google Scholar]
- Thomsen W, Frazer J, Unett D. Functional assays for screening GPCR targets. Curr Opin Biotechnol. 2005;16(6):655–665. doi: 10.1016/j.copbio.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Kunishima N, Kamiya N, Jingami H, Morikawa K. Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+. Proc Natl Acad Sci U S A. 2002;99(5):2660–2665. doi: 10.1073/pnas.052708599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23(3):583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320(1):1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Valant C, Felder CC, Sexton PM, Christopoulos A. Probe dependence in the allosteric modulation of a G protein-coupled receptor: implications for detection and validation of allosteric ligand effects. Mol Pharmacol. 2012;81(1):41–52. doi: 10.1124/mol.111.074872. [DOI] [PubMed] [Google Scholar]
- Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, Kinney GG, Conn PJ. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J Neurosci. 2003;23(18):7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A, Ferraboli S, Paterlini M, Spano P, Barlati S. Identification of novel alternatively-spliced mRNA isoforms of metabotropic glutamate receptor 6 gene in rat and human retina. Gene. 2001;262(1-2):99–106. doi: 10.1016/s0378-1119(00)00547-3. [DOI] [PubMed] [Google Scholar]
- Varney MA, Cosford ND, Jachec C, Rao SP, Sacaan A, Lin FF, Bleicher L, Santori EM, Flor PJ, Allgeier H, Gasparini F, Kuhn R, Hess SD, Velicelebi G, Johnson EC. SIB-1757 and SIB-1893: selective, noncompetitive antagonists of metabotropic glutamate receptor type 5. J Pharmacol Exp Ther. 1999;290(1):170–181. [PubMed] [Google Scholar]
- Varney MA, Gereau R. W. t. Metabotropic glutamate receptor involvement in models of acute and persistent pain: prospects for the development of novel analgesics. Curr Drug Targets CNS Neurol Disord. 2002;1(3):283–296. doi: 10.2174/1568007023339300. [DOI] [PubMed] [Google Scholar]
- Vieira E, Huwyler J, Jolidon S, Knoflach F, Mutel V, Wichmann J. Fluorinated 9H-xanthene-9-carboxylic acid oxazol-2-yl-amides as potent, orally available mGlu1 receptor enhancers. Bioorg Med Chem Lett. 2009;19(6):1666–1669. doi: 10.1016/j.bmcl.2009.01.108. [DOI] [PubMed] [Google Scholar]
- Watkins JC, Krogsgaard-Larsen P, Honore T. Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci. 1990;11(1):25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]
- Wenthur CJ, Morrison R, Felts AS, Smith KA, Engers JL, Byers FW, Daniels JS, Emmitte KA, Conn PJ, Lindsley CW. Discovery of (R)-(2-Fluoro-4-((-4-methoxyphenyl)ethynyl)phenyl) (3-Hydroxypiperidin-1-yl)methanone (ML337), An mGlu Selective and CNS Penetrant Negative Allosteric Modulator (NAM). J Med Chem. 2013;56(12):5208–5212. doi: 10.1021/jm400439t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Marino MJ, Bradley SR, Conn PJ. Activation of group III mGluRs inhibits GABAergic and glutamatergic transmission in the substantia nigra pars reticulata. J Neurophysiol. 2001;85(5):1960–1968. doi: 10.1152/jn.2001.85.5.1960. [DOI] [PubMed] [Google Scholar]
- Woltering TJ, Adam G, Wichmann J, Goetschi E, Kew JN, Knoflach F, Mutel V, Gatti S. Synthesis and characterization of 8-ethynyl-1,3-dihydro-benzo[b][1,4]diazepin-2-one derivatives: part 2. New potent non-competitive metabotropic glutamate receptor 2/3 antagonists. Bioorg Med Chem Lett. 2008;18(3):1091–1095. doi: 10.1016/j.bmcl.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Woltering TJ, Wichmann J, Goetschi E, Adam G, Kew JN, Knoflach F, Ballard TM, Huwyler J, Mutel V, Gatti S. Synthesis and characterization of 1,3-dihydro-benzo[b][1,4]diazepin-2-one derivatives: Part 3. New potent non-competitive metabotropic glutamate receptor 2/3 antagonists. Bioorg Med Chem Lett. 2008;18(8):2725–2729. doi: 10.1016/j.bmcl.2008.02.076. [DOI] [PubMed] [Google Scholar]
