Abstract
Purpose
The creatine kinase rate of metabolic adenosine triphosphate (ATP) synthesis is an important metabolic parameter but is challenging to measure in vivo due to limited signal-to-noise ratio and long measurement time.
Methods
This study reports the implementation of an accelerated 31P Four Angle Saturation Transfer (FAST) method to measure the forward creatine kinase (CK) rate of ATP synthesis. Along with a high-field scanner (11.7 Tesla) and a small sensitive surface coil, the forward CK rate in the rat brain was measured in ∼5 mins.
Results
Under 1.2% isoflurane, the forward CK rate constant and metabolic flux were, respectively, kf,CK = 0.26±0.02 s-1 and Ff,CK = 70.8±4.6 (μmol/g/min). As a demonstration of utility and sensitivity, measurements were made under graded isoflurane. Under 2.0% isoflurane, kf,CK = 0.16±0.02 s-1 and Ff,CK = 41.0±4.2 μmol/g/min, corresponding to a 38% and 42% reduction, respectively, relative to 1.2% isoflurane. By contrast, the ATP and phosphocreatine concentrations were unaltered.
Conclusion
This study demonstrated the 31P FAST measurement of creatine kinase rate of ATP synthesis in rat brain with reasonable temporal resolution. Different isoflurane levels commonly used in animal models significantly alter the CK reaction rate but not ATP and phosphocreatine concentrations.
Keywords: rats, high fields, metabolic flux, MRS
Introduction
The majority of the metabolic adenosine triphosphate (ATP) yield in the brain is produced in the mitochondria by oxidative phosphorylation via the ATPase pathway (Pi + ADP ⇔ ATP). The adult human brain produces and consumes 5 times its weight (∼5.7 kg) in ATP daily, whereas at a given moment the brain contains only about 2g of ATP (1-3). Thus ATP is rapidly cycled to meet the energetic demands.
Creatine kinase (CK) catalyses the conversion of creatine and consumes ATP to create phosphocreatine (PCr) and adenosine diphosphate (ADP): (ATP + Cr ⇔ PCr + ADP). This CK reaction is reversible and thus ATP can also be generated from PCr and ADP. As such, PCr serves as energy storage for the rapid buffering and regeneration of ATP. Moreover, CK enzymes also facilitate the transfer of the high-energy bond out of the mitochondria to the cytosol in the form of PCr. The high-energy phosphate bond in the cytosol is cycled between the high-energy phosphates (PCr and ATP). The rate of ATP synthesis via the CK pathway is 5-6 times higher than the metabolic rate of ATP synthesis via the ATPase pathway (4,5). Such high rate of chemical exchange ensures diffusion of the high-energy phosphate bond, so that energy produced at the mitochondria is available at the cell membrane to maintain ionic gradients. Disruption of ATP energy pathways could affect normal cellular function and has been associated with a number of metabolic diseases (6-8).
31P magnetization transfer (MT) by magnetic resonance offers a unique, non-invasive tool for directly measuring the CK rate of ATP synthesis in vivo. 31P MT measures the forward creatine kinase rate (kf,CK) by using frequency-selective RF energy to saturate γ-ATP. Saturating the γ-ATP resonance results in the attenuation of the PCr amplitude due to chemical exchanges. By measuring the change in signal amplitude of the PCr resonance, the forward creatine kinase rate can be calculated.
31P MT experiments to measure CK rate are inherently challenging. 31P has approximately 1/1000th of the signal-to-noise ratio of the 1H2O signal in vivo. The 31P high-energy phosphates are characterized by long longitudinal magnetization recovery (T1, on the order of seconds) and short transverse magnetization relaxation (T2, on the order of tens of milliseconds). These constraints have prevented widespread use of 31P MT techniques. Nonetheless, 31P MT has been used to measure CK rates under different anesthetics, pharmacologic and functional stimulations (4,5,9-11), and in association with stroke (8). The protocols for these applications of 31P MT ranged from half an hour to ten hours.
