Abstract
Severe refractory asthma is associated with enhanced nitrative stress. To determine the mechanisms for high nitrative stress in human severe asthma, 3-nitrotyrosine (3NT) was compared with Th1 and Th2 cytokine expression. In severe asthma, high 3NT levels were associated with high IFN-γ and low IL-13 expression, both of which have been reported to increase inducible nitric oxide synthase (iNOS) in human airway epithelial cells (HAEC). We found IL-13 and IFN-γ synergistically enhanced iNOS, nitrite and 3NT, corresponding with increased H2O2. Catalase inhibited while superoxide dismutase enhanced 3NT formation, supporting a critical role for H2O2 but not peroxynitrite, in 3NT generation. Dual oxidase-2 (DUOX2), central to H2O2 formation, was also synergistically induced by IL-13 and IFN-γ. The catalysis of nitrite and H2O2 to nitrogen dioxide radical (NO2•) requires an endogenous peroxidase in this epithelial cell system. Thyroid peroxidase (TPO) was identified by microarray analysis ex vivo as a gene distinguishing HAEC of severe asthma from controls. IFN-γ induced TPO in HAEC and siRNA knockdown decreased nitrated tyrosine residues. Ex vivo, DUOX2, TPO and iNOS were higher in severe asthma and correlated with 3NT. Thus a novel iNOS-DUOX2-TPO-NO2• metabolome drives nitrative stress in HAEC and likely in severe asthma.
INTRODUCTION
High levels of nitrated proteins, widely recognized to contribute to altered protein function and disease, are formed by the reaction of reactive nitrogen species (RNS) with tyrosine residues at the ortho position.1–2 These 3-nitrotyrosines (3NT) have been consistently reported in asthma, particularly in severe asthma (SA),.3–5 where they have been associated with worsening airway obstruction.5 In human airway epithelial cells (HAEC), inducible nitric oxide synthase (iNOS), is the most important source of the nitrites and NO required for formation of RNS.6–7 However, the oxidative metabolic pathways by which these iNOS products become RNS in human diseases such as asthma are less clear. Two pathways are generally believed to be important, one involving generation of superoxide radical, the other H2O2. Superoxide radical reacts with NO to form peroxynitrite, while H2O2 reacts with nitrite to form nitrogen dioxide radical (NO2•), either of which can nitrate tyrosine residues.1, 8–12 In the case of the H2O2 pathway, however, an endogenous or exogenous peroxidase is also required for the formation of NO2•.
While the specifics of the oxidative stress pathways involved remain poorly understood in primary human airway epithelial cells and in association with disease, it is likely that the family of NADPH oxidases and Dual oxidases (DUOX) contribute.13–14 DUOX1, is the most abundant oxidase, while DUOX2 (also known as NAD/NADPH thyroid oxidase) is reported to be the primary source of H2O2 in human airway epithelium.15–16 Both were originally described in the thyroid where their H2O2 production is tightly regulated by thyroid peroxidase (TPO), and through interactions with TPO control the formation of iodide radical essential for thyroid hormone synthesis.17–18 Despite the high levels of DUOX enzymes in human airway epithelium, and their known interactions with TPO in thyroid epithelium, TPO has been thought to be a thyroid specific enzyme, not previously described in other epithelial structures. However, microarray analysis identified TPO in HAEC as the only peroxidase distinguishing SA from healthy controls (and milder asthma). In environments, such at the airway, where high levels of nitrite (as opposed to iodide) exist, DUOX2, interacting with its known partner in the thyroid epithelium, TPO, could enhance production of NO2• and 3NT.
Although asthma, or at least an endotype of it, has consistently been thought of as a Th2 disease, iNOS expression has long been associated with Th1 immunity, with an earlier report linking airway epithelial Stat1 expression to iNOS.19 More recently, the Th2 cytokine IL-13 has also been shown to induce iNOS in vitro, and indeed, specific inhibitors of IL-4 and/or IL-13 decrease levels of NO by 50% in exhaled breath of human asthmatics.20–21 Interestingly, long before iNOS was confirmed to be upregulated by Th2 cytokines alone, the combination of IL-4 and IFN-γ were reported to jointly increase iNOS expression.7, 22 Corticosteroid (CS) refractory SA has been associated with sustained elevations in iNOS expression,23 but the mechanisms for the high levels remain poorly understood, especially in view of the general effect of CSs to suppress Th2 immunity. Whether certain phenotypes of SA exhibit high levels of iNOS and exhaled NO due to a mixed Th1 and Th2 immune process is not yet clear. However, interestingly, several studies have shown that, similar to iNOS, DUOX enzymes are regulated by Th1 and Th2 cytokines.15–16, 24
We therefore hypothesized that increases in nitrative stress in SA would be seen in the presence of increasing Th1 cytokines, in association with a Th2 background. This combined Th1 and Th2 immunity would increase nitrative stress through synergistic increases in iNOS and the expression and activation of the oxidizing enzyme DUOX2, in the presence of an unknown endogenous epithelial peroxidase. Thus, the biochemistry would involve a H2O2-nitrite (endogenous) peroxidase nitration reaction. This hypothesis was addressed by determining: 1) the levels of 3NT in association with Th1 and Th2 cytokines in SA, 2) the combined effects of Th1 (IFN-γ) and Th2 (IL-13) cytokines on 3NT generation and the central importance of nitrite and H2O2 to this process, 3) the critical importance of 2 novel proteins, DUOX2 and TPO to the NO2• and nitrated protein formation and 4) the recapitulation of this novel nitrative metabalome in severe asthmatic epithelium.
RESULTS
Subject Demographics
Fresh airway epithelial and bronchoalveolar lavage (BAL) cells were obtained from 91 subjects (Table 1). Twenty-three healthy controls (HC), 23 mild-moderate (MMA) and 29 SA contributed to BAL cell studies. Fresh epithelial cells were collected from 15 HC, 15 MMA and 31 SA. In vitro studies were performed on epithelial cells from 4 HC, 16 MMA and 8 SA. SA were older than control groups, had the lowest forced expiratory volume in 1 second (FEV1) percent predicted despite lower levels of atopy than MMA. Thirty-five (83%) of SA used regular systemic CS. There were no differences in the demographics of the subgroups used for BAL, epithelial, or in vitro studies, nor between MMA by use of inhaled CS.
Table 1.
Demographics of subjects in study (n = 91)
| Healthy control (n = 25) |
Mild-Moderate asthma (n = 24) |
Severe asthma (n = 42) |
P-values | |
|---|---|---|---|---|
| Female gender | 11 (44%) | 17 (71%) | 30 (71%) | 0.05 |
| Age (years)a | 28 (23–44) | 29 (23–37) | 47 (35–55) | < 0.001 |
| FEV1% predicted a | 97 (93–107) | 90 (83–97) | 56 (40–71) | < 0.001 |
| ICS | 0 | 14 of 24 | 42 of 42 | NA |
| Atopy | 13 of 25 | 23 of 24 | 31 of 42 | 0.002 |
Median with 25th-75th percentiles.
FEV1% predicted, forced expiratory volume in 1 second expressed as a percentage of the predicted value; ICS, inhaled corticosteroid; NA, not applicable.
3NT is increased in SA lungs in association with increases in IFN-γ
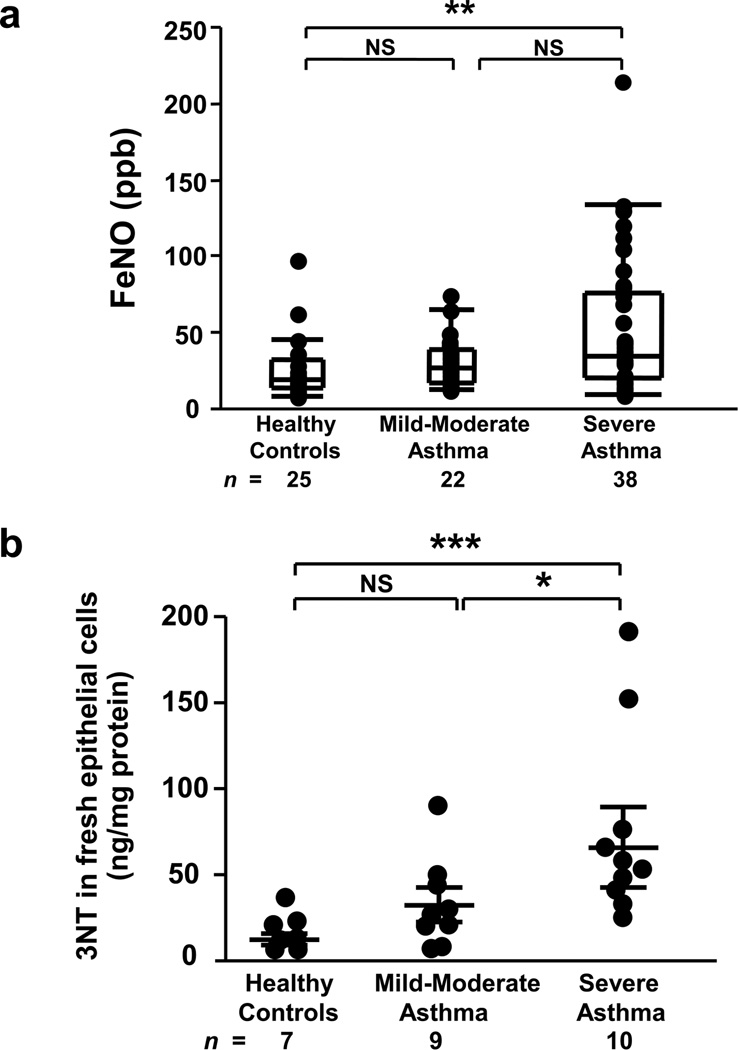
Both fractional exhaled nitric oxide (FeNO) and epithelial 3NT levels were elevated in SA, however, 3NT better distinguished severity (Figure 1). Similarly, IFN-γ mRNA was elevated in BAL cells from asthmatics and highest in SA [median arbitrary units (25th-75th percentile), HC=0.2 (0.09–0.36), MMA=0.4 (0.22–2.48), SA=3.1 (0.70–6.27); overall P<0.001, age-adjusted P<0.05 for all comparisons, n=53]. The levels of 3NT and IFN-γ did not differ by atopy or CS use. In contrast, BAL cell IL-13 mRNA levels were 2–3 log lower than IFN-γ and did not differentiate the 3 groups [HC=0.005 (0–0.103), MMA=0.010 (0–0.033), SA=0.002 (0–0.041); overall P=0.97, n=59]. However, unlike IFN-γ, atopy was associated with IL-13 levels, with IL-13 being significantly higher in atopic [0.057 (0.003–0.139), n=8] versus non-atopic HC [0 (0–0.037), n=8; P=0.046), suggesting a strong influence of background atopy alone. (85% of asthmatics were atopic limiting comparisons.) Thus, although low levels of IL-13 mRNA were present, and higher in atopic HCs, there were no increases with asthma or its severity . However, IFN-γ mRNA levels increased with increasing severity of disease, in association with increasing 3NT levels.
