Abstract
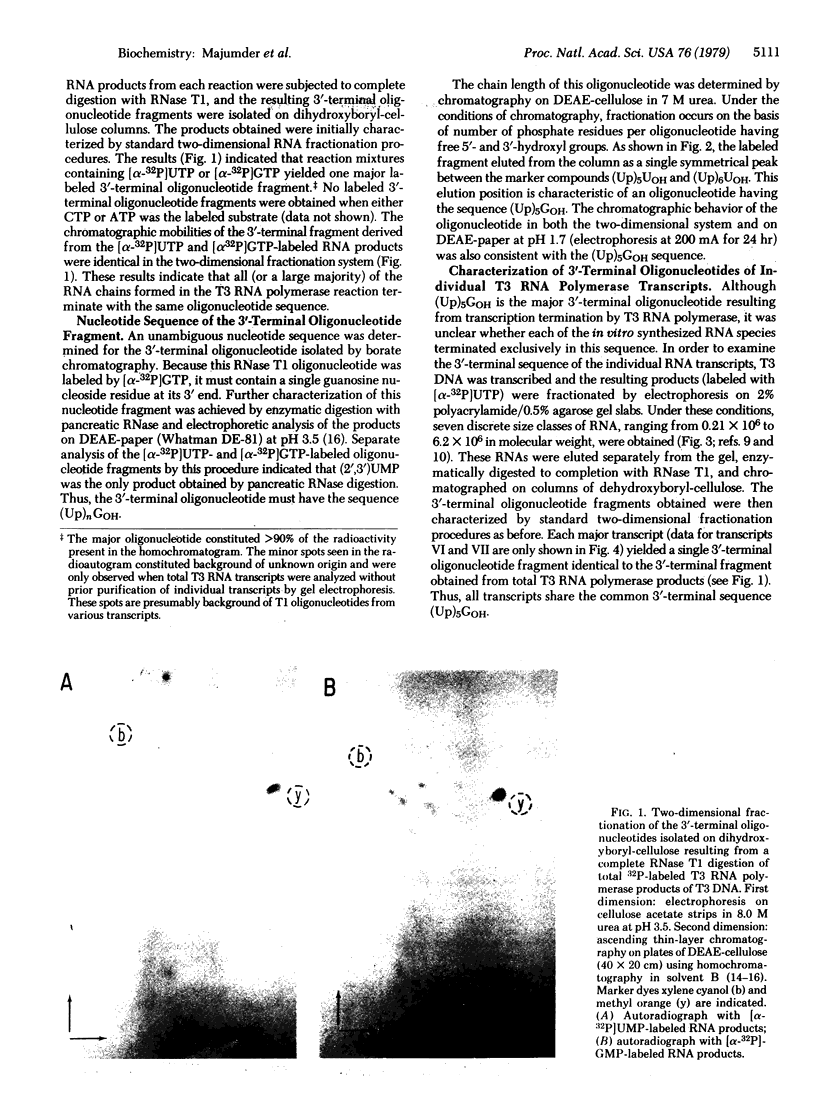
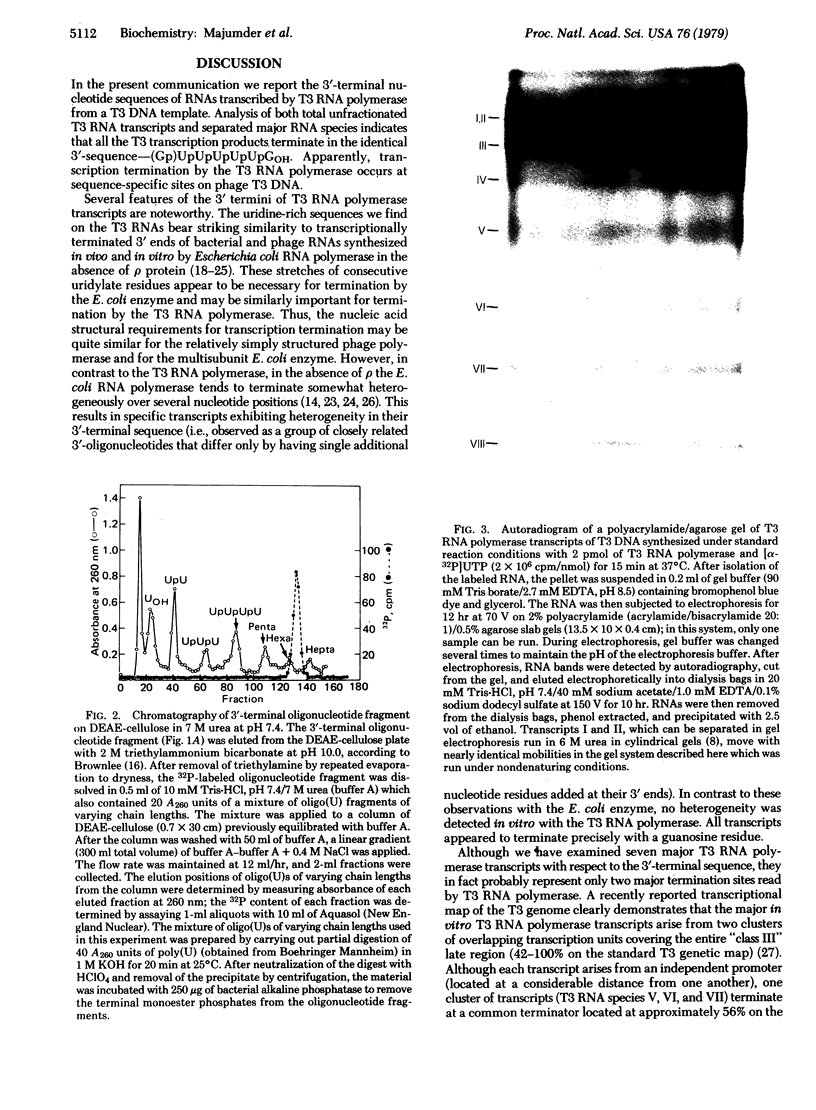
RNA was synthesized in vitro from a T3 DNA template by T3 RNA polymerase and subsequently separated into seven discrete size classes (molecular weights ranging between 0.21 x 10(6) and 6.2 x 10(6)) by electrophoresis in polyacrylamide slab gels. RNase T1-generated 3'-terminal oligonucleotide fragments were then selectively isolated from either the unfractionated total RNA or the gel-purified specific transcripts by chromatography on columns of dihydroxyboryl-cellulose. Sequence analysis of these oligonucleotide products indicated that the unfractionated transcripts as well as all the individual major RNA species examined had a unique sequence, (Gp)UpUpUpUpUpGOH, at their 3' termini. The specificity of this sequence, as well as the total lack of any sequence heterogeneity at the ends of these transcripts, indicates a high degree of specificity of termination during transcription in this system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Adhya S., Sarkar P., Valenzuela D., Maitra U. Termination of transcription by Escherichia coli RNA polymerase: influence of secondary structure of RNA transcripts on rho-independent and rho-dependent termination. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1613–1617. doi: 10.1073/pnas.76.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Golomb M., Chamberlin M. Isolation of recombinants between T7 and T3 bacteriophages and their use in vitro transcriptional mapping. J Virol. 1977 Feb;21(2):753–765. doi: 10.1128/jvi.21.2.753-765.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty P. R., Bandyopadhyay P., Huang H. H., Maitra U. Fidelity of in vitro transcription of T3 deoxyribonucleic acid by bacteriophage T3-induced ribonucleic acid polymerase and by Escherichia coli ribonucleic acid polymerase. J Biol Chem. 1974 Nov 10;249(21):6901–6909. [PubMed] [Google Scholar]
- Chakraborty P. R., Salvo R. A., Majumder H. K., Maitra U. Further characterization of bacteriophage T3-induced ribonucleic acid polymerase. Studies on the size of in vitro transcripts and interaction of T3 RNA polymerase with T3 DNA. J Biol Chem. 1977 Sep 25;252(18):6485–6493. [PubMed] [Google Scholar]