This study implemented the accelerated 31P Four Angle Saturation Transfer (FAST) (12) technique to evaluate the brain high-energy phosphates and the forward creatine kinase synthesis rate under graded isoflurane anesthesia. High field (11.7 Tesla) and a small sensitive surface coil were used to improve 31P signal sensitivity. BIRP radiofrequency excitation was used to overcome radiofrequency B1 field inhomogeneity associated with the use of surface coil. The temporal resolution of the 31P FAST approach was 5 mins.
Theory
31P MT measurements can be used to measure the forward CK rate constant (kf,CK in units s-1) of ATP synthesis (PCr → ATP). The modified Bloch equation for the MT experiment can be written as (12):
| (1) |
where M'0 is the magnetization of PCr in the presence of RF saturation, M0 is the magnetization of PCr in the absence of RF saturation, and T1int is the longitudinal relaxations constant for PCr in the presence of saturating RF irradiation of γ-ATP. In addition, the forward metabolic flux Ff,CK (μmol/g/min) is related to kf,CK as 60 ×kf,CK ×[PCr]/1.1, where [PCr] is the PCr concentration which is usually taken as 5mM in normal rats (4,13) and the brain tissue density is assumed to be 1.1 g/ml. Absolute measurements of [PCr] using NMR are challenging, requiring a carefully calibrated scheme using an external reference, and often are not necessary. It is valuable to report the metabolic flux, compared to the rate constant only, when altered physiology can be measured in the same animal, thereby observing reliable changes in metabolite concentrations. One caveat, is that the magnetization transfer experiment must be fast relative to the changes of metabolite concentrations, so that the physiological concentrations can be assumed stable over the duration of the measurement.
In vivo 31P MT experiments are generally a variation of the saturation transfer (ST) experiment. However, conventional saturation transfer experiments require acquisition of many data points at long TR, requiring lengthy scan times. In this study, we implemented an accelerated magnetization transfer experiment, 31P Four Angle Saturation Transfer (FAST) (12), to measure kf,CK in approximately 5 mins, instead of hours. The FAST approach uses acquisitions with low flip angles allowing shorter TR. Measuring the parameters M'0, M0 and T1int requires only four spectra, acquired with and without saturation at two flip angles: α and β. The PCr signal from each spectra (M(α), M(β), M'(α) and M'(β), where the prime denotes acquisitions with γ-ATP saturated) are used to calculate M0, M'0 and T1int according to:
| (2) |
| (3) |
| (4) |
where R=M(α)/M(β) and R'=M'(α)/M'(β).
Sensitivity for 31P experiments is improved by the use of surface coils. However, using inhomogeneous resonators typically results in a range of flip angle distributions over the sensitive volume. Accurate calculation of the rate constant measured by 31P FAST require very accurate flip angles. Accurate flip angles associated with a surface coil can be achieved using a phase alternated-B1 insensitive rotation (BIRP) (14) acquisition scheme that subtraction averages FID acquired using pairs of four segment B1 insensitive rotation (BIR-4) (15) excitation pulses 180° out of phase. The BIR-4 adiabatic pulses are amplitude and phase modulated (Figure 1) to produce homogeneous flip angle distributions using inhomogeneous resonators. Commonly, adiabatic pulses are constrained to give 90° or 180° flip angles. However, BIR-4 pulses provide user selected adiabatic plane wave rotation by employing abrupt phase discontinuities after the first and third segments. Generally, system limitations implementing the abrupt phase changes result in flip angle errors on the order on 10°. The BIRP, phase alternated, scheme has been shown to average out positive and negative flip-angle errors Whereas, BIR-4 pulses resulted in flip angle errors of -7° to +9°, in a direct comparison the nominal flip angle differed from the actual value by only -1.7° to +1.3° when using BIRP (14).
Figure 1. Modulation of BIR4 Excitation Pulses.
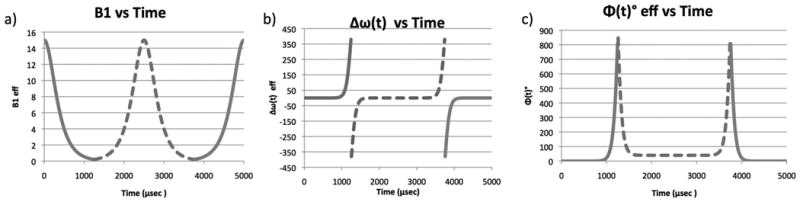
Three graphs of TANH/TAN modulation of the BIR4 pulses used for 31P excitation (a) amplitude B1, (b) frequency ω, (c) phase Φ. the discontinuity of the phase modulations after segment 1 and 3 determine the flip angle.
