Abstract
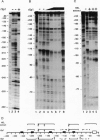
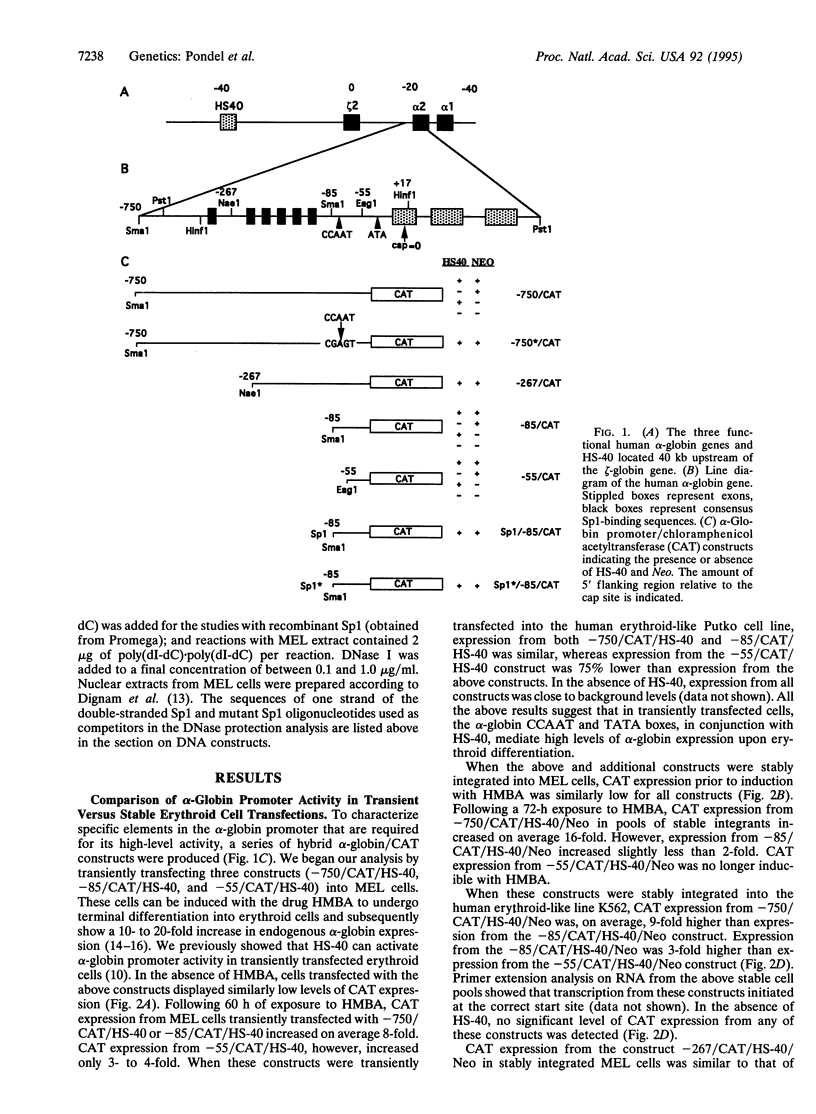
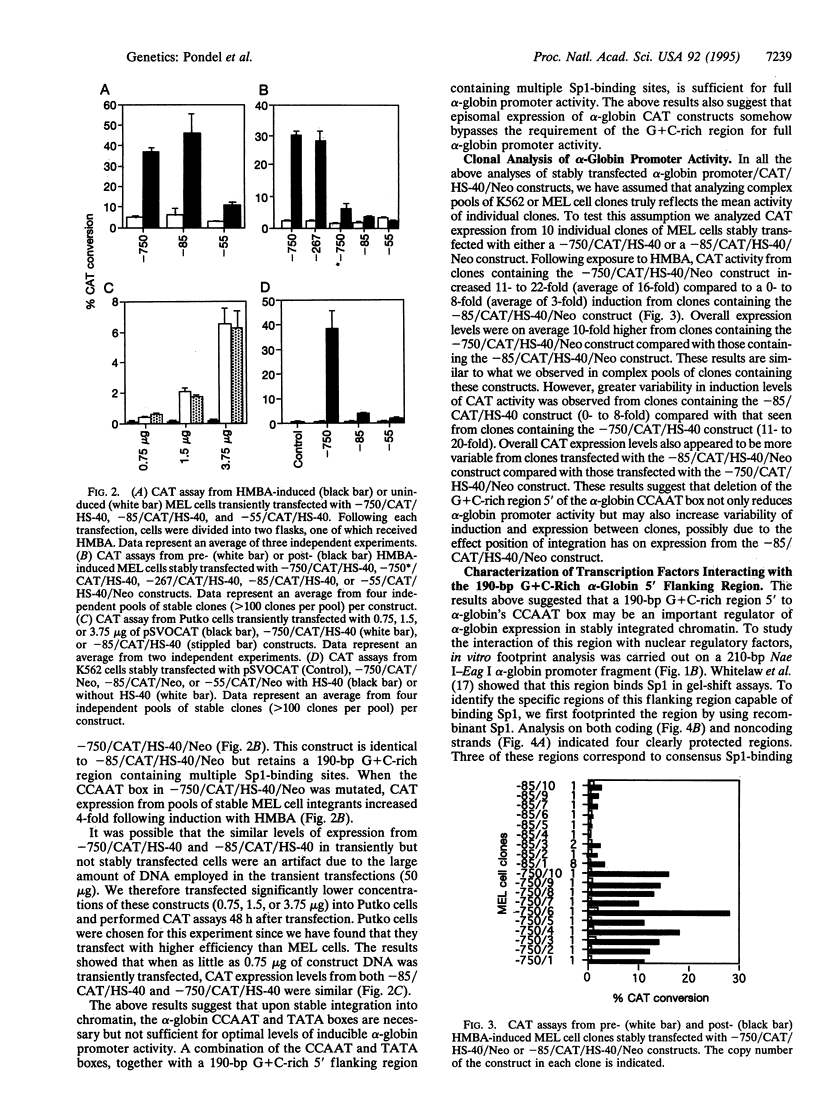
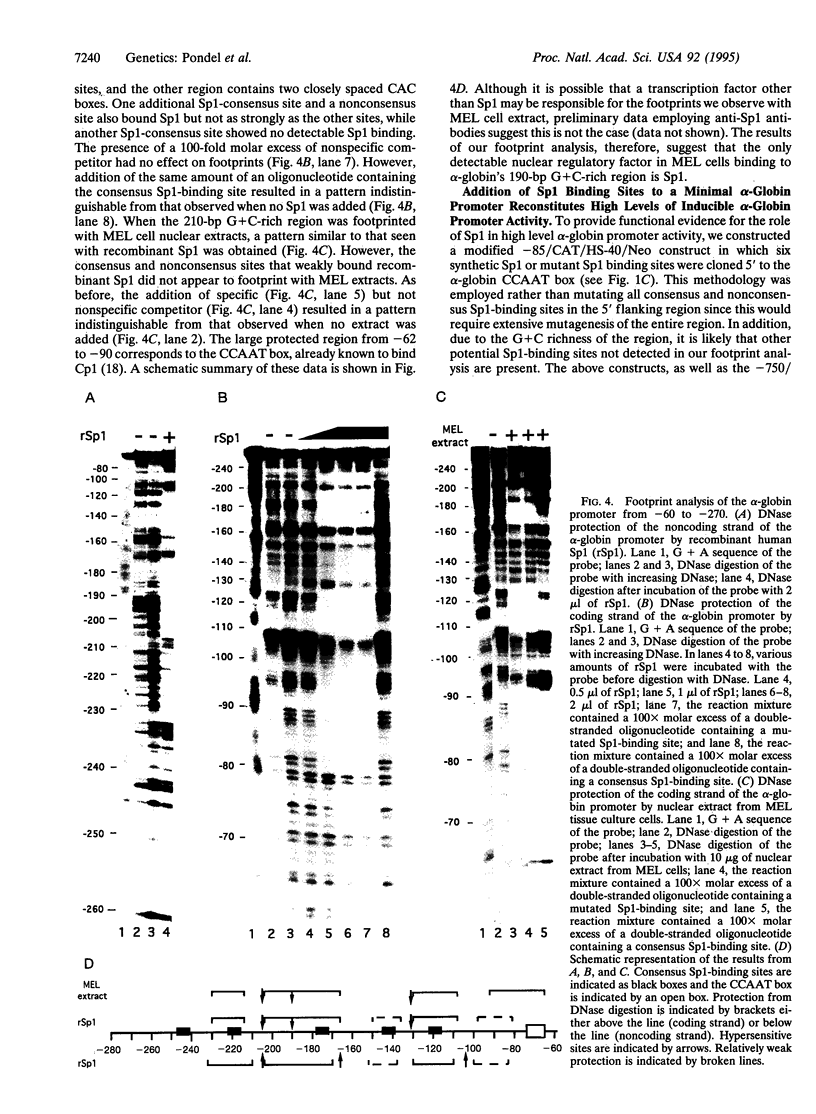
The 5' flanking region of the human alpha-globin gene is highly G + C rich and contains multiple copies of the consensus sequence for the Sp1 binding site. We investigated the role of this G + C-rich region in augmenting alpha-globin promoter activity in the presence of the far-upstream alpha-globin enhancer, HS-40. We show that in transiently transfected erythroid cells, deletion of the alpha-globin G + C-rich 5' flanking region has no effect on alpha-globin promoter activity. However, upon stable integration into chromatin, deletion of this region causes a nearly 90% decrease in promoter activity compared with expression from an alpha-globin promoter retaining this region. These results suggest that the alpha-globin G + C-rich 5' flanking region augments alpha-globin promoter activity in a chromatin-dependent manner. We further show that this G + C-rich region is required for the activation of alpha-globin gene expression during erythroid differentiation. Finally, we show by both footprint analysis and functional assays that the ability of the G + C-rich region to increase alpha-globin promoter activity from a stably integrated alpha-globin gene is mediated by its multiple binding sites for the transcription factor Sp1.