Figure 1.
Elevated FeNO and epithelial 3NT levels in SA. (a) FeNO levels were increases in SA compared to HC. Overall P<0.01, **P<0.01; Kruskal-Wallis with Bonferroni correction; NS, not significant. Data were shown as median (25–75 percentiles). (b) 3NT levels were highest in fresh bronchial epithelial cells from SA compared to MMA and HC. Overall P<0.001, *P<0.05, ***P<0.001; adjusted for age. Results were shown as mean±SEM.
The combination of Th1 and Th2 cytokines increases iNOS expression, and selectively increases nitrite in association with increases in 3NT over that of either cytokine alone
iNOS mRNA and protein
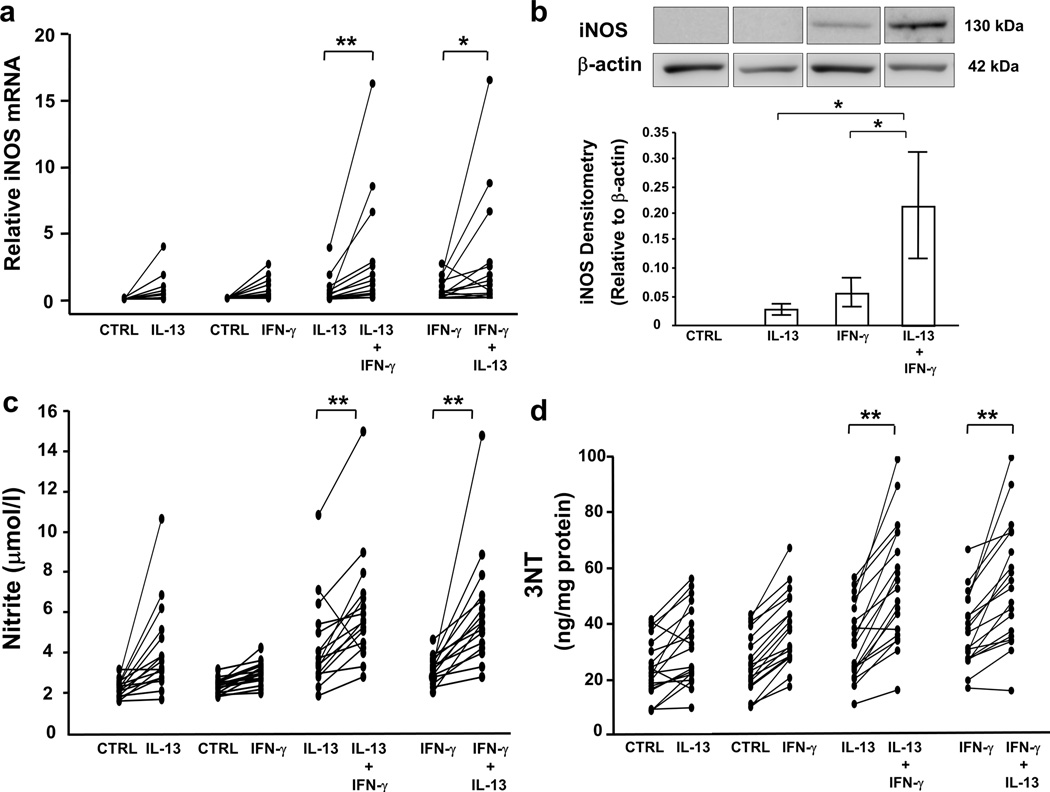
To confirm the combined impact of Th1 and Th2 cytokines on iNOS expression, cultured HAEC were stimulated for 8 d with/without IL-13 (1,10 ng/ml) and then stimulated (or not) with IFN-γ (10,100 ng/ml) for last 72 h. iNOS mRNA and protein expression were highest in the presence of low-dose IL-13 (1 ng/ml) and either low- or high-dose IFN-γ (Figure 2a and b).
Figure 2.
The combination of low-dose IL-13 and IFN-γ enhances iNOS expression, nitrite, and 3NT levels. ALI-cultured HAEC were treated with low-dose IL-13 (1 ng/ml) or media for 8 d, with/without exposure to IFN-γ (10 ng/ml) for final 72 h. mRNA was harvested for real-time PCR. Total proteins were harvested for Western blot analysis and 3NT measurement. Nitrite levels were measured in the lower supernatants. (a) iNOS mRNA, (b) iNOS protein, (c) nitrite, and (d) 3NT expression were augmented in the presence of IL-13 and IFN-γ compared to either cytokine alone. P for interaction <0.05 for all markers; *P<0.025, **P<0.001; n=16, 15, 16, 19 for iNOS mRNA, iNOS protein, nitrite, and 3NT, respectively. Densitometry values were shown as mean±SEM.
iNOS activity
Nitrite and nitrate were utilized as markers of iNOS activity. Nitrite levels were consistently and synergistically higher with the combination of low-dose IL-13 and IFN-γ (any dose) (Figure 2c). In contrast, nitrate did not increase with the combination (Supplementary Figure 1a). Although nitrate was the predominant metabolite generated by both cytokines, IFN-γ treatment resulted in a greater ratio of nitrite/nitrate (IFN-γ 10 ng/ml: 0.3±0.06, IL-13 10 ng/ml: 0.1±0.01; P<0.01, n=12). High-dose IFN-γ together with low-dose IL-13 similarly enhanced nitrite/nitrate levels compared to IL-13 alone (P<0.025). However, there were no differences in nitrite/nitrate ratios between treatment with IFN-γ alone or IFN-γ plus IL-13.
3NT expression
Both low- and high-dose IFN-γ increased 3NT levels (control: 24±2, IFN-γ 10 ng/ml: 36±3, IFN-γ 100 ng/ml: 33±4 ng/mg protein; P<0.017 vs. control for both doses, n=19), while only low-dose IL-13 significantly increased their levels (32±3; P<0.017 vs. control). Similar to iNOS and nitrite, 3NT levels were synergistically enhanced in the presence of low-dose IL-13 and IFN-γ (any dose) (Figure 2d). The percent increase in 3NT correlated with the percent increase in nitrite (from baseline) with low-dose IFN-γ (r=0.75, P<0.01, n=11) and low-dose IFN-γ plus low-dose IL-13 (r=0.7, P<0.05), but not under other conditions.
Generation of 3NT is dependent on H2O2 and nitrite as opposed to superoxide and NO
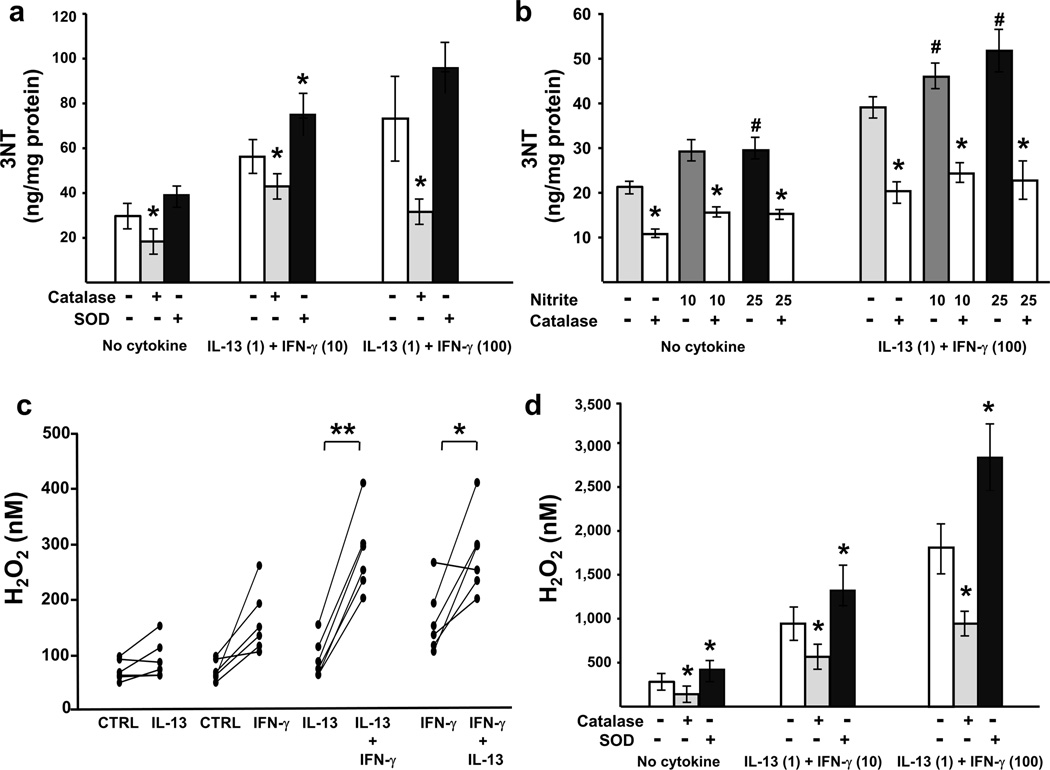
To explore the oxidative pathways involved in 3NT generation, specifically peroxynitrite dependent vs. H2O2-nitrite-peroxidase dependent, catalase (H2O2 scavenger) or superoxide dismutase (SOD; superoxide scavenger) was added apically for 1 h. Catalase addition decreased 3NT levels. Conversely, treatment with SOD paradoxically increased 3NT in the presence of low-dose IL-13 and low-dose IFN-γ (Figure 3a), supporting the contribution of H2O2, but not superoxide, to epithelial 3NT formation. The addition of supplemental nitrite further increased the levels of 3NT in the presence of SOD (Supplementary Figure 3).