Methods
Animal preparation
Animal experiments were performed in accordance with the ARRIVE guidelines on ethics and were approved by the Institutional Animal Care and Use Committee (IACUC). Male Sprague-Dawley rats (200-300 g, n = 4) were initially anesthetized with 2% isoflurane in air. Animals were secured in a holder with a stereotaxic headset and placed in the magnet. Once in the magnet, isoflurane was reduced to 1.2% for 30 mins prior to beginning data acquisition. Isoflurane was delivered in air at a flow rate was 1-2 L/min using a calibrated vaporizer. Rectal temperature was monitored and maintained at 37.0 ± 0.5° C throughout. Heart rate and blood oxygen saturation level (SaO2) were recorded using a MouseOx system (STARR Life Science, Oakmont, PA) and parameters were maintained within normal physiological ranges.
Shimming and positioning were performed using the 1H frequency. The first 31P data sets were acquired after 30min of exposure to 1.2% isoflurane. The isoflurane was raised to 2% for 30 mins and 31P measurements were repeated. The 31P measurements were repeated at 1.2% and 2.0% isoflurane with 30 mins exposures to stabilize prior to acquiring each of the subsequent data sets.
MR experiments
MRI was performed on an 11.7 T Bruker Biospin Magnet using a custom-made concentric loop 1H/31P (500/202.5 MHz) 2/1.5-cm diameter transceiver surface coil. The 1H (500MHz) element was used for positioning and shimming prior to 31P NMR. 31P magnetization transfer data was acquired using the FAST method to determine the creatine kinase rate. Four spectra acquired with α=30° and β=60° flip angles with and without γ-ATP saturation (TR=1100s, NA=64, DS=6). Accurate flip angles throughout the brain were set using BIRP excitation scheme. Narrowband ATP saturation with negligible bleed over was achieved using the BISTRO (16) saturation scheme with eight 50 ms hyperbolic secant RF pulses interleaved with dephasing gradients. Total acquisition time for a kf,CK measurement was ∼5 mins.
Data analysis
31P spectra were analyzed using Bruker Topspin software. The chemical shift of the PCr resonance peak was set to zero. Integral values for seven resonance peaks (PME, Pi, PDE, PCr and the three adenosine triphosphate peaks: α-, β- and γ-ATP) were acquired in the data set. The spectra baseline and zero and first order phase were corrected. kf,CK is calculated using Equation 1-4, using four spectra acquired with α=30° and β=60° flip angles with and without γ-ATP saturation. Data analysis employed codes written in Matlab (MathWorks Inc, Natick, MA). A paired two-tailed Student t-test was used to evaluate metabolic biomarkers between isoflurane conditions. Values in text and in graphs are mean ± SEM with P<0.05 considered significant.
Results
The BIR-4 pulses used were 50x the length of optimized square excitation pulses. The increased pulse duration allows T2 dephasing in the rotating frame during excitation. Compared to conventional square pulses, BIR-4 pulses eliminated the short T2 phospholipids that contaminated metabolite signals, thereby improving quantification of signal amplitudes (Figure 2). The BIRP acquisition scheme with phase alternation scheme averaged out positive and negative flip-angle errors of the BIR-4 acquisition, thereby improving T1 fitting, as demonstrated by measurements of the Pi peak in a dead rat (Figure 3).
Figure 2. Square versus BIR4 RF Pulse.
