Abstract
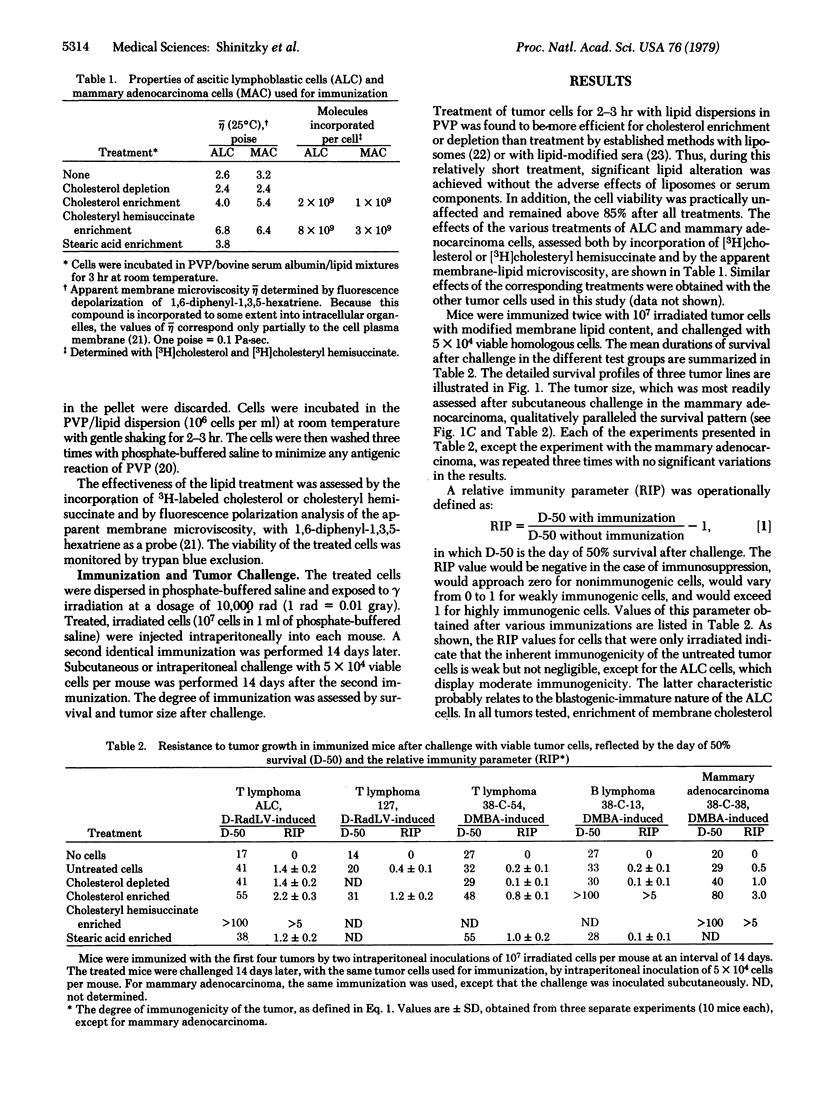
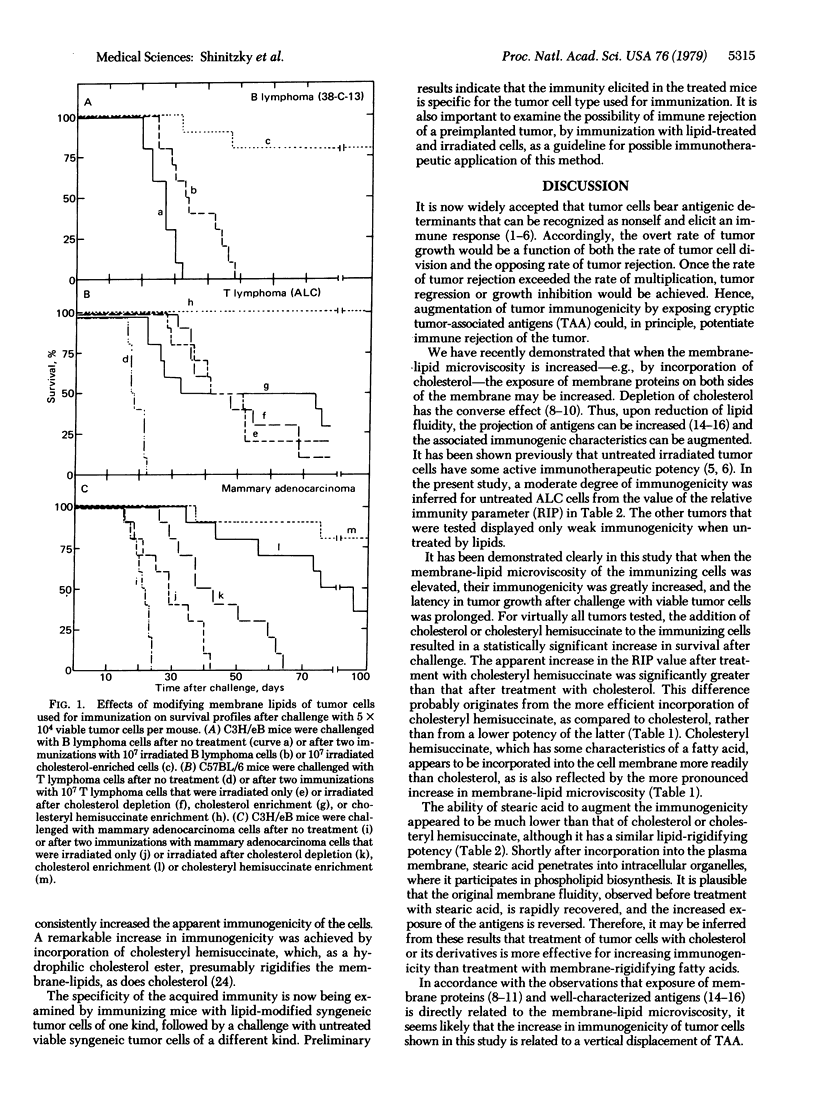
The immunogenicity of a series of mouse tumor lines propagated in vivo (T and B lymphomas and mammary adenocarcinoma) was tested after alteration of the cell membrane-lipid microviscosity. Tumor cells used for immunization were first treated to alter the lipid content, then irradiated and injected intraperitoneally into syngeneic mice. A second identical immunization was performed 14 days later. The degree of immunization in the treated mice was assessed by survival time after challenge with untreated viable tumor cells of the same origin as the immunizing cells. For all tumors tested, enrichment of the immunizing cells with cholesterol or cholesteryl hemisuccinate, which increased the membrane-lipid microviscosity significantly, afforded a marked increase in immunization, compared to that obtained with cells that were only irradiated. Furthermore, in over 90% of the mice that were pretreated with cholesteryl hemisuccinate-enriched cells, tumor growth after the challenge was not detectable. Because the lipid-modifying treatments of the immunizing cells involve no toxic substances, these results may provide the basis for a potent approach to immunotherapy of human cancer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B. Induction of immunity and immunologic paralysis in mice against polyvinyl pyrrolidone. J Immunol. 1969 May;102(5):1309–1313. [PubMed] [Google Scholar]
- Baldwin R. W., Embleton M. J., Price M. R., Vose B. M. Embryonic antigen expression on experimental rat tumours. Transplant Rev. 1974;20(0):77–99. doi: 10.1111/j.1600-065x.1974.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Bergman Y., Haimovich J. Characterization of a carcinogen-induced murine B lymphocyte cell line of C3H/eB origin. Eur J Immunol. 1977 Jul;7(7):413–417. doi: 10.1002/eji.1830070702. [DOI] [PubMed] [Google Scholar]