Figure 3.
3NT expression changes in parallel with levels of H2O2 and nitrite. ALI-cultured HAEC were treated with low-dose IL-13 (1 ng/ml) or media for 8 d, with/without exposure to IFN-γ for the last 72 h. Culture medium from the apical chamber was removed, and 100 µl PBS with/without one of the following was added: catalase (150 U/ml), SOD (150 U/ml), nitrite (10,25 µM), or the combination of catalase and nitrite. After 1-h incubation, extracellular H2O2 levels were measured in the upper chamber supernatants. Total proteins were harvested for 3NT measurement. (a) Addition of catalase decreased 3NT levels in the presence/absence of IL-13 and IFN-γ while SOD increased 3NT when cells were stimulated with IL-13 and IFN-γ (10 ng/ml). *P<0.05 vs. no catalase/SOD. (b) Exogenous nitrite upregulated 3NT expression while catalase attenuated this effect. #P<0.017 vs. no nitrite, *P<0.05 vs. no catalase. (c) H2O2 levels were enhanced in the presence of low-dose IL-13 and IFN-γ (10 ng/ml) compared to either cytokine alone. P for interaction <0.05, *P<0.025, **P<0.001. (d) H2O2 production followed a similar trend to 3NT, with the levels decreasing with catalase and increasing with SOD. *P<0.05 vs. no catalase/SOD. All n=6. Data represented mean±SEM.
The role of H2O2/nitrite-mediated tyrosine nitration was further investigated by adding exogenous nitrite in the presence/absence of IL-13 (1 ng/ml) plus IFN-γ (100 ng/ml). 3NT increased following addition of nitrite, an effect only marginally dependent on the presence of IL-13 + IFN-γ (12.6±1.99 vs. 6.5±1.82 ng/mg protein, P=0.1 for differences following 25 µM nitrite addition; Figure 3b). The increase in 3NT was attenuated ~50% by catalase under all conditions, confirming a role for H2O2.
IFN-γ alone, and in combination with low-dose IL-13, increases H2O2 production which correlates with 3NT levels
To determine whether Th1 or Th2 cytokines, alone or in combination, enhance H2O2 production, extracellular H2O2 production was measured apically after 1-h with PBS under the various IFN-γ and IL-13 conditions. IFN-γ robustly enhanced H2O2 production in a dose-dependent manner (control: 76±8, IFN-γ 10 ng/ml: 165±24, IFN-γ 100 ng/ml: 230±29 nM; overall P<0.001, P<0.017 for all comparisons, n=6). H2O2 also increased following high-dose, but not low-dose, IL-13 stimulation (IL-13 1 ng/ml: 96±15, IL-13 10 ng/ml: 179±28; overall P<0.01, P<0.017 for IL-13 10 ng/ml vs. control). Similar to 3NT expression, the combination of low-dose IL-13 and IFN-γ (any dose) synergistically augmented apical H2O2 production (Figure 3c). Furthermore, H2O2 levels strongly correlated with 3NT (r=0.82, P<0.05, n=6) with stimulation by low-dose IL-13 plus low-dose IFN-γ, but not with low-dose IL-13 plus high-dose IFN-γ (r=0.61, P=0.2). There were no correlations under other conditions (all r<0.4), perhaps due to small sample size. As expected, and in parallel with the changes in 3NT, H2O2 was decreased by catalase, while SOD increased H2O2 in the presence/absence of IL-13 and IFN-γ (Figure 3d).
Expression of the H2O2 producing enzyme DUOX2 is increased by IFN-γ, alone and in combination with IL-13, while knockdown reduces H2O2 and 3NT levels
DUOX2 mRNA and protein
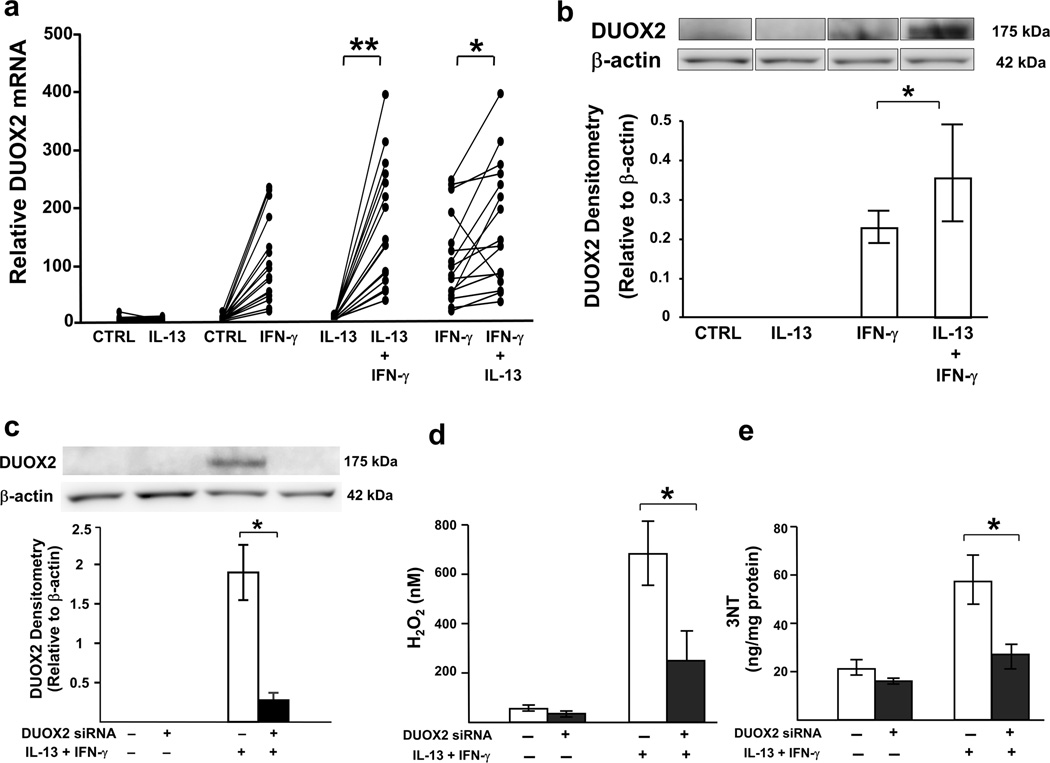
As previously reported,15 IFN-γ dose-dependently increased DUOX2 mRNA and protein expression from HAEC (Supplementary Figure 1b and c). In the presence of low-dose IL-13, there was a synergistic increase in DUOX2 mRNA and protein compared to IFN-γ alone (any dose) (Figure 4a and b).
Figure 4.
IFN-γ in combination with low-dose IL-13 enhances DUOX2 expression and knockdown of DUOX2 decreases H2O2 as well as 3NT levels. ALI-cultured HAEC were stimulated for 8 d with or without low-dose IL-13 (1 ng/ml) and then stimulated (or not) with IFN-γ (10 ng/ml) for the last 72 h. For knockdown experiment, ALI-cultured cells transfected with DUOX2 siRNA were stimulated with IL-13 (1 ng/ml) and IFN-γ (100 ng/ml) for 48 h. The culture medium from the upper chamber was removed, and 100 µl PBS was added. Extracellular H2O2 production was measured in the upper chamber supernatants after 1-h incubation. mRNA was harvested for real-time PCR. Total proteins were harvested for Western blot analysis and 3NT measurement. In the concomitant presence of IL-13 and IFN-γ, there were increases in (a) DUOX2 mRNA and (b) DUOX2 protein over that of either IFN-γ or IL-13 alone. P for interaction< 0.001 for both markers, *P<0.025, **P<0.001; n=16 for DUOX2 mRNA, n=11 for DUOX2 protein. (c) Western blot demonstrating DUOX2 knockdown in the presence of IL-13 and IFN-γ stimulation. Inhibition of DUOX2 by siRNA transfection suppressed (d) H2O2, and (e) 3NT production induced by IL-13 and IFN-γ. *P<0.05; n=4. Data represented mean±SEM.