- Chakraborty P. R., Sarkar P., Huang H. H., Maitra U. Studies on T3-induced ribonucleic acid polymerase. 3. Purification and characterization of the T3-induced ribonucleic acid polymerase from bacteriophage T3-infected Escherichia coli cells. J Biol Chem. 1973 Oct 10;248(19):6637–6646. [PubMed] [Google Scholar]
- Dunn J. J., Bautz F. A., Bautz E. K. Different template specificities of phage T3 and T7 RNA polymerases. Nat New Biol. 1971 Mar 17;230(11):94–96. doi: 10.1038/newbio230094a0. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., McAllister W. T., Bautz E. K. In vitro transcription of T3 DNA by Escherichia coli and T3 polymerases. Virology. 1972 Apr;48(1):112–125. doi: 10.1016/0042-6822(72)90119-5. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb M., Chamberlin M. J. T7- and T3-specific RNA polymerases: characterization and mapping of the in vitro transcripts read from T3 DNA. J Virol. 1977 Feb;21(2):743–752. doi: 10.1128/jvi.21.2.743-752.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H., Sekiya T., Rosenberg M., Egan J., Landy A. A rho-dependent termination site in the gene coding for tyrosine tRNA su3 of Escherichia coli. Nature. 1978 Mar 30;272(5652):423–428. doi: 10.1038/272423a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz P., Weissman S. M., Radding C. M. Nucleotide sequence of a ribonucleic acid transcribed in vitro from lambda phage deoxyribonucleic acid. J Biol Chem. 1971 Aug 25;246(16):5120–5139. [PubMed] [Google Scholar]
- Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U., Huang H. H. Initiation, release, and reinitiation of RNA chains by bacteriophage-T3-induced polymerase from T3 DNA templates (E. coli-guanosine triphosphate terminus-purified polymerase). Proc Natl Acad Sci U S A. 1972 Jan;69(1):55–59. doi: 10.1073/pnas.69.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U. Induction of a new RNA polymerase in Escherichia coli infected with bacteriophage T3. Biochem Biophys Res Commun. 1971 Apr 16;43(2):443–450. doi: 10.1016/0006-291x(71)90773-x. [DOI] [PubMed] [Google Scholar]
- Maitra U., Salvo R. A., Chakraborty P. R. Specificity of ribonucleic acid chain initiation by bacteriophage T3-induced ribonucleic acid polymerase. J Biol Chem. 1974 Sep 25;249(18):5835–5839. [PubMed] [Google Scholar]
