Abstract
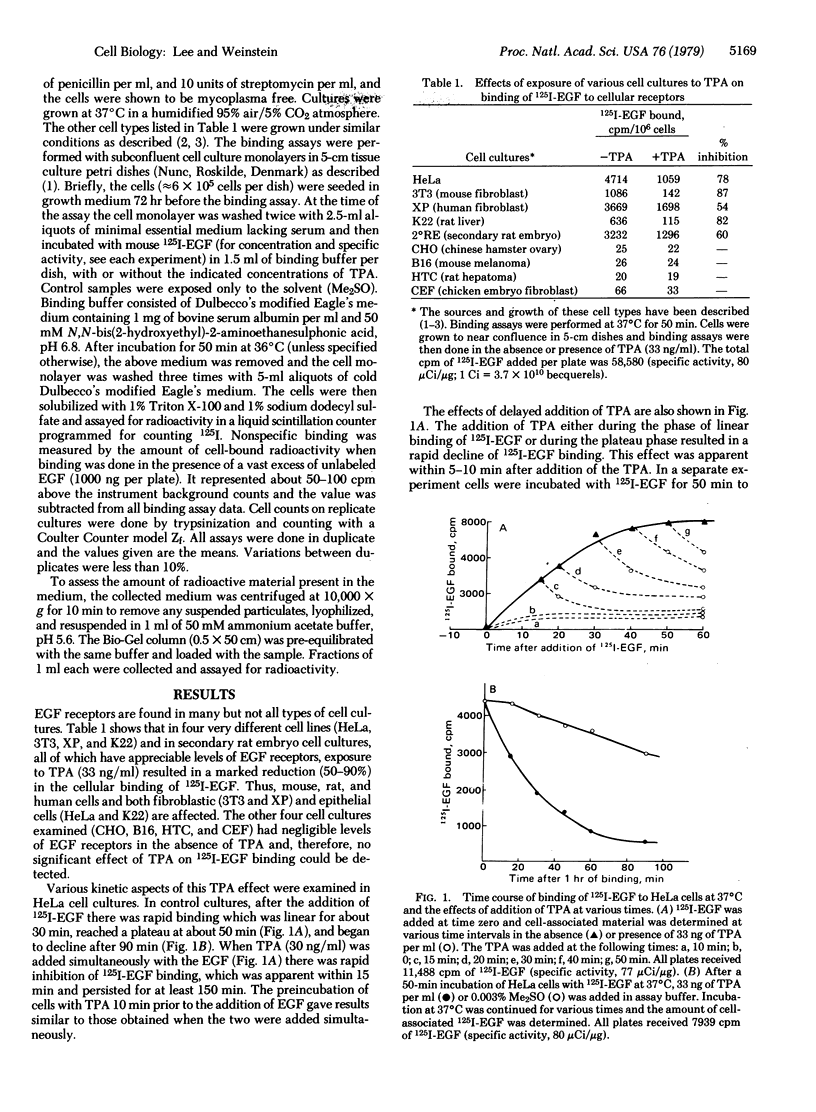
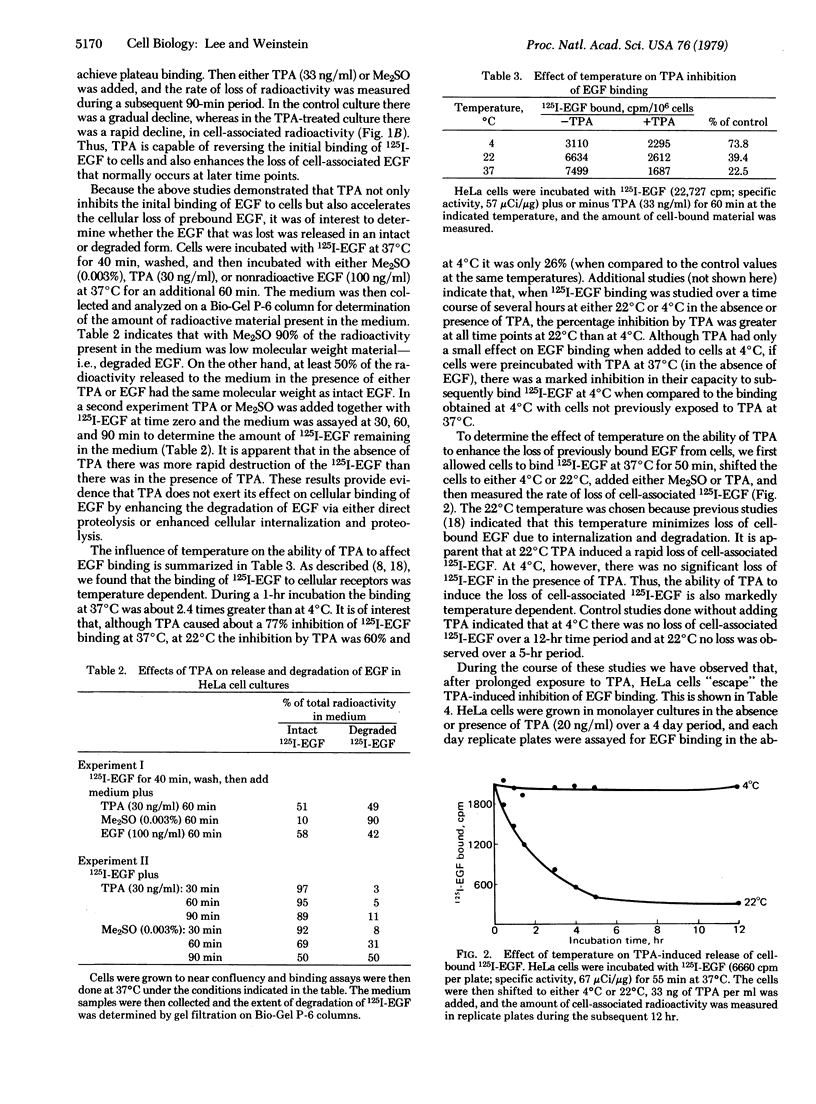
In previous studies we demonstrated that the tumor-promoting agent 12-O-tetradecanoyl phorbol 13-acetate (TPA) and related macrocyclic diterpenes are potent inhibitors of the binding of epidermal growth factor (EGF) to its cell surface receptors in HeLa cells. The present study explores the specificity and mechanism of this effect. We have found that the same effect is observed in various cell types derived from mice, rats, or humans. In HeLa cells TPA inhibits the initial binding of EGF and also accelerates the loss of previously bound EGF from cells. The released EGF is recovered largely intact in the medium, indicating that TPA does not induce increased proteolysis or increased cellular internalization and degradation of EGF. The TPA effect on EGF receptors is mediated by a highly temperature-dependent process because TPA inhibition of EGF binding, and TPA-induced release of prebound EGF, are much greater at 37°C or 22°C than at 4°C. A curious feature is that when cells are grown in TPA for one or more days they escape or become refractory to TPA inhibition of EGF binding. Taken together, these results suggest that TPA inhibits EGF binding not by binding directly to the “active site” of the EGF receptor but by indirectly altering the conformation or inducing the clustering of EGF receptors. These and other membrane effects of this tumor promoter suggest that it is a valuable probe for elucidating complex aspects of membrane structure and function.
Keywords: phorbol esters, receptors, carcinogenesis, cell surface
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A. Pituitary extracts and steroid hormones in the control of 3T3 cell growth. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2702–2706. doi: 10.1073/pnas.70.9.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D., Colowick S. P. Stimulation of sugar uptake in cultured fibroblasts by epidermal growth factor (EGF) and EGF-binding arginine esterase. J Cell Physiol. 1976 Dec;89(4):633–639. doi: 10.1002/jcp.1040890420. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Dicker P., Rozengurt E. Inhibition of epidermal growth factor binding to surface receptors by tumor promotors. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1037–1043. doi: 10.1016/0006-291x(79)90221-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Regulation of the activity of the low density lipoprotein receptor in human fibroblasts. Cell. 1975 Nov;6(3):307–316. doi: 10.1016/0092-8674(75)90182-8. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., King L., Jr, Cohen S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature. 1978 Nov 23;276(5686):409–410. doi: 10.1038/276409a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. The stimulation of epidermal proliferation by a specific protein (EGF). Dev Biol. 1965 Dec;12(3):394–407. doi: 10.1016/0012-1606(65)90005-9. [DOI] [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Fisher P. B., Flamm M., Schachter D., Weinstein I. B. Tumor promoters induce membrane changes detected by fluorescence polarization. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1063–1068. doi: 10.1016/0006-291x(79)90225-0. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J., Wong K. Y., Jones A. L. Cellular uptake and nuclear binding of insulin in human cultured lymphocytes: evidence for potential intracellular sites of insulin action. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1368–1372. doi: 10.1073/pnas.74.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory H. Isolation and structure of urogastrone and its relationship to epidermal growth factor. Nature. 1975 Sep 25;257(5524):325–327. doi: 10.1038/257325a0. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Epidermal growth factor: receptors in human fibroblasts and modulation of action by cholera toxin. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2964–2968. doi: 10.1073/pnas.70.10.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Cohen S. Epidermal growth factor. I. The stimulation of protein and ribonucleic acid synthesis in chick embryo epidermis. Biochim Biophys Acta. 1967 Apr 18;138(2):347–356. [PubMed] [Google Scholar]
- Ishii D. N., Fibach E., Yamasaki H., Weinstein I. B. Tumor promoters inhibit morphological differentiation in cultured mouse neuroblastoma cells. Science. 1978 May 5;200(4341):556–559. doi: 10.1126/science.644318. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Epidermal growth factor, like phorbol esters, induces plasminogen activator in HeLa cells. Nature. 1978 Aug 17;274(5672):696–697. doi: 10.1038/274696a0. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Membrane effects of tumor promoters: stimulation of sugar uptake in mammalian cell cultures. J Cell Physiol. 1979 Jun;99(3):451–460. doi: 10.1002/jcp.1040990319. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Tumor-promoting phorbol esters inhibit binding of epidermal growth factor to cellular receptors. Science. 1978 Oct 20;202(4365):313–315. doi: 10.1126/science.308698. [DOI] [PubMed] [Google Scholar]
