Abstract
Globally, the rate of development of myocardial diseases and hypertension is very common, which is responsible for incremental morbidity and mortality statistics. Treatment of ischemic hypertensive patients with diuretics such as hydrochlorothiazide (HCTZ) can precipitate myocardial infarction due to hypokalemia. This study was undertaken to evaluate the pharmacodynamic interaction of green tea extract (GTE) with HCTZ against ischemia-reperfusion induced myocardial toxicity. Wistar albino rats of either sex were taken and pretreated with high (500 mg/kg, p.o.) and low (100 mg/kg, p.o.) dose of GTE for 30 days. Standard, high and low dose of interactive groups received HCTZ (10 mg/kg, p.o.) for last 7 days. Ischemia-reperfusion injury was induced by modified Lagendorff apparatus, and the effect of different treatments was evaluated by percentage recovery in terms of heart rate and developed tension, serum biomarkers, and heart tissue antioxidant levels. Prophylactic treatment groups, such as high and low dose of GTE and their interactive groups with HCTZ, exhibited significant percentage recovery in terms of heart rate and developed tension. Apart from that, significant increase in superoxide dismutase and catalase, decrease in thiobarbituric acid reactive species in heart tissue, as well as significant decrease in serum lactate dehydrogenase, creatinine phosphokinase-MB and N-acetylcysteine levels have also been documented. The present findings clearly suggest that GTE dose-dependently reduces myocardial toxicity due to ischemia, and combination with HCTZ can reduce the associated side-effects and exhibits myocardial protection.
Keywords: Antioxidant, biomarker, green tea extract, hydrochlorothiazide, ischemic-reperfusion injury
INTRODUCTION
From the ancient times, in many societies, herbs and herb-based therapy played an important role to treat different diseased condition and to improve the quality-of-life.[1] When synthetic drug is combined with herb, it may influence the pharmacokinetic and pharmacodynamic profile of each other and can mimic, magnify or oppose the action of each other.[2,3]
The treatment of ischemic hypertensive patients with diuretic like hydrochlorothiazide (HCTZ) require utmost care, because mild to moderate level of hypokalemia may lead to the development of cardiac arrhythmias.[4] The combination of HCTZ with angiotensin-converting enzyme inhibitor-I, aldosterone antagonist or angiotensin Type-I receptor blocker can be beneficial.[5]
Green tea is one of the most consumed beverages in the world. It is obtained from the nonfermented leaves of Camellia sinensis belonging to family Theaceae, which contains more catechins than black tea or oolong tea. Due to the presence of high levels of catechin, certain minerals and vitamins increase the antioxidant potential of this type of tea. Since ancient times, green tea has been considered as a healthful beverage by the traditional Chinese medicine. Recent studies have been reported that green tea may contribute to the reduction in risk of cardiovascular diseases and some forms of cancer, as well as to the promotion of oral health. It plays an important role in the control of body weight and has shown anti-hypertensive, antibacterial, antiviral, neuroprotective and anti-fibrotic properties. It is reported that green tea gives protection from solar ultraviolet rays and increase bone mineral density.[6,7]
Green tea has been reported to prevent left ventricular hypertrophy, hypertension, cardiovascular damage and endothelial dysfunction. One of the clinical study showed that the consumption of green tea is responsible for reducing the occurrence of cardiovascular disease by increasing plasma antioxidant capacity.[8,9,10]
Until now, no study has been carried out indicating the combined effect of green tea and HCTZ on myocardial cell. Hence, this study has been designed to evaluate the combined effect of green tea and HCTZ against ischemia-reperfusion injury (IRI) induced myocardial stress.
MATERIALS AND METHODS
Chemicals
All chemicals used were of analytical grade and purchased from standard companies. Pure sample of HCTZ was gifted by Bangalore Test House (Bangalore, India). Biochemical kits were procured from Crest Biosystems (Goa, India).
Experimental animals
Healthy adult Wistar albino rats of either sex weighing 175-250 g, were housed in polypropylene cages, maintained under standardized condition (12 h L: D cycles, 25 ± 5°C) with paddy husk bedding at the Central Animal House, Shree Devi College of Pharmacy, Mangalore; were provided with standard pellet food and had free access to purified drinking water. The guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Social Justice and Empowerment, Government of India were followed and prior permission was sought from the Institutional Animal Ethics Committee for conducting the study (SDCP/IAEC-19/2012-13).