- Wood MR, Hopkins CR, Brogan JT, Conn PJ, Lindsley CW. Molecular switches” on mGluR allosteric ligands that modulate modes of pharmacology. Biochemistry. 2011;50(13):2403–2410. doi: 10.1021/bi200129s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD. Binding of [3H](2S,1'S,2'S)-2-(9-xanthylmethyl)-2-(2'-carboxycyclopropyl) glycine ([3H]LY341495) to cell membranes expressing recombinant human group III metabotropic glutamate receptor subtypes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(6):546–554. doi: 10.1007/s002100000305. [DOI] [PubMed] [Google Scholar]
- Wright RA, Johnson BG, Zhang C, Salhoff C, Kingston AE, Calligaro DO, Monn JA, Schoepp DD, Marek GJ. CNS distribution of metabotropic glutamate 2 and 3 receptors: transgenic mice and [(3)H]LY459477 autoradiography. Neuropharmacology. 2013;66:89–98. doi: 10.1016/j.neuropharm.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Wu H, Wang C, Gregory KJ, Han GW, Cho HP, Xia Y, Niswender CM, Katritch V, Meiler J, Cherezov V, Conn PJ, Stevens RC. Structure of a Class C GPCR Metabotropic Glutamate Receptor 1 Bound to an Allosteric Modulator. Science. 2014 doi: 10.1126/science.1249489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa M, Yamashita T, Shichida Y. Glutamate acts as a partial inverse agonist to metabotropic glutamate receptor with a single amino acid mutation in the transmembrane domain. J Biol Chem. 2013;288(14):9593–9601. doi: 10.1074/jbc.M112.437780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Noetzel MJ, Johnson KA, Zamorano R, Jalan-Sakrikar N, Gregory KJ, Conn PJ, Niswender CM. Selective actions of novel allosteric modulators reveal functional heteromers of metabotropic glutamate receptors in the CNS. J Neurosci. 2014;34(1):79–94. doi: 10.1523/JNEUROSCI.1129-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Zamorano R, Conn PJ, Niswender CM. Functional selectivity induced by mGlu(4) receptor positive allosteric modulation and concomitant activation of Gq coupled receptors. Neuropharmacology. 2013;66:122–132. doi: 10.1016/j.neuropharm.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz C, Forster U, Neidhardt J, Feil S, Kalin S, Leifert D, Flor PJ, Berger W. Night blindness-associated mutations in the ligand-binding, cysteine-rich, and intracellular domains of the metabotropic glutamate receptor 6 abolish protein trafficking. Hum Mutat. 2007;28(8):771–780. doi: 10.1002/humu.20499. [DOI] [PubMed] [Google Scholar]
- Zhai J, Tian MT, Wang Y, Yu JL, Koster A, Baez M, Nisenbaum ES. Modulation of lateral perforant path excitatory responses by metabotropic glutamate 8 (mGlu8) receptors. Neuropharmacology. 2002;43(2):223–230. doi: 10.1016/s0028-3908(02)00087-4. [DOI] [PubMed] [Google Scholar]
- Zhang CS, Bertaso F, Eulenburg V, Lerner-Natoli M, Herin GA, Bauer L, Bockaert J, Fagni L, Betz H, Scheschonka A. Knock-in mice lacking the PDZ-ligand motif of mGluR7a show impaired PKC-dependent autoinhibition of glutamate release, spatial working memory deficits, and increased susceptibility to pentylenetetrazol. J Neurosci. 2008;28(34):8604–8614. doi: 10.1523/JNEUROSCI.0628-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rodriguez AL, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther. 2005;315(3):1212–1219. doi: 10.1124/jpet.105.090308. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Manka JT, Rodriguez AL, Weaver CD, Days EL, Vinson PN, Jadhav S, Hermann EJ, Jones CK, Conn PJ, Lindsley CW, Stauffer SR. Discovery of N-Aryl Piperazines as Selective mGlu(5) Potentiators with Efficacy in a Rodent Model Predictive of Anti-Psychotic Activity. ACS Med Chem Lett. 2010;1(8):433–438. doi: 10.1021/ml100181a. [DOI] [PMC free article] [PubMed] [Google Scholar]