- Levine L., Hassid A. Epidermal growth factor stimulates prostaglandin biosynthesis by canine kidney (MDCK) cells. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1181–1187. doi: 10.1016/0006-291x(77)90980-9. [DOI] [PubMed] [Google Scholar]
- Mufson R. A., Simsiman R. C., Boutwell R. K. The effect of the phorbol ester tumor promoters on the basal and catecholamine-stimulated levels of cyclic adenosine 3':5'-monophosphate in mouse skin and epidermis in vivo. Cancer Res. 1977 Mar;37(3):665–669. [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3918–3921. doi: 10.1073/pnas.74.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. P., Stahn R., Passovoy D. S., Herschman H. Epidermal growth factor enhancement of skin tumor induction in mice. Experientia. 1976;32(7):913–915. doi: 10.1007/BF02003764. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Inagami T., Cohen S. The primary structure of epidermal growth factor. J Biol Chem. 1972 Dec 10;247(23):7612–7621. [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Cuatrecasas P., Willingham M. C., Pastan I. Quantitative determination of the lateral diffusion coefficients of the hormone-receptor complexes of insulin and epidermal growth factor on the plasma membrane of cultured fibroblasts. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5353–5357. doi: 10.1073/pnas.75.11.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Hernaez L., Cuatrecasas P. Epidermal growth factor: biological activity requires persistent occupation of high-affinity cell surface receptors. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5788–5791. doi: 10.1073/pnas.75.12.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Stastny M., Cohen S. Epidermal growth factor. IV. The induction of ornithine decarboxylase. Biochim Biophys Acta. 1970 Apr 15;204(2):578–589. [PubMed] [Google Scholar]
- Süss R., Kreibich G., Kinzel V. Phorbol esters as a tool in cell research? Eur J Cancer. 1972 Jun;8(3):299–304. doi: 10.1016/0014-2964(72)90024-2. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E. Growth factors produced by sarcoma virus-transformed cells. Cancer Res. 1978 Nov;38(11 Pt 2):4147–4154. [PubMed] [Google Scholar]
- Yamasaki H., Fibach E., Nudel U., Weinstein I. B., Rifkind R. A., Marks P. A. Tumor promoters inhibit spontaneous and induced differentiation of murine erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3451–3455. doi: 10.1073/pnas.74.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Weinstein I. B., Fibach E., Rifkind R. A., Marks P. A. Tumor promoter-induced adhesion of the DS19 clone of murine erythroleukemia cells. Cancer Res. 1979 Jun;39(6 Pt 1):1989–1994. [PubMed] [Google Scholar]