Knockdown of DUOX2
To confirm the role of DUOX2 in the increase in H2O2 and 3NT, siRNA knockdown of DUOX2 was utilized in the presence/absence of combined IL-13 (1 ng/ml) and IFN-γ (100 ng/ml). High-dose IFN-γ was used because it gave the most robust increase in DUOX2. DUOX2-targeted siRNA decreased DUOX2 mRNA (5.6±1.38 vs. 6.1±1.42, P<0.05, n=3) and protein expression following stimulation with IL-13 and IFN-γ (Figure 4c). Knockdown of DUOX2 led to ~50% lower H2O2 and 3NT levels as compared to scramble siRNA (Figure 4d and e).
TPO, an endogenous epithelial peroxidase, is identified as a highly differentiating gene in epithelial microarrays
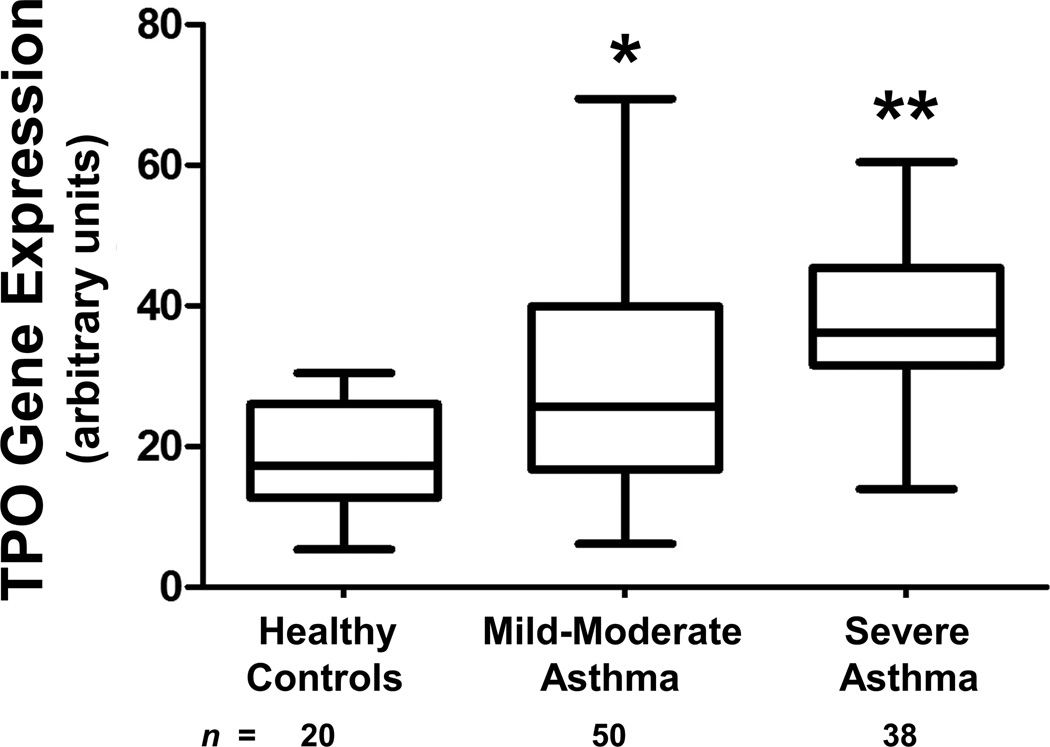
A heme peroxidase is required to generate the NO2•, which nitrates tyrosine residues to form 3NT. The presence of 3NT in the isolated cell culture system suggests the presence of an endogenous epithelial peroxidase. A large scale microarray dataset generated from airway epithelial brushings of asthmatics and HC (Subject Demographics in Supplementary Table 1) recruited through the Severe Asthma Research Program (SARP) network was probed for the presence of peroxidases. When comparing the subjects with SA to HC and looking at 27,958 genes, the differentially expressed gene with the sixth highest linear fold ratio difference was TPO with a fold ratio of 3.65 (Figure 5). No other peroxidases were differentially expressed between SA and HC.
Figure 5.
TPO is identified as a highly differentiating gene in epithelial microarrays. Box-whisker plot showing linear-scale microarray gene expression of TPO for HC, MMA, and SA. The boxes represented the mean and standard deviation and the whiskers represented the minimum and maximum values of the distribution. *P<0.05; 2-tailed unpaired t-test vs. HC, **P<0.05; 2-tailed unpaired t-test vs. HC and MMA.
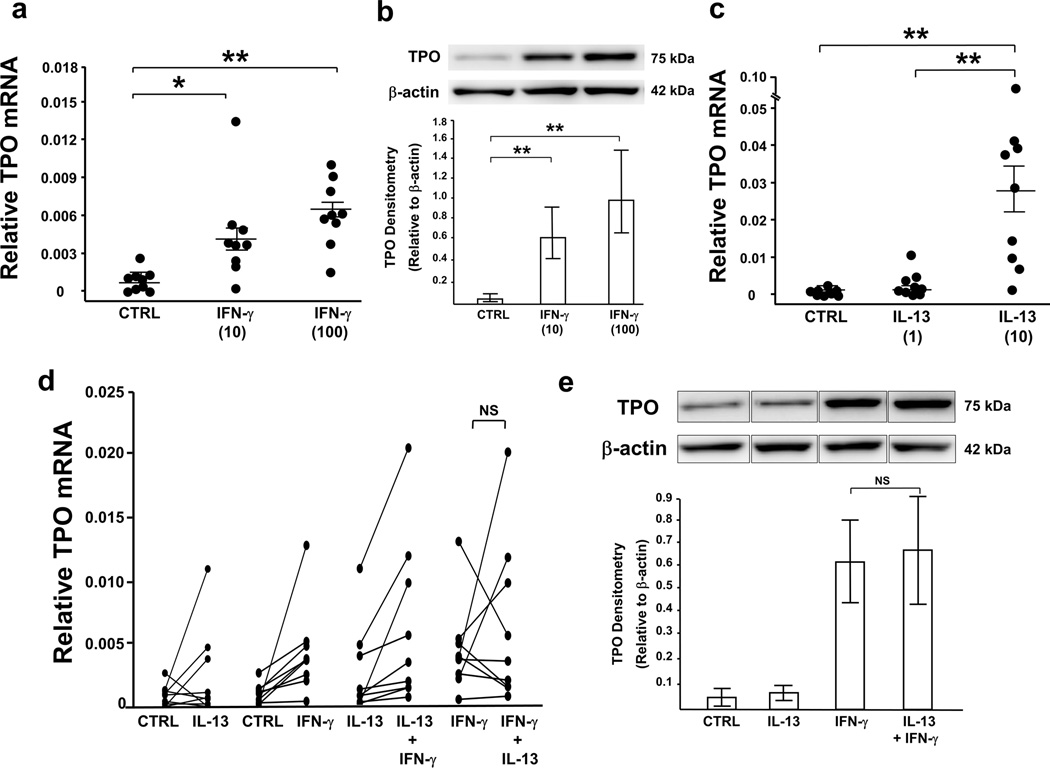
HAEC endogenously express TPO, a heme peroxidase which is primarily enhanced by Th1 cytokine IFN-γ
IFN-γ stimulation enhanced both TPO mRNA and protein expression (Figure 6a and b). TPO in air-liquid interface (ALI)-cultured cells consistently appeared as a single ~75–80 kDa band (25–35 kDa less than the previously reported ~100–110 kDa full-length protein), suggesting a potential new TPO isoform expressed in HAEC. TPO mRNA, but not protein, was induced by high-dose IL-13 (Figure 6c). Unlike DUOX2 and iNOS, only high-dose (100 ng/ml), but not low-dose (10 ng/ml), IFN-γ in combination with low-dose IL-13 further increased TPO mRNA (overall P<0.001, P<0.025 between combined low-dose IL-13 and high-dose IFN-γ vs. IL-13 alone or IFN-γ alone; n=9), however, no synergistic effect on protein was observed (Figure 6d and e). TPO mRNA correlated with DUOX2 mRNA under combined low-dose IL-13 and low-dose IFN-γ (r=0.92, P<0.001, n=9). Under low-dose IL-13 and high-dose IFN-γ, TPO mRNA levels in cultured cells from SA (n=4) tended to be higher than those from non-severe subjects (1 HC, 4 MMA; P=0.07).
Figure 6.
TPO expression is enhanced by IFN-γ. ALI-cultured cells were treated with IL-13 or media for 8 d, with/without exposure to IFN-γ for final 72 h. mRNA was harvested for real-time PCR and total proteins were harvested for Western blot analysis. Both concentrations of IFN-γ (10,100 ng/ml) induced (a) TPO mRNA and (b) protein, while (c) only high-dose IL-13 (10 ng/ml), but not low-dose (1 ng/ml) enhanced TPO mRNA. Overall P<0.001, *P<0.017, **P<0.001. Unlike INOS and DUOX2, IFN-γ (10 ng/ml) in combination with low-dose IL-13 (1 ng/ml) did not further increase (d) TPO mRNA or (e) protein. n=9 for TPO mRNA, n=8 for TPO protein. Results were shown as mean±SEM.