- Borochov H., Abbott R. E., Schachter D., Shinitzky M. Modulation of erythrocyte membrane proteins by membrane cholesterol and lipid fluidity. Biochemistry. 1979 Jan 23;18(2):251–255. doi: 10.1021/bi00569a002. [DOI] [PubMed] [Google Scholar]
- Borochov H., Shinitzky M. Vertical displacement of membrane proteins mediated by changes in microviscosity. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4526–4530. doi: 10.1073/pnas.73.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brûlet P., McConnell H. M. Structural and dynamical aspects of membrane immunochemistry using model membranes. Biochemistry. 1977 Mar 22;16(6):1209–1217. doi: 10.1021/bi00625a028. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Arner E. C., Wiley J. S., Shattil S. J. Modification of red cell membrane structure by cholesterol-rich lipid dispersions. A model for the primary spur cell defect. J Clin Invest. 1975 Jan;55(1):115–126. doi: 10.1172/JCI107901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Haran-Chera N., Peled A. Thymus and bone marrow derived lymphatic leukaemia in mice. Nature. 1973 Feb 9;241(5389):396–398. doi: 10.1038/241396a0. [DOI] [PubMed] [Google Scholar]
- Haran-Ghera N., Ben-Yaakov M., Peled A. Immunologic characteristics in relation to high and low leukemogenic activity of radiation leukemia virus variants. I. Cellular analysis of immunosuppression. J Immunol. 1977 Feb;118(2):600–606. [PubMed] [Google Scholar]
- Herberman R. B. Cell-mediated immunity to tumor cells. Adv Cancer Res. 1974;19(0):207–263. doi: 10.1016/s0065-230x(08)60055-x. [DOI] [PubMed] [Google Scholar]
- Juillard G. J., Boyer P. J., Yamashiro C. H. A phase I study of active specific intralymphatic immunotherapy (ASILI). Cancer. 1978 Jun;41(6):2215–2225. doi: 10.1002/1097-0142(197806)41:6<2215::aid-cncr2820410622>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lyte M., Shinitzky M. Cholestryl-phosphoryl-choline in lipid bilayers. Chem Phys Lipids. 1979 Apr;24(1):45–55. doi: 10.1016/0009-3084(79)90094-x. [DOI] [PubMed] [Google Scholar]