Plant materials
Green tea (C. sinensis) leaves were purchased in the month of June, 2013 from the local market of Mangalore bearing the brand name green tea, manufactured by New hilltop traders (Vandiperiyar, Kerala). The authentication was done by Dr. Neoline J. Pinto, H.O.D., Department of Botany, St. Agnes College, Mangalore (SAC/MNG/SMP/Drug/2013-06/52). The Aqueous extract was prepared by mixing green tea leaves gently in distilled water maintained at 70°C to 80°C for 30 min. Thereafter, it was filtered and evaporated in the same temperature to get a thick gummy mass. The yield was found to be 24.76% (W/W). Extract was freshly dissolved in distilled water before giving each dose to animals.
Phytochemical estimations of the extract
Aqueous extract of green tea (GTE) was subjected to qualitative analysis to investigate the presence of various phytochemical constituents such as alkaloids, glycosides, steroids, flavonoids, gallic tannins, catecholic tannin, terpenoid, and saponins.[11,12]
Acute toxicity study
Acute toxicity study was carried out according to Office of Prevention, Pesticide and Toxic Substance guidelines following the limit test procedure.[13,14]
Mice were fasted overnight prior to the studies, and then divided into two groups of three each. Test dose of 2 g/kg body weight and 5 g/kg body weight were given orally to either group of mice, and then observed for 72 h for mortality. 1/10th and 1/50th of the maximum safe dose corresponding to 500 and 100 mg/kg orally were selected as high and low doses, respectively.
Experimental protocol
The animals were divided into six different treatment groups of eight animals each. Group I was served as IRI control and the Group II received HCTZ orally at a dose of 10 mg/kg for 7 days.[5] Groups III and IV were prophylactically treated with GTE at a dose of 100 and 500 mg/kg for 30 days through oral route. The animals of Groups V and VI were treated with GTE at a dose of 100 and 500 mg/kg for 30 days, along with that HCTZ (10 mg/kg) was incorporated for last 7 days.
Experimental procedure
Two hours after the last treatment, heart was isolated from the animals of different groups under ketamine (70 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) anesthesia. Isolated heart was subjected to modified Lagendroff apparatus as mentioned in earlier reported article. The isolated heart was perfused with Kreb-Henseleit (K-H) solution, gassed with carbogen (95% O2 and 5% CO2) at 37°C, at a constant flow rate of 5 mL/min. The composition of K-H solution was (mM) NaCl 118, KCl 4.7, NaHCO3 25, NaHPO4 1.0, MgSO4 7H2 O 0.57, CaCl2 2.5, and glucose 11. The pH of K-H solution was adjusted to 7.4 to avoid K-H buffer acidosis that may occur after prolonged gassing with carbogen. The heart was allowed to equilibrate for 10 min and then regular recordings were taken for a perfusion period of 15 min. The heart was kept for equilibrium for a period of 10 min and then regular reading was taken for 15 min. The measurement of contractile force was determined with the help of force displacement transducer and recording was taken in digital physiograph (model no - DI-2, INCO, Ambala city, India). After the initial preischemic period, the isolated heart was subjected to no flow global ischemia by stopping the flow of carbogenated K-H solution for 15 min. Then, the heart was reperfused for a period of 15 min. The heart rate and force of contraction was determined during the pre- and post-ischemic period, and the percentage recovery of heart rate and force of contraction was calculated. The levels of creatine kinase-MB (CK-MB), creatine kinase-N-acetylcysteine (CK-NAC), lactate dehydrogenase (LDH) activity were determined in perfusate collected during postischemic condition.[15] Then half of the isolated hearts from all the groups were subjected for the preparation of heart tissue homogenate (HTH) using sucrose. Superoxide dismutase (SOD), catalase and thiobarbituric acid reactive substances (TBARS) were measured in HTH. For remaining heart samples, histological study was carried out.
Histological analysis
Heart sections were prepared from the remaining half of the heart samples in each group, stained with hematoxylin and eosin and change in histology were observed. The myocardial damage was determined by scoring method depending on the severity as follows, no change - 0 score, mild - 1 score (focal myocytes damage or small multifocal degeneration with a slight degree of inflammation), moderate - 2 score (extensive myofibrillar degeneration) and marked - 3 score (necrosis with diffuse inflammation).[16]
Statistical analysis
Results are expressed as mean ± standard error of the mean. Statistical significance was assessed using one-way analysis of variance followed by Tukey–Karmer multiple comparison tests. P < 0.05 was considered to be significant.
RESULTS
Preliminary phytochemical investigation
The preliminary phytochemical investigation of the GTE extract showed the presence of alkaloids, flavonoids, steroids, gallic tannins, and catecholic tannins. The percentage yield of GTE was found to be 24.76%.