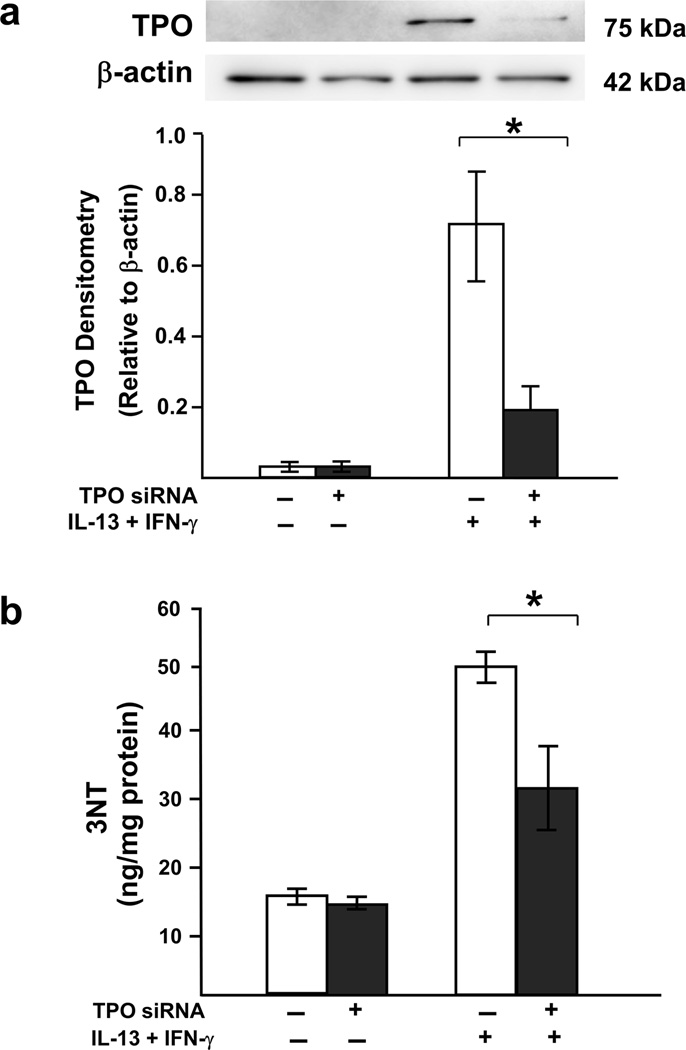
Knockdown of TPO decreases 3NT expression confirming its involvement
To investigate the role of TPO in 3NT generation, cells were transfected with TPO siRNA in the presence/absence of combined IL-13 (1 ng/ml) and IFN-γ (100 ng/ml; as used for DUOX2 siRNA experiments). TPO siRNA reduced TPO protein and 3NT (Figure 7) supporting the contribution of TPO to epithelial 3NT formation.
Figure 7.
TPO siRNA reduces 3NT formation. ALI-cultured cells transfected with TPO siRNA were stimulated with IL-13 (1 ng/ml) and IFN-γ (100 ng/ml) for 48 h. Total proteins were harvested for Western blot analysis and 3NT measurement. (a) Western blot of TPO knockdown in the presence of IL-13 and IFN-γ stimulation. (b) Suppression of TPO by siRNA transfection reduced 3NT production induced by IL-13 and IFN-γ. *P<0.05; one-tailed t-test, n=3. Data represented mean±SEM.
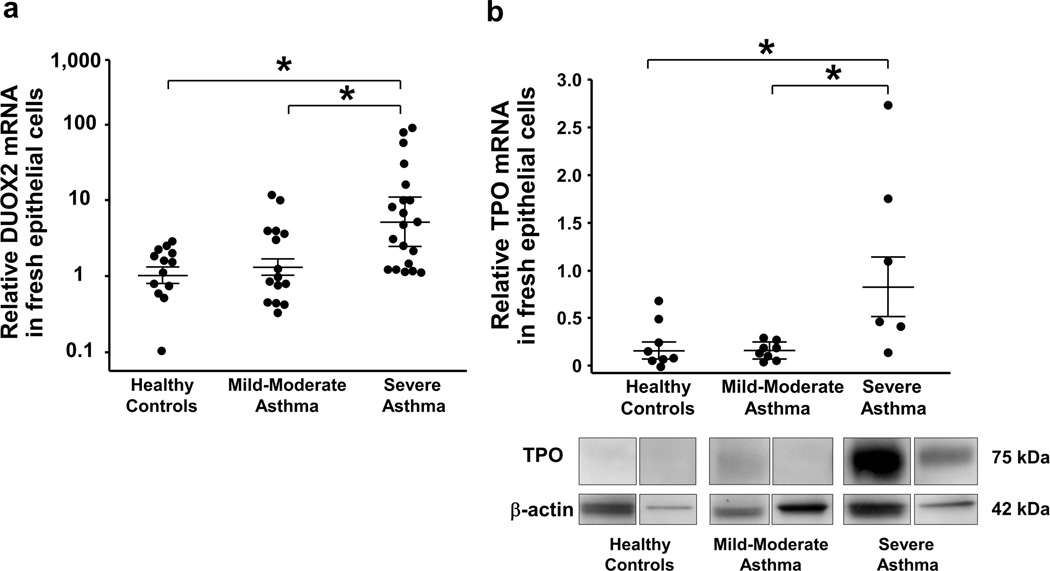
DUOX2 and TPO expression are increased in fresh bronchial epithelial cells from SA
We confirmed previously reported increase in iNOS expression in epithelial cells of asthma, including SA (Supplementary Figure 2).19, 23 Similarly, DUOX2, and TPO expression were highest in SA compared to MMA and HC (Figure 8). Use of inhaled CS did not impact results. DUOX2 mRNA in fresh epithelial cells correlated with IFN-γ mRNA in BAL cells (r=0.58, P<0.001, n=30). Positive correlations between DUOX2 and iNOS mRNA (r=0.46, P<0.001, n=48), DUOX2 and 3NT (r=0.57, P<0.01, n=23), as well as DUOX2 and TPO mRNA (rho=0.46, P<0.05, n=19) were observed. 3NT correlated with iNOS mRNA (r=0.45, P<0.05, n=26) and FeNO (rho=0.48, P<0.05, n=26). TPO mRNA did not correlate with 3NT. No correlation was found between BAL IL-13 mRNA and epithelial 3NT, DUOX2 and TPO mRNA.
Figure 8.
Enhanced DUOX2 and TPO mRNA in epithelial cells from SA. Expression of (a) DUOX2 mRNA and (b) TPO mRNA were increased in fresh bronchial epithelial cells from SA compared to MMA and HC. Overall P<0.05 for both markers, *P<0.05; adjusted for age. Results were shown as mean±SEM.
DISCUSSION
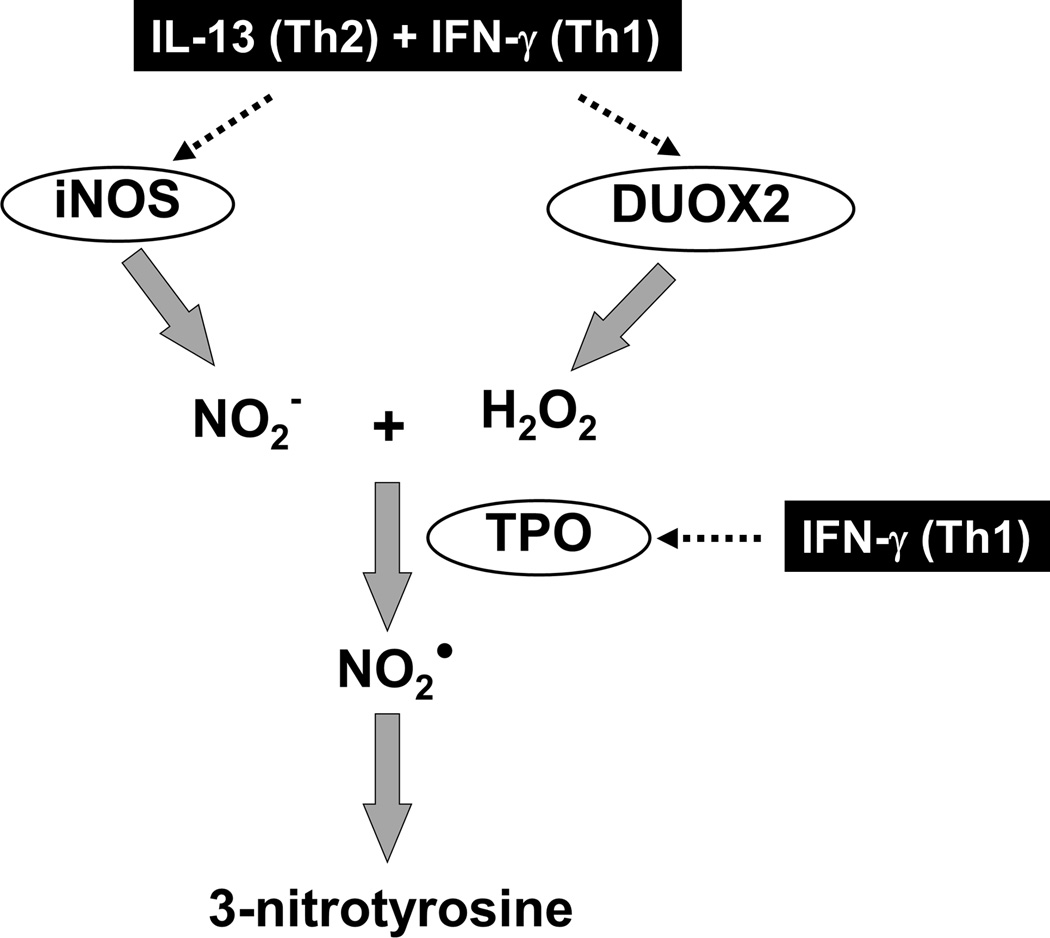
This present study identifies a highly novel metabolic pathway by which combined Th1 and Th2 cytokines, as observed in SA, enhance nitrative stress in HAEC. It links these in vitro data to in vivo data supporting the relevance of these findings to more severe forms of asthma, where the degree of airway nitrative (and oxidative stress) is greatest. The Th1 cytokine (IFN-γ), together with a Th2 (IL-13) inflammatory background not only amplifies epithelial DUOX2 expression leading to a robust increase in its reactive oxygen species (ROS) product H2O2, but also potentiates the production of one specific iNOS generated nitrogen species, nitrite, required for reactions with H2O2. Reaction of these two specific molecules, H2O2 and nitrite, when catalyzed by peroxidases, specifically the novel airway epithelial peroxidase TPO identified here, forms NO2•, a powerful reactive nitrogen radical which nitrates tyrosine residues identified as 3NT. Thus, generation of 3NT in this airway epithelial culture system occurs primarily through a novel iNOS/DUOX2/TPO nitrite oxidation metabolome, and not through a superoxide dependent peroxynitrite reaction (Figure 9). Thus, in addition to its well-known beneficial oxidative function in innate pulmonary host defense, DUOX2 appears critical to airway nitrative-oxidative injury through this H2O2-induced 3NT formation. Collectively, we propose that the concomitant presence of Th1 and Th2 cytokines, as observed in SA, augments airway nitro-oxidative stress through novel pathways, contributing to epithelial dysfunction and worsening asthma.