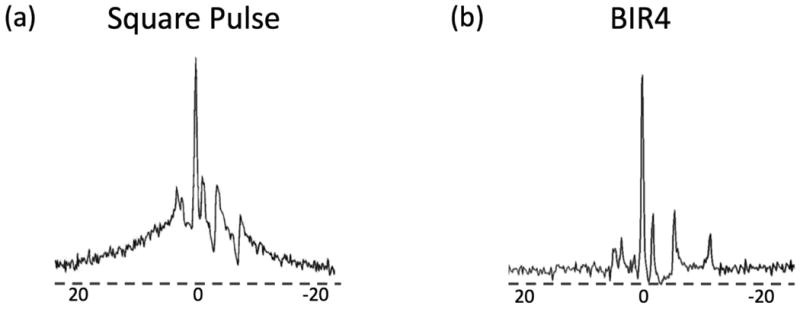
In vivo 31P spectra acquired using a (a) square and (b) BIR4 pulse. With the square pulse, the 31P spectrum is heavily contaminated by phospholipid signal. With BIR4, phospholipid signal was eliminated, improving quantification of all metabolites.
Figure 3. Saturation Recovery using BIRP and BIR4 ±30.
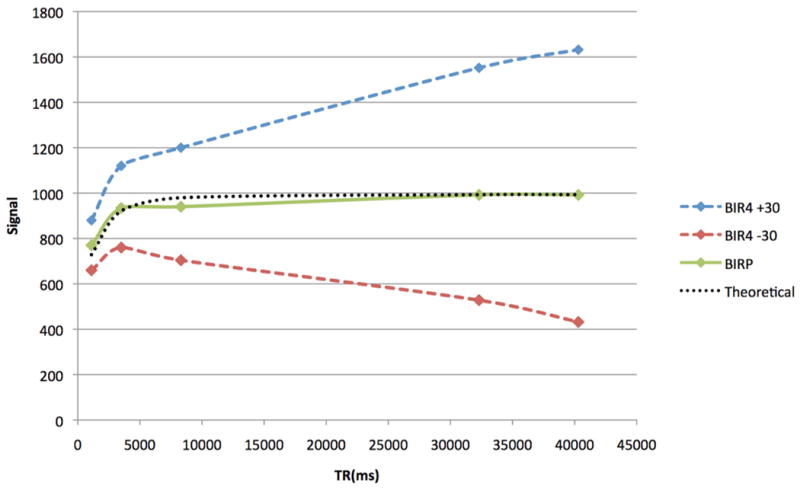
T1 measurements of inorganic phosphorus made on a dead rat brain. BIR4 T1 measurements are skewed due to positive or negative contributions of phospholipid signal.
A typical 31P data set consisting of the four spectra used to calculate kf,CK in the FAST method is shown in Figure 4. Spectra were acquired at 60° and 30°, with and without BISTRO saturation of the γ-ATP resonance (-2.3ppm). The pair of spectra acquired without saturation was used to calculate M0 of PCr. The pair of spectra acquired with saturation was used to calculate M'0 and T1int of PCr. The change in PCr signal was robustly detected, allowing for reproducible measurements of the forward CK rate (kf,CK).
Figure 4.
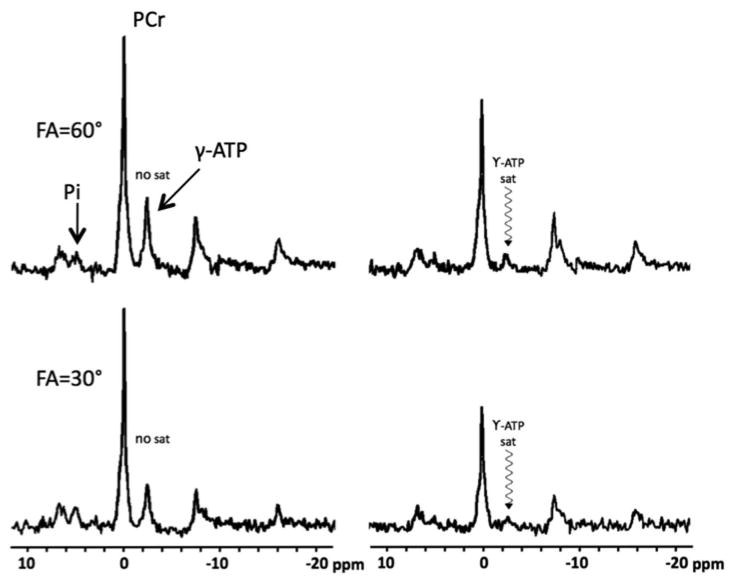
31P FAST spectra using flip angles of 60° and 30° with and without saturation of γ-ATP (in vivo whole brain).