Full text
PDF
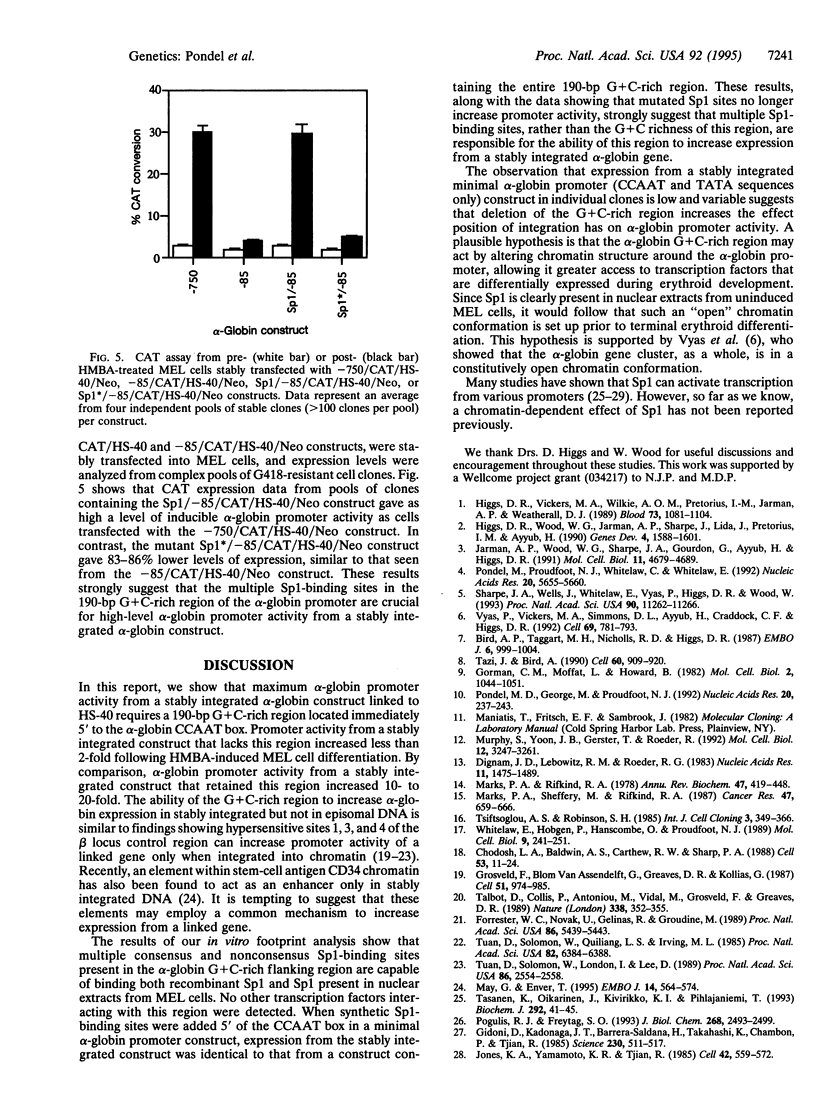
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. P., Taggart M. H., Nicholls R. D., Higgs D. R. Non-methylated CpG-rich islands at the human alpha-globin locus: implications for evolution of the alpha-globin pseudogene. EMBO J. 1987 Apr;6(4):999–1004. doi: 10.1002/j.1460-2075.1987.tb04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Novak U., Gelinas R., Groudine M. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidoni D., Kadonaga J. T., Barrera-Saldaña H., Takahashi K., Chambon P., Tjian R. Bidirectional SV40 transcription mediated by tandem Sp1 binding interactions. Science. 1985 Nov 1;230(4725):511–517. doi: 10.1126/science.2996137. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Higgs D. R., Vickers M. A., Wilkie A. O., Pretorius I. M., Jarman A. P., Weatherall D. J. A review of the molecular genetics of the human alpha-globin gene cluster. Blood. 1989 Apr;73(5):1081–1104. [PubMed] [Google Scholar]
- Higgs D. R., Wood W. G., Jarman A. P., Sharpe J., Lida J., Pretorius I. M., Ayyub H. A major positive regulatory region located far upstream of the human alpha-globin gene locus. Genes Dev. 1990 Sep;4(9):1588–1601. doi: 10.1101/gad.4.9.1588. [DOI] [PubMed] [Google Scholar]
- Jarman A. P., Wood W. G., Sharpe J. A., Gourdon G., Ayyub H., Higgs D. R. Characterization of the major regulatory element upstream of the human alpha-globin gene cluster. Mol Cell Biol. 1991 Sep;11(9):4679–4689. doi: 10.1128/mcb.11.9.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Sheffery M., Rifkind R. A. Induction of transformed cells to terminal differentiation and the modulation of gene expression. Cancer Res. 1987 Feb 1;47(3):659–666. [PubMed] [Google Scholar]
- May G., Enver T. Targeting gene expression to haemopoietic stem cells: a chromatin-dependent upstream element mediates cell type-specific expression of the stem cell antigen CD34. EMBO J. 1995 Feb 1;14(3):564–574. doi: 10.1002/j.1460-2075.1995.tb07032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S., Yoon J. B., Gerster T., Roeder R. G. Oct-1 and Oct-2 potentiate functional interactions of a transcription factor with the proximal sequence element of small nuclear RNA genes. Mol Cell Biol. 1992 Jul;12(7):3247–3261. doi: 10.1128/mcb.12.7.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogulis R. J., Freytag S. O. Contribution of specific cis-acting elements to activity of the mouse pro-alpha 2(I) collagen enhancer. J Biol Chem. 1993 Feb 5;268(4):2493–2499. [PubMed] [Google Scholar]