Effect on lactate dehydrogenase, creatine kinase-MB and creatine kinase-N-acetylcysteine activities
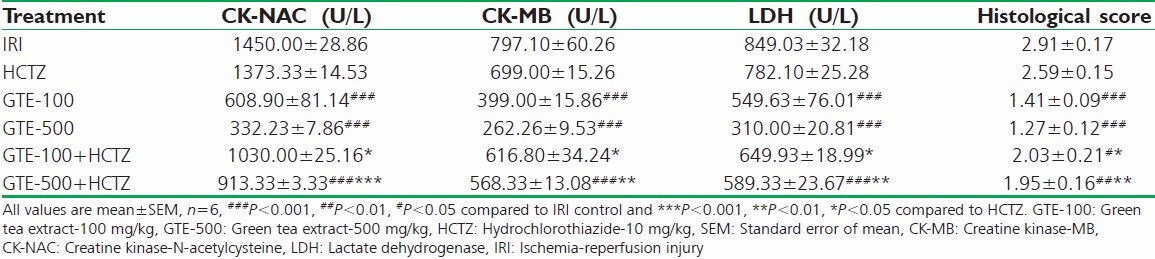
The experimental group prophylactically treated with GTE-100, GTE-500 and GTE-500 + HCTZ showed a significant decrease in LDH, CK-MB and CK-NAC levels in perfusate compared with IRI control alone.
High (GTE-500) and low dose (GTE-100) of GTE when incorporated with HCTZ, showed a significant decrease in LDH, CK-MB and CK-NAC activities in perfusate compared to HCTZ alone treated group [Table 1].
Table 1.
Effect on CK-NAC, CK-MB, LDH in perfusate using IRI in isolated rat heart
Effect on histological score
The groups pretreated with GTE-100, GTE-500 and GTE-500 combined with HCTZ, witnessed significant decrease in histological score compared to IRI control group. Groups treated with GTE-100 + HCTZ, GTE-500 + HCTZ showed a significant decrease in histological score compared to HCTZ alone treated group [Table 1].
Effect on superoxide dismutase, catalase and thiobarbituric acid reactive substances activities
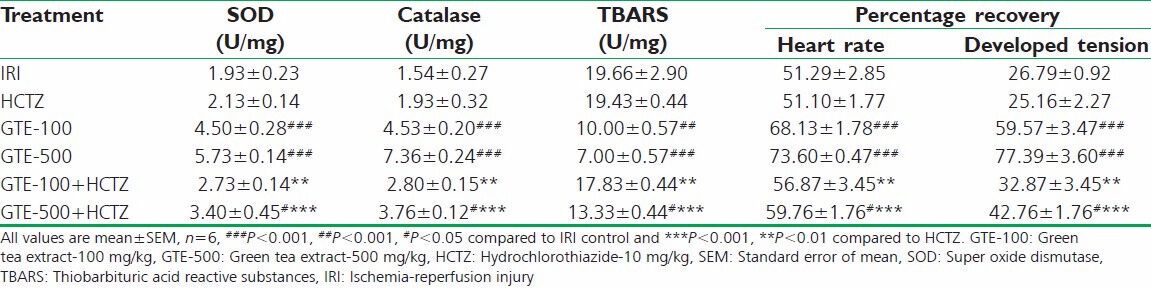
Experimental groups such as GTE-100, GTE-500 and GTE-500 combined with HCTZ reported a significant increase in both SOD and catalase activities, and decrease in TBARS compared to IRI control group.
Groups treated with GTE-100 + HCTZ, GTE-500 + HCTZ showed a significant increase in both SOD and catalase, and decrease in TBARS activities compared to HCTZ alone treated group [Table 2].
Table 2.
Effect on SOD, catalase, TBARS in heart tissue homogenate and percentage recovery of developed tension and heart rate using IRI in isolated rat heart
Developed tension and heart rate
The treatment groups such as GTE-100, GTE-500 and GTE-500 + HCTZ showed significant recovery in ischemic heart in terms of heart rate and developed tension compared to IRI control. GTE-500 + HCTZ and GTE-100 + HCTZ showed significant recovery compared to HCTZ alone treated group [Table 2].
Effect on histopathological study
Under ischemia, the isolated hearts showed different pathological changes such as severe myocardial edema, separation of fibers, and loss of striation. HCTZ alone treated group also associated with these severe pathological changes. GTE-100, GTE-500 and GTE-500 + HCTZ showed a significant decrease in histopathological score. GTE-100 + HCTZ and GTE-500 + HCTZ both documented significant reduction in histopathological score compared to HCTZ alone treated group [Figure 1].