- Muller C., Shinitzky M. Modulation of transferrin receptors in bone marrow cells by changes in lipid fluidity. Br J Haematol. 1979 Jul;42(3):355–362. doi: 10.1111/j.1365-2141.1979.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Terry W. D. Passive immunotherapy of cancer in animals and man. Adv Cancer Res. 1977;25:323–388. doi: 10.1016/s0065-230x(08)60637-5. [DOI] [PubMed] [Google Scholar]
- Shiku H., Takahashi T., Oettgen H. F. Cell surface antigens of human malignant melanoma. II. Serological typing with immune adherence assays and definition of two new surface antigens. J Exp Med. 1976 Oct 1;144(4):873–881. doi: 10.1084/jem.144.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M. An efficient method for modulation of cholesterol level in cell membranes. FEBS Lett. 1978 Jan 15;85(2):317–320. doi: 10.1016/0014-5793(78)80482-7. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Shinitzky M. Membrane changes in malignant cells--modulation of receptors and antigens by lipids. Bull Schweiz Akad Med Wiss. 1976 Dec;32(4-6):203–207. [PubMed] [Google Scholar]
- Shinitzky M., Rivnay B. Degree of exposure of membrane proteins determined by fluorescence quenching. Biochemistry. 1977 Mar 8;16(5):982–986. doi: 10.1021/bi00624a027. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Souroujon M. Passive modulation of blood-group antigens. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4438–4440. doi: 10.1073/pnas.76.9.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Dancey G. F., Kinsky S. C. Immunogenicity of liposomal model membranes in mice: dependence on phospholipid composition. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1234–1236. doi: 10.1073/pnas.74.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]