- McAllister W. T., Küpper H., Bautz E. K. Kinetics of transcription by the bacteriophage-T3 RNA polymerase in vitro. Eur J Biochem. 1973 May 2;34(3):489–501. doi: 10.1111/j.1432-1033.1973.tb02785.x. [DOI] [PubMed] [Google Scholar]
- Neff N. F., Chamberlin M. J. Termination of transcription by Escherichia coli RNA polymerase in vitro is affected by ribonucleoside triphosphate base analogs. J Biol Chem. 1978 Apr 10;253(7):2455–2460. [PubMed] [Google Scholar]
- Pieczenik G., Barrell B. G., Gefter M. L. Bacteriophage phi 80-induced low molecular weight RNA. Arch Biochem Biophys. 1972 Sep;152(1):152–165. doi: 10.1016/0003-9861(72)90203-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D., Shimatake H., Brady C., Wulff D. L. The relationship between function and DNA sequence in an intercistronic regulatory region in phage lambda. Nature. 1978 Mar 30;272(5652):414–423. doi: 10.1038/272414a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Isolation and sequence determination of the 3'-terminal regions of isotopically labelled RNA molecules. Nucleic Acids Res. 1974 May;1(5):653–671. doi: 10.1093/nar/1.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Weissman S., deCrombrugghe B. Termination of transcription in bacteriophage lambda. Heterogeneous, 3'-terminal oligo-adenylate additions and the effects of rho factor. J Biol Chem. 1975 Jun 25;250(12):4755–4764. [PubMed] [Google Scholar]
- Rosenberg M., de Chrombrugghe B., Musso R. Determination of nucleotide sequences beyond the sites of transcriptional termination. Proc Natl Acad Sci U S A. 1976 Mar;73(3):717–721. doi: 10.1073/pnas.73.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Pace N. R. Nucleotide sequence of 5 S ribosomal RNA precursor from Bacillus subtilis. J Biol Chem. 1976 Jun 10;251(11):3480–3488. [PubMed] [Google Scholar]
- Weith H. L., Wiebers J. L., Gilham P. T. Synthesis of cellulose derivatives containing the dihydroxyboryl group and a study of their capacity to form specific complexes with sugars and nucleic acid components. Biochemistry. 1970 Oct 27;9(22):4396–4401. doi: 10.1021/bi00824a021. [DOI] [PubMed] [Google Scholar]