Figure 9.
Proposed biochemical pathway of 3NT generation in airway epithelia.
In a previous study we reported significant increases in iNOS expression in SA, despite high-dose inhaled and even systemic CS use.23 While this increase in iNOS is likely partly due to persistent Th2 cytokines, such as IL-13 (known to induce iNOS)7, IL-13 and IL-4 have been difficult to measure in consistently higher quantities in severe CS treated asthmatics. However, asthmatics have higher than normal levels of IFN-γ in BAL that increase with allergen exposure, with evidence for induction of IFN-γ signal transduction pathways.19 IFN-γ gene expression in the airways was similarly evaluated in this study revealing significantly higher levels in SA compared to milder asthmatics, supporting a role of this cytokine in iNOS expression as well In HAEC, IFN-γ synergized with IL-13 to enhance iNOS expression. Thus, it is plausible that the presence of IL-13, together with IFN-γ might play a large role in expression of iNOS in SA. Furthermore, the increase in INOS was associated with increases in both FeNO and 3NT. Increases in 3NT expression necessitate the additional presence of ROS. Excessive ROS (and RNS) has been consistently reported in the airways of asthmatics.3–5, 25–27 These ROS contribute to the formation of RNS which then nitrate tyrosine residues forming 3NT.
There are two well-described pathways for protein nitration. One uses peroxynitrite, which is the reaction product of NO and superoxide, and the other uses NO2• formed by the reaction of nitrite and H2O2, typically in the presence of a heme peroxidase.8–12 Although tyrosine nitration in asthmatic airways is generally attributed to peroxynitrite, there is substantial evidence supporting the generation of 3NT through peroxynitrite-independent mechanisms.8–12 For this pathway to be active, typically, the presence of heme peroxidases, such as those found in eosinophils (eosinophil peroxidase; EPO) and neutrophils (myeloperoxidase; MPO) is believed to be mandatory.9–12, 28–30 While these enzymes contribute to the generation of bromous and hypochlorous acid through interactions with halides, in the presence of nitrite, EPO and MPO also drive 3NT formation via oxidation of nitrite to NO2•.9–12, 27–30 The data from this study strongly suggest that the 3NT measured both in vitro and ex vivo is formed via this peroxidase/H2O2/NO2− system. However, as the isolated epithelial cell culture system used here does not contain granulocytes, it suggested that alternate novel and non-inflammatory cell linked peroxidase sources also exist and contribute.
Elevated H2O2 in exhaled breath has been observed in various airway diseases including asthma.26 The primary enzyme contributing to H2O2 in airway epithelium is reported to be DUOX2.15–16 Intriguingly, DUOX2 (like iNOS) was strongly upregulated in response to IFN-γ in ALI cell culture, was elevated in freshly obtained airway epithelial cells from SA and strongly correlated with IFN-γ in this compartment. Thus, we hypothesized that IFN-γ, alone or in combination with the Th2 cytokine IL-13 might be contributing to the significantly higher levels of 3NT in SA reported here.
In fact, in an ALI epithelial system, IL-13 and IFN-γ in combination induced the higher expression of iNOS and DUOX2 than either cytokine alone, with the effect being most consistently seen in the presence of a low IL-13 concentration, as might be seen in patients treated with high-dose CS. DUOX2 expression was associated with increases in activation as measured by H2O2. In the case of iNOS, while total NO metabolites increased only slightly, the generation of nitrite specifically distinguished the combination from IL-13 alone. Elevated levels of nitrite have been found in exhaled condensate of asthmatics supporting the activity of this pathway in vivo in enhancing nitro-oxidative stress.31
As nitrite specifically interacts with H2O2 to form NO2•, and DUOX2, unlike the other NADPH oxidases, exclusively generates H2O2,13 the relative importance of H2O2 (as compared to superoxide) for the generation of 3NT was addressed by adding SOD (to metabolize superoxide) and catalase (to metabolize H2O2). The addition of catalase significantly decreased both H2O2 and 3NT while the addition of SOD paradoxically increased H2O2 and 3NT, likely due to the metabolism of superoxide to H2O2, thereby increasing the levels even further. Thus, in this system, H2O2 contributed to the generation of 3NT while superoxide clearly did not. In fact, these results, in human cells, may account for the variable success of SOD mimetic drugs for asthma.32–33
In this airway epithelial system, there were strong correlations between 3NT and H2O2, as well as DUOX2. When H2O2 levels were reduced by DUOX2 knockdown, 3NT levels decreased as well, confirming the importance of H2O2 and DUOX2 to increases in 3NT. H2O2 is known to be a key ROS that reacts with heme peroxidases to form ferryl intermediates specifically oxidizing nitrite to generate NO2•.34 With its high affinity for the iron center in these peroxidases, nitrite enhances the oxidizing capacity of peroxidase/H2O2.35 This is supported by our findings that the addition of nitrite resulted in increased 3NT expression which tends to be more pronounced in the presence of H2O2. These results strongly predicted the presence of a yet unidentified heme peroxidase, endogenous to the airway epithelium, which could contribute to enhanced oxidative stress, even in the absence of inflammatory cells.
Intriguingly, DUOX2 was first described in thyroid epithelium and has alternatively been known as thyroid oxidase.36 While DUOX2 is reported to have its own peroxidase activity24, in the thyroid epithelium it co-localizes with TPO. Although not previously reported in lung tissue or any epithelium outside the thyroid, TPO was highly expressed in epithelial microarrays, and, in fact, was the 6th most differentiating gene between SA and HC and the only peroxidase differentially expressed in the epithelium. Quantitative real-time PCR of epithelial cell brushing, Western Blot identification of protein and selective knockdown by TPO siRNA confirmed that TPO was not only present in the airway epithelium, but increased in SA. Interestingly, Western blot analysis for TPO revealed a single band at ~75–80 kDa, approximately 25–35 kDa less than the reported full length TPO,37 thereby suggesting a novel isoform may be present in the airway epithelium. Given the widely appreciated diversity of human TPO, including a variant between 80–85 kDa, this possibility is highly plausible.38 Presently, at least 7 transcript variants encoding TPO isoforms, ranging from 760 to 933 amino acids have been identified.38 The identity and functional significance of this airway TPO was further addressed by silencing it with siRNA, which resulted in reduced 3NT production, supporting TPO as a key endogenous peroxidase in epithelial 3NT generation. In the thyroid, TPO promotes the production of reactive iodine species through its interaction (and co-localization) with DUOX2 induced H2O2 and iodide. These studies suggest similar interactions occur in the airway epithelium, whereby TPO utilizes DUOX2-derived H2O2 to catalyze oxidation of nitrite (instead of iodide) to subsequently form NO2•, which then nitrates tyrosine residues. The upregulation of both DUOX2 and TPO by IFN-γ in the presence of IL-13 (or IFN-γ alone) emphasizes that multiple immune pathways can increase nitro-oxidative stress in asthmatic epithelial cells, even perhaps in the absence of an active Th2/eosinophilic process. Whether activation of this pathway represents a process common to both Th2-Hi and Th2-Lo (or non-eosinophilic) asthma awaits further study.39
Although a knockout mouse might be useful to understand the functional contribution of the iNOS/DUOX2/TPO/3NT metabalome to the pathobiology of asthmatic inflammation, this approach is not possible given the opposing effects of Th1 and Th2 cytokines on iNOS expression in mice compared to humans, and the likely differences in the cell source of the iNOS. In mice, IL-4 and IL-13, alone or in combination with IFN-γ, inhibit iNOS expression and nitrite production.40–41 Further, iNOS in mice is found in the airway epithelium and alveolar macrophage, while in asthmatic humans, the epithelium is the primary source.6, 19, 23 Overall, these factors limit the utility of the study of iNOS-DUOX interactions in murine models.
Post-translational modification of epithelial proteins by ROS or RNS can alter their expression, structure, and biological function. Studies using human airway epithelial cells show that H2O2 induces aberrant phosphorylation of epidermal growth factor (EGF) receptor, a receptor involved in wound repair, resulting in impaired canonical dimerization, trafficking and degradation.42 Similarly nitration of the EGF-like domain of neuregulin-1, an EGF-related growth factor, diminishes its ability to bind and activate the EGF receptor.43 Further, H2O2-mediated tyrosine nitration could also promote CS insensitivity through decreased histone deacetylase-2 activity resulting in enhanced inflammatory cytokine production.44 Nitrotyrosine incorporated into alpha-tubulin caused microtubular dysfunction, changes in cell morphology and loss of epithelial barrier function, critical to airway diseases.45 Perhaps most importantly, RNS have also been reported to nitrate catalase, the H2O2 scavenger used in this study. This nitrated catalase, reported to be increased in BAL fluid and corresponding with increases in nitrotyrosine activity, has decreased function and could further perpetuate increases in H2O2.27 Thus, the increase in nitro-oxidative stress observed in asthma and particularly in SA, could be due to both increases in production of ROS and RNS, as well as decreases in ROS catabolism.