- Pondel M. D., George M., Proudfoot N. J. The LCR-like alpha-globin positive regulatory element functions as an enhancer in transiently transfected cells during erythroid differentiation. Nucleic Acids Res. 1992 Jan 25;20(2):237–243. doi: 10.1093/nar/20.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondel M. D., Proudfoot N. J., Whitelaw C., Whitelaw E. The developmental regulation of the human zeta-globin gene in transgenic mice employing beta-galactosidase as a reporter gene. Nucleic Acids Res. 1992 Nov 11;20(21):5655–5660. doi: 10.1093/nar/20.21.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe J. A., Wells D. J., Whitelaw E., Vyas P., Higgs D. R., Wood W. G. Analysis of the human alpha-globin gene cluster in transgenic mice. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11262–11266. doi: 10.1073/pnas.90.23.11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot D., Collis P., Antoniou M., Vidal M., Grosveld F., Greaves D. R. A dominant control region from the human beta-globin locus conferring integration site-independent gene expression. Nature. 1989 Mar 23;338(6213):352–355. doi: 10.1038/338352a0. [DOI] [PubMed] [Google Scholar]
- Tasanen K., Oikarinen J., Kivirikko K. I., Pihlajaniemi T. Interaction of transcription factor Sp1 with the promoter of the gene for the multifunctional protein disulphide isomerase polypeptide. Biochem J. 1993 May 15;292(Pt 1):41–45. doi: 10.1042/bj2920041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazi J., Bird A. Alternative chromatin structure at CpG islands. Cell. 1990 Mar 23;60(6):909–920. doi: 10.1016/0092-8674(90)90339-g. [DOI] [PubMed] [Google Scholar]
- Tsiftsoglou A. S., Robinson S. H. Differentiation of leukemic cell lines: a review focusing on murine erythroleukemia and human HL-60 cells. Int J Cell Cloning. 1985 Nov;3(6):349–366. doi: 10.1002/stem.5530030602. [DOI] [PubMed] [Google Scholar]
- Tuan D. Y., Solomon W. B., London I. M., Lee D. P. An erythroid-specific, developmental-stage-independent enhancer far upstream of the human "beta-like globin" genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2554–2558. doi: 10.1073/pnas.86.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas P., Vickers M. A., Simmons D. L., Ayyub H., Craddock C. F., Higgs D. R. Cis-acting sequences regulating expression of the human alpha-globin cluster lie within constitutively open chromatin. Cell. 1992 May 29;69(5):781–793. doi: 10.1016/0092-8674(92)90290-s. [DOI] [PubMed] [Google Scholar]
- Whitelaw E., Hogben P., Hanscombe O., Proudfoot N. J. Transcriptional promiscuity of the human alpha-globin gene. Mol Cell Biol. 1989 Jan;9(1):241–251. doi: 10.1128/mcb.9.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]