The modulation in Pi amplitude, theoretically, also allows for similar calculations of the forward ATPase rate (kf,ATPase). However due to the much smaller signal amplitude and contamination from the phosphomonoesters, reproducible kf,ATPase measurements were not achievable and thus not reported.
Under 1.2% isoflurane, the CK rate kf,CK was 0.26±0.02 and the forward metabolic flux Ff,CK was 70.8±4.6 μmol/g/min (Table 1). Under 2.0% isoflurane, kf,CK = 0.16±0.02 s-1 and Ff,CK = 41.0±4.2 μmol/g/min, corresponding to 38% and 42% reduction, respectively, compared to 1.2% isoflurane. By contrast, the ATP and PCr concentrations were unaltered. After the isoflurane level was returned from 2% to 1.2% for 30 mins, the CK rate recovered but did not reach the prior 1.2% isoflurane level. The CK rate dropped again after another 30 mins exposure to 2.0% isoflurane.
Table 1. [PCr], [ATP], kf,CK(s-1) and Ff,CK(μmol/g/min) at 1.2% and 2.0% isoflurane.
Acclimation of 30 mins was given after switching isoflurane concentration. Baseline concentration of PCr was assumed to be 5 mM and ATP 3 mM.
| isoflurane | PCr, mM | ATP, mM | kf,CK(s-1) | Ff,CK(μmol/g/min) |
|---|---|---|---|---|
| 1.20% | 5 | 3 | 0.26±0.02 | 70.8±4.6 |
| 2.0% | 4.75±0.05 | 2.78±0.13 | 0.16±0.01* | 41.0±4.2* |
| 1.20% | 5.25±0.10 | 2.87±0.09 | 0.20±0.01 | 58.0±2.6 |
| 2.0% | 5.05±0.15 | 2.91±0.18 | 0.17±0.02 | 45.1±6.8 |
N=4, mean ± sem
p<0.05 with unpaired t-test for comparison with initial 1.2% isoflurane condition.
Discussion
We implemented the accelerated 31P FAST protocol at 11.7 T and measured the concentrations of ATP and PCr, and the forward CK rate of ATP synthesis with a temporal resolution of 5 mins. The major findings were: i) the forward creatine kinase rate and the metabolic flux of the rat brain were reliably measured, and ii) changing isoflurane concentration from 1.2% to 2.0% did not change the PCr and ATP concentrations but significantly decreased the forward creatine kinase synthesis rate and the metabolic flux. This approach has potential applications in studying neurological disorders with metabolic dysfunction.
Sauter and Rudin (11) and Du et al. (4) have previously reported kf,CK albeit at much lower temporal resolution. Sauter and Rudin used a conventional 31P saturation transfer method at 4.7T to measure forward CK rate and high-energy phosphate concentrations under 1-2% halothane, thiopental sodium and graded bicuculline (0.4 mg/kg and 0.8 mg/kg) and found kf,CK to be 0.25±0.02 s-1, 0.21±0.03 s-1, 0.30±0.04 s-1 and 0.49±0.04 s-1, respectively, in normal animals. kf,CK linearly correlated with EEG activity. The ATP levels remained constant, while PCr decreased with increased EEG activity. In contrast to expectations, PCr did not increase with decreased EEG activity. These finding demonstrated that kf,CK is a sensitive reliable indicator of changes in metabolic activity, whereas the concentrations of ATP and PCr did not provide consistent useful information.