In summary, results from this study reveal an interaction between airway epithelial iNOS, DUOX2, and TPO, which contribute to airway nitro-oxidative regulation. This regulation occurs primarily through a H2O2/endogenous peroxidase dependent nitrite oxidation reaction, leading to increases in 3NT. These biochemical events, influenced by the presence of Th1 and Th2 cytokines associated with SA, and involving the highly novel peroxidase TPO, are likely to alter epithelial function and contribute to the pathobiology of this disease. Whether inhibition of these pathways in vivo will improve outcomes in asthma and SA awaits further study.
METHODS
Subjects
All asthmatic subjects met the American Thoracic Society (ATS) criteria for the diagnosis of asthma. MMA had an FEV1 of >60% predicted, with/without low-moderate dose inhaled CS. SA subjects met the ATS workshop definition for severe refractory asthma, including the continuous use of high-dose inhaled CS and/or frequent use of oral CS with continuing symptoms and/or chronic airflow limitation.46 HC had no history of respiratory disease or recent respiratory infection, normal lung function and no evidence of bronchial hyperresponsiveness. No subject smoked within the last year or had a history of smoking >10 pack years. The study was approved by the National Jewish and the University of Pittsburgh Institutional Review Boards and all subjects gave informed consent. Pulmonary function testing, atopy and FeNO were evaluated as previously described.23, 47
In addition to the local Pittsburgh SARP cohort, microarray studies were done on epithelial brushings from HC, MMA and SA subjects from 4 SARP sites (Cleveland Clinics, University of Pittsburgh, University of Wisconsin and Wake Forest University). These subjects were characterized identically to the University of Pittsburgh subjects described above.
Bronchoscopy and BAL
Bronchoscopy with endobronchial epithelial brushing and BAL was performed as previously described.48–49 The bronchial brushings generally comprised >90% epithelial cells.
Primary HAEC Culture and siRNA transfection
Primary HAEC were cultured in ALI and siRNA transfection was performed as previously described.49 From day 0 of ALI, cells were stimulated with IL-13 (1,10 ng/ml; R&D Systems, MN) or medium alone added to the lower chamber every 48 h for 8 d, with/without exposure to IFN-γ (10,100 ng/ml; R&D Systems, MN) for the last 72 h. DUOX2 siRNA (Invitrogen, TX) or TPO siRNA (Santa Cruz, CA) was transfected into cells using Mirus siQUEST transfection reagent (Mirus, WI). For transfection experiment, combination of IL-13 (1 ng/ml) and IFN-γ (100 ng/ml) was initiated at the beginning of ALI culture and continued for 48 h. Scramble siRNA was transfected at the same time as the negative control.
H2O2 measurements
Culture medium from the upper chamber was removed on day 8, and 100 µl PBS with/without one of the following was added: catalase (150 U/ml; Sigma, MO), superoxide dismutase-polyethylene glycol from bovine erythrocytes (SOD, 150 U/ml; Sigma, MO), nitrite (10,25 µM; Sigma, MO), or the combination of catalase and nitrite. Following 1 h incubation, H2O2 was then measured in the upper chamber supernatants using the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Molecular Probes, OR). The fluorescent signal was read at 530 nm excitation, 590 nm emission, using the Infinite 200 PRO (Tecan, Männedorf, Switzerland).
Microarray methods
mRNA was extracted from bronchial epithelial cells suspended in Trizol solution using the QIACube system (QIAGEN INC, Valencia, CA). Microarray experiments were performed as we previously reported.50 RNA quality was determined using the Agilent Bioanalyzer 2100 (Agilent Technologies Inc, Santa Clara, CA). RNA was hybridized to Agilent Human GE 4×44K V2 Gene Expression microarrays. The microarrays were scanned using the Agilent Microarray Scanner and the data was extracted using the Agilent Feature Extraction software 10.7.3.1. The data was then normalized using a cyclic loess algorithm authored in the R programming environment using the Bioconductor suite of tools. The resultant data was analyzed using BRB ArrayTools version 4.3.0 developed by Dr. Richard Simon and the BRB-ArrayTools Development Team. Differentially expressed genes were determined by t-test using a false discovery rate of 0.05 with a confidence interval of 90%. The data discussed in this manuscript have been deposited in NCBI’s Gene Expression Omnibus database (GEO, http://www.ncbi.nlm.nih.gov/geo) and are accessible through GEO Series accession number GSE43696.
Quantitative Real-Time PCR
Real-time PCR was performed on the ABI Prism 7700 sequence detection system (Applied Biosystems) as described previously.7 The primers and probes were obtained from Applied Biosystems (IFN-γ: Hs00989291_m1, IL-13: Hs00174379_m1, iNOS: Hs00167248_m1, DUOX2: Hs00204187_m1, TPO: Hs00374171_m1, GAPDH: 4310884E). TPO primers covered the junction of exons 16 and 17. The mRNAs of interest were indexed to GAPDH using the formula 1/(2ΔCT) × 1000.
Western Blotting
Samples were resolved on 4–12% SDS-PAGE and immuno-probed with antibodies for iNOS (1:500, BD Transduction Laboratories, NJ), DUOX2 (1:250, Abcam, MA), TPO (1:100, Santa Cruz, CA), and β-actin (1:5000, Sigma, MO). The membrane was developed and densitometry performed using Multi Gauge software (FujiFilm, Stamford, CT). Each image was obtained from the same blot, however, some blot conditions were shown individually as they were rearranged for conceptual clarity.
Nitrite and Nitrate measurement
Lower supernatant nitrite and nitrate concentrations were quantified using a colorimetric assay based on the Griess reaction (Parameter Total Nitric Oxide and Nitrite/Nitrate Assay; R&D Systems, MN). Briefly, nitrite was quantified and nitrate was converted to nitrite using nitrate reductase, followed by the addition of Griess reagent to produce an azo dye compound. The absorbance was measured at 540 nm. Nitrate concentrations were indirectly determined by subtracting the concentration of nitrite from the basal and converted nitrite concentrations.
3NT measurement
3NT concentrations in lysates were quantified spectrophotometrically at 600 nm, using a sandwich ELISA kit (Abcam, MA), with a sensitivity of 8 ng/ml, intra- and inter-assay variation of 6.9% and 13% respectively. Samples were measured in duplicate against known standards. Levels of 3NT were normalized to cellular protein content and expressed in ng/mg protein.
Data and Statistical Analysis
Statistical analysis was performed using JMP SAS software. When possible, data were log transformed to achieve linearity. Ex vivo data were assessed by ANCOVA (adjusted for age) followed by the Tukey–Kramer test to compare intergroup difference. A two-way repeated measures ANOVA was used to determine significant main effects and interactions between IL-13 and IFN-γ in in vitro experiments. The Bonferroni procedure was used to conservatively test for the multiple post-hoc comparisons between groups using: 1) α = 0.05/3 = 0.017 for comparisons between control vs. IL-13 (1,10 ng/ml) or vs. IFN-γ (10,100 ng/ml), 2) α = 0.05/2 = 0.025 for comparisons between combined IL-13 plus IFN-γ vs. IL-13 alone or vs. IFN-γ alone. Correlations were assessed using either the Pearson r (normally distributed data) or the Spearman rho (non-normally distributed data) coefficients.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank F Holguin and L Wang for statistical advice; H Hu and C Uvalle for excellent technical assistance; AS Larkin, X Zhou, ML Fajt, and RS Traister for helpful contributions; and L Wang for technical advice and providing nitrite. This study was supported by grants AI67780, HL69174, CTSI UL1-RR024153, RC2HL101487, HL69167, HL69170, HL69116, and the Dellenback Fund.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/mi
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.van der Vliet A, Eiserich JP, O'Neill CA, Halliwell B, Cross CE. Tyrosine modification by reactive nitrogen species: a closer look. Arch Biochem Biophys. 1995;319(2):341–349. doi: 10.1006/abbi.1995.1303. [DOI] [PubMed] [Google Scholar]
- 2.Hurst JK. Whence nitrotyrosine? J Clin Invest. 2002;109(10):1287–1289. doi: 10.1172/JCI15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, et al. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci U S A. 2001;98(5):2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacPherson JC, Comhair SA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, et al. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. 2001;166(9):5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- 5.Sugiura H, Komaki Y, Koarai A, Ichinose M. Nitrative stress in refractory asthma. J Allergy Clin Immunol. 2008;121(2):355–360. doi: 10.1016/j.jaci.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Guo FH, De Raeve HR, Rice TW, Stuehr DJ, Thunnissen FB, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci U S A. 1995;92(17):7809–7813. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chibana K, Trudeau JB, Mustovich AT, Hu H, Zhao J, Balzar S, et al. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy. 2008;38(6):936–946. doi: 10.1111/j.1365-2222.2008.02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel RP, McAndrew J, Sellak H, White CR, Jo H, Freeman BA, et al. Biological aspects of reactive nitrogen species. Biochim Biophys Acta. 1999;1411(2–3):385–400. doi: 10.1016/s0005-2728(99)00028-6. [DOI] [PubMed] [Google Scholar]
- 9.van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen 640 species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272(12):7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 10.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391(6665):393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 11.Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, et al. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277(20):17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 12.Govindaraju K, Shan J, Levesque K, Hussain SN, Powell WS, Eidelman DH. Nitration of respiratory epithelial cells by myeloperoxidase depends on extracellular nitrite. Nitric Oxide. 2008;18(3):184–194. doi: 10.1016/j.niox.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17(11):1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 14.Forteza R, Salathe M, Miot F, Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;32(5):462–469. doi: 10.1165/rcmb.2004-0302OC. [DOI] [PubMed] [Google Scholar]
- 15.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, et al. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579(21):4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Gattas MV, Forteza R, Fragoso MA, Fregien N, Salas P, Salathe M, et al. Oxidative epithelial host defense is regulated by infectious and inflammatory stimuli. Free Radic Biol Med. 2009;47(10):1450–1458. doi: 10.1016/j.freeradbiomed.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Y, Ruf J, Lothaire P, Dequanter D, Andry G, Willemse E, et al. Association of duoxes with thyroid peroxidase and its regulation in thyrocytes. J Clin Endocrinol Metab. 2010;95(1):375–382. doi: 10.1210/jc.2009-1727. [DOI] [PubMed] [Google Scholar]
- 18.Fortunato RS, Lima de Souza EC, Ameziane-el Hassani R, Boufraqech M, Weyemi U, Talbot M, et al. Functional consequences of dual oxidase-thyroperoxidase interaction at the plasma membrane. J Clin Endocrinol Metab. 2010;95(12):5403–5411. doi: 10.1210/jc.2010-1085. [DOI] [PubMed] [Google Scholar]
- 19.Guo FH, Comhair SA, Zheng S, Dweik RA, Eissa NT, Thomassen MJ, et al. Molecular mechanisms of increased nitric oxide (NO) in asthma: evidence for transcriptional and post-translational regulation of NO synthesis. J Immunol. 2000;164(11):5970–5980. doi: 10.4049/jimmunol.164.11.5970. [DOI] [PubMed] [Google Scholar]
- 20.Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181(8):788–796. doi: 10.1164/rccm.200909-1448OC. [DOI] [PubMed] [Google Scholar]
- 21.Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370(9596):1422–1431. doi: 10.1016/S0140-6736(07)61600-6. [DOI] [PubMed] [Google Scholar]
- 22.Guo FH, Uetani K, Haque SJ, Williams BR, Dweik RA, Thunnissen FB, et al. Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J Clin Invest. 1997;100(4):829–838. doi: 10.1172/JCI119598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto M, Tochino Y, Chibana K, Trudeau JB, Holguin F, Wenzel SE. Nitric oxide and related enzymes in asthma: relation to severity, enzyme function and inflammation. Clin Exp Allergy. 2012;42(5):760–768. doi: 10.1111/j.1365-2222.2011.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harper RW, Xu C, McManus M, Heidersbach A, Eiserich JP. Duox2 exhibits potent heme peroxidase activity in human respiratory tract epithelium. FEBS Lett. 2006;580(22):5150–5154. doi: 10.1016/j.febslet.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 25.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003;35(3):213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 26.Horvath I, Donnelly LE, Kiss A, Kharitonov SA, Lim S, Chung KF, et al. Combined use of exhaled hydrogen peroxide and nitric oxide in monitoring asthma. Am J Respir Crit Care Med. 1998;158(4):1042–1046. doi: 10.1164/ajrccm.158.4.9710091. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh S, Janocha AJ, Aronica MA, Swaidani S, Comhair SA, Xu W, et al. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J Immunol. 2006;176(9):5587–5597. doi: 10.4049/jimmunol.176.9.5587. [DOI] [PubMed] [Google Scholar]
- 28.Duguet A, Iijima H, Eum SY, Hamid Q, Eidelman DH. Eosinophil peroxidase mediates protein nitration in allergic airway inflammation in mice. Am J Respir Crit Care Med. 2001;164(7):1119–1126. doi: 10.1164/ajrccm.164.7.2010085. [DOI] [PubMed] [Google Scholar]
- 29.van Dalen CJ, Winterbourn CC, Senthilmohan R, Kettle AJ. Nitrite as a substrate and 700 inhibitor of myeloperoxidase. Implications for nitration and hypochlorous acid production at sites of inflammation. J Biol Chem. 2000;275(16):11638–11644. doi: 10.1074/jbc.275.16.11638. [DOI] [PubMed] [Google Scholar]
- 30.Baldus S, Eiserich JP, Brennan ML, Jackson RM, Alexander CB, Freeman BA. Spatial mapping of pulmonary and vascular nitrotyrosine reveals the pivotal role of myeloperoxidase as a catalyst for tyrosine nitration in inflammatory diseases. Free Radic Biol Med. 2002;33(7):1010. doi: 10.1016/s0891-5849(02)00993-0. [DOI] [PubMed] [Google Scholar]
- 31.Hunt J, Byrns RE, Ignarro LJ, Gaston B. Condensed expirate nitrite as a home marker for acute asthma. Lancet. 1995;346(8984):1235–1236. doi: 10.1016/s0140-6736(95)92947-9. [DOI] [PubMed] [Google Scholar]
- 32.Assa'ad AH, Ballard ET, Sebastian KD, Loven DP, Boivin GP, Lierl MB. Effect of superoxide dismutase on a rabbit model of chronic allergic asthma. Ann Allergy Asthma Immunol. 1998;80(3):215–224. doi: 10.1016/S1081-1206(10)62960-2. [DOI] [PubMed] [Google Scholar]
- 33.Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2010;12(1):93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bian K, Gao Z, Weisbrodt N, Murad F. The nature of heme/iron-induced protein tyrosine nitration. Proc Natl Acad Sci U S A. 2003;100(10):5712–5717. doi: 10.1073/pnas.0931291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reszka KJ, McCormick ML, Britigan BE. Peroxidase- and nitrite-dependent metabolism of the anthracycline anticancer agents daunorubicin and doxorubicin. Biochemistry. 2001;40(50):15349–15361. doi: 10.1021/bi011869c. [DOI] [PubMed] [Google Scholar]
- 36.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275(30):23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 37.Magnusson RP, Chazenbalk GD, Gestautas J, Seto P, Filetti S, DeGroot LJ, et al. Molecular cloning of the complementary deoxyribonucleic acid for human thyroid peroxidase. Mol Endocrinol. 1987;1(11):856–861. doi: 10.1210/mend-1-11-856. [DOI] [PubMed] [Google Scholar]
- 38.Ferrand M, Le Fourn V, Franc JL. Increasing diversity of human thyroperoxidase generated by alternative splicing. Characterized by molecular cloning of new transcripts with single- and multispliced mRNAs. J Biol Chem. 2003;278(6):3793–3800. doi: 10.1074/jbc.M209513200. [DOI] [PubMed] [Google Scholar]
- 39.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 40.Paludan SR, Lovmand J, Ellermann-Eriksen S, Mogensen SC. Effect of IL-4 and IL-13 on IFN-gamma-induced production of nitric oxide in mouse macrophages infected with herpes simplex virus type 2. FEBS Lett. 1997;414(1):61–64. doi: 10.1016/s0014-5793(97)00987-3. [DOI] [PubMed] [Google Scholar]
- 41.Ruetten H, Thiemermann C. Interleukin-13 is a more potent inhibitor of the expression of inducible nitric oxide synthase in smooth muscle cells than in macrophages: a comparison with interleukin-4 and interleukin-10. Shock. 1997;8(6):409–414. [PubMed] [Google Scholar]
- 42.Filosto S, Khan EM, Tognon E, Becker C, Ashfaq M, Ravid T, et al. EGF receptor exposed to oxidative stress acquires abnormal phosphorylation and aberrant activated conformation that impairs canonical dimerization. PLoS One. 2011;6(8):e23240. doi: 10.1371/journal.pone.0023240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nethery DE, Ghosh S, Erzurum SC, Kern JA. Inactivation of neuregulin-1 by nitration. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L287–L293. doi: 10.1152/ajplung.00058.2006. [DOI] [PubMed] [Google Scholar]
- 44.Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun. 2004;315(1):240–245. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 45.Eiserich JP, Estevez AG, Bamberg TV, Ye YZ, Chumley PH, Beckman JS, et al. Microtubule dysfunction by posttranslational nitrotyrosination of alpha-tubulin: a nitric oxide-dependent mechanism of cellular injury. Proc Natl Acad Sci U S A. 1999;96(11):6365–6370. doi: 10.1073/pnas.96.11.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. American Thoracic Society. Am J Respir Crit Care Med. 2000;162(6):2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 47.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu HW, Balzar S, Westcott JY, Trudeau JB, Sun Y, Conrad DJ, et al. Expression and activation of 15-lipoxygenase pathway in severe asthma: relationship to eosinophilic phenotype and collagen deposition. Clin Exp Allergy. 2002;32(11):1558–1565. doi: 10.1046/j.1365-2222.2002.01477.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhao J, O'Donnell VB, Balzar S, St Croix CM, Trudeau JB, Wenzel SE. 15-Lipoxygenase 1 interacts with phosphatidylethanolamine-binding protein to regulate MAPK signaling in human airway epithelial cells. Proc Natl Acad Sci U S A. 2011;108(34):14246–14251. doi: 10.1073/pnas.1018075108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milosevic J, Pandit K, Magister M, Rabinovich E, Ellwanger DC, Yu G, et al. Profibrotic Role of miR-154 in Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2012;47(6):879–887. doi: 10.1165/rcmb.2011-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.