Du et al. (4) used variations of the saturation transfer technique at 9.4T to measure ATP synthesis, including the forward CK and ATPase rates in rats under different depths of anesthesia. The concentrations of the high-energy phosphates, forward ATPase and CK rates and the Spectral Entropy Index of EEG were measured in rats anesthetized using isoflurane (2%), α-chloralose, low dose pentobarbital and high dose pentobarbital (from low to high (isoelectric) anesthetic depth). They found kf,CK to be 0.24±0.02 s-1, 0.21±0.03 s-1, 0.21±0.02 s-1 and 0.19±0.03 s-1 for animals anesthetized with 2.0% isoflurane, α-chloralose, low dose pentobarbital and high dose pentobarbital, respectively. [PCr] decreased 8±2% and [Pi] increased 42±6% in the high dose pentobarbital (isoelectric state) compared to low dose pentobarbital anesthesia. It was concluded that the ATP metabolic rates measured by 31P MT are more sensitive measures of brain bioenergetics than concentrations of the high-energy phosphates.
Our reported values for the creatine kinase rates under graded isoflurane anesthesia are in general agreement with studies by Sauter and Rudin and Du et al. (4,11), although the experimental conditions and type or level of anesthesia differed. In addition, our results also showed that after the isoflurane level was returned from 2% to 1.2% for 30 mins, the CK rate recovered but did not reach the prior 1.2% isoflurane level. The CK rate dropped again after another 30 mins exposure to 2.0% isoflurane. These findings suggest that 30 mins may not be sufficient for metabolic rate to fully recover and that commonly used isoflurane levels can significantly alter cerebral metabolism.
Alternative techniques
Alternatively to 31P MT, magnetic resonance measurements of cerebral metabolism can be made by 13C or 17O or blood oxygen level dependent (BOLD) functional MR techniques (17,18), all measure different aspects of metabolism. 13C studies use 13C labeled glucose infusions to measure glucose consumption in the GABAergic tricarboxylic acid cycle (19). 17O measures cerebral metabolic rate of oxygen. 17O NMR techniques resemble positron emission measurements of oxygen consumption, both using inhaled 17O labeled oxygen gas (20). Alternatively, 17O labeled water (H217O) may be injected prior to spectroscopic measurement. It may be of interest to compare different measures of metabolic parameters.
Conclusions
This study implemented and employed the 31P FAST technique at 11.7T to evaluate cerebral high-energy phosphates and creatine kinase synthesis rate under graded isoflurane anesthesia. The advantage of the 31P FAST technique is that the measurement of creatine kinase synthesis is made practical. A drawback of the 31P FAST technique is that it is likely less robust than the full saturation recovery MT technique with very long acquisition time. However, the use of high field and small surface coil as well as optimized 31P FAST acquisition parameters and radiofrequency pulses enable robust measurement of the CK synthesis rate. Further improvement in sensitivity is needed in order to robustly measure ATPase rate (kf,ATPase). Future studies will incorporate localization by single voxel spectroscopy and chemical shift imaging.
Acknowledgments
Grant support: This work was supported by the NIH (NINDS, R01-NS45879), the American Heart Association (EIA 0940104N and PRE4450009), a Clinical Translational Science Award Pilot Grant (parent grant NIH UL1TR000149), and a Translational Technology Resource grant (parent grant NIH UL1TR000149).
Abbreviations
- PCr
phosphocreatine
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- Cr
creatine
- CK
creatine kinase
Footnotes
Disclosure/Conflict of Interest: The authors declare no conflict of interest
References
- 1.Lajtha A, Gibson G, Dienel G, Purdon AD, Rapoport SI. Handbook of Neurochemistry and Molecular Neurobiology. Springer; US: 2007. 4.6 Energy Consumption by Phospholipid Metabolism in Mammalian Brain; pp. 401–427. [Google Scholar]
- 2.Raichle ME, Gusnard DA. Appraising the brain's energy budget. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(16):10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu XH, Qiao H, Du F, Xiong Q, Liu X, Zhang X, Ugurbil K, Chen W. Quantitative imaging of energy expenditure in human brain. Neuroimage. 2012;60(4):2107–2117. doi: 10.1016/j.neuroimage.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A. 2008;105(17):6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoubridge EA, Briggs RW, Radda GK. 31p NMR saturation transfer measurements of the steady state rates of creatine kinase and ATP synthetase in the rat brain. FEBS Lett. 1982;140(2):289–292. doi: 10.1016/0014-5793(82)80916-2. [DOI] [PubMed] [Google Scholar]
- 6.Amaral AU, Cecatto C, Seminotti B, Zanatta A, Fernandes CG, Busanello EN, Braga LM, Ribeiro CA, de Souza DO, Woontner M, Koeller DM, Goodman S, Wajner M. Marked reduction of Na(+), K(+)-ATPase and creatine kinase activities induced by acute lysine administration in glutaryl-CoA dehydrogenase deficient mice. Molecular genetics and metabolism. 2012;107(1-2):81–86. doi: 10.1016/j.ymgme.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Boeck CR, Carbonera LS, Milioli ME, Constantino LC, Garcez ML, Rezin GT, Scaini G, Streck EL. Mitochondrial respiratory chain and creatine kinase activities following trauma brain injury in brain of mice preconditioned with N-methyl-D-aspartate. Molecular and cellular biochemistry. 2013;384(1-2):129–37. doi: 10.1007/s11010-013-1790-8. [DOI] [PubMed] [Google Scholar]
- 8.Mlynarik V, Kasparova S, Liptaj T, Dobrota D, Horecky J, Belan V. Creatine kinase reaction rates in rat brain during chronic ischemia. MAGMA. 1998;7(3):162–165. doi: 10.1007/BF02591333. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Zhu XH, Adriany G, Ugurbil K. Increase of creatine kinase activity in the visual cortex of human brain during visual stimulation: a 31P magnetization transfer study. Magn Reson Med. 1997;38(4):551–557. doi: 10.1002/mrm.1910380408. [DOI] [PubMed] [Google Scholar]
- 10.Mora B, Narasimhan PT, Ross BD, Allman J, Barker PB. 31P saturation transfer and phosphocreatine imaging in the monkey brain. Proc Natl Acad Sci U S A. 1991;88(19):8372–8376. doi: 10.1073/pnas.88.19.8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauter A, Rudin M. Determination of creatine kinase kinetic parameters in rat brain by NMR magnetization transfer. Correlation with brain function. J Biol Chem. 1993;268(18):13166–13171. [PubMed] [Google Scholar]
- 12.Bottomley PA, Ouwerkerk R, Lee RF, Weiss RG. Four-angle saturation transfer (FAST) method for measuring creatine kinase reaction rates in vivo. Magn Reson Med. 2002;47(5):850–863. doi: 10.1002/mrm.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erecinska M, Silver IA. ATP and brain function. J Cereb Blood Flow Metab. 1989;9(1):2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- 14.Bottomley PA, Ouwerkerk R. BIRP, an Improved Implementation of Low-Angle Adiabatic (BIR-4) Excitation Pulses. Journal of Magnetic Resonance, Series A. 1993;103(2):242–244. [Google Scholar]
- 15.Staewen RS, Johnson AJ, Ross BD, Parrish T, Merkle H, Garwood M. 3-D FLASH imaging using a single surface coil and a new adiabatic pulse, BIR-4. Investigative radiology. 1990;25(5):559–567. doi: 10.1097/00004424-199005000-00015. [DOI] [PubMed] [Google Scholar]
- 16.de Graaf RA, Luo Y, Garwood M, Nicolay K. B1-Insensitive, Single-Shot Localization and Water Suppression. Journal of Magnetic Resonance, Series B. 1996;113(1):35–45. doi: 10.1006/jmrb.1996.0152. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Zhu X-H. Dynamic study of cerebral bioenergetics and brain function using in vivo multinuclear MRS approaches. Concepts in Magnetic Resonance Part A. 2005;27A(2):84–121. [Google Scholar]
- 18.Lin AL, Fox PT, Yang Y, Lu H, Tan LH, Gao JH. Evaluation of MRI models in the measurement of CMRO2 and its relationship with CBF. Magn Reson Med. 2008;60(2):380–389. doi: 10.1002/mrm.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci U S A. 2005;102(15):5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu XH, Zhang N, Zhang Y, Zhang X, Ugurbil K, Chen W. In vivo 17O NMR approaches for brain study at high field. NMR Biomed. 2005;18(2):83–103. doi: 10.1002/nbm.930. [DOI] [PubMed] [Google Scholar]