Figure 1.
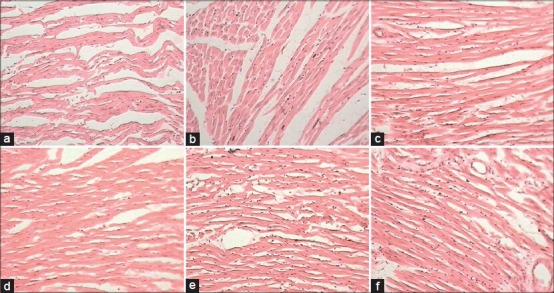
Microscopic section of (a) ischemia-reperfusion injury control – (1) Myocardial edema, (2) separation of fibers, (3) loss of striation, (4) diffuse inflammation (5) necrosis. (b) Hydrochlorothiazide (HCTZ) treated – (1) Myocardial edema, (2) separation of fibers, (3) loss of striation. (c) Green tea extract-100 (GTE)-100 – (1) Myocardial edema, (2) separation of fibers, (3) loss of striation. (d) GTE-500 – (1) Myocardial edema, (2) separation of fibers. (e) GTE-100 + HCTZ – (1) Myocardial edema, (2) separation of fibers, (3) loss of striation. (f) GTE-500 + HCTZ – (1) Myocardial edema, (2) separation of fibers, (3) loss of striation (H and E, ×400)
DISCUSSION
The aim of this study was to elucidate the pharmacodynamic interaction of GTE with HCTZ using ischemia-reperfusion induced myocardial injury. Observed results suggested that GTE (100 and 500 mg/kg, p.o.) showed beneficial results dose-dependently. Apart from that, GTE when combined with HCTZ indicated better results compared to HCTZ alone treated group against ischemia-reperfusion induced myocardial injury.
In this experimental model, myocardial toxicity was induced by ischemia-reperfusion. Ischemia, which is an acute or chronic form of cardiac disability, occurred due to imbalance between the supply and demand of oxygen in the blood. In IRI model, global ischemia causes immediate biological alteration, such as increase in intracellular Na+, which results an increase in intracellular Ca2+ via Na+/Ca2+ exchange. Increased intracellular Ca2+ is responsible for the formation of irreversible damage in the cardiac cell at the end of 15 min global ischemia.[17,18,19]
Hydrochlorothiazide alters the renal tubular mechanisms of electrolyte reabsorption. The direct action of HCTZ is responsible for an increase in the excretion of sodium and chloride. The indirect action causes reduction in plasma volume, consequent increase in urinary potassium loss, plasma renin activity, aldosterone secretion, and decrease in serum potassium loss. It has been observed that in patients with cardiac ischemia, heart failure or left ventricular hypertrophy, the likelihood of cardiac arrhythmia is increased because of mild-to-moderate hypokalemia. It has been reported that HCTZ induced hypokalemia is responsible for the increase in serum biomarker levels, such as LDH, CK-MB, and decrease in heart tissue antioxidant levels like SOD and catalase in rat. Therefore, it is proved that their undesirable metabolic consequences have been suspected to contribute an increase in cardiovascular morbidity and mortality. Hence, search for concurrently administered safe therapeutic medicament continues, which can ameliorate the hypokalemia in patients with ischemic heart diseases.[5,20]
Camellia sinensis (green tea) is having a rich source of polyphenols, which is responsible for strong free radical-scavenging activity. Green tea has reported its potency against cardiovascular disease risk factors. It reduces body weight by interfering within the sympathoadrenal system, reduction in fatty acid synthesis and decrease in cholesterol absorption. It possesses antithrombotic activity by inhibiting platelet aggregation. Green tea inhibits low density lipid oxidation, reduce adhesion molecule expression and reduce systolic as well as diastolic blood pressures.[21]
In this study, ischemia-reperfusion induced injury in the myocardial cell generates oxygen free radicals. Oxygen free radical is extensively associated with the loss of membrane integrity, which causes leaking of different biomarker enzymes from the cell. Apart from that, antioxidants, such as SOD and catalase also come out, which is responsible for inhibition of free radicals neutralization. As a result, further accumulation of free radicals takes place, leading to exaggerated tissue damage.[22]
Prophylactic treatment with GTE in both high and low dose, reported to have a beneficial role against the ischemic condition in myocardial cell. GTE dose-dependently decreased serum biomarker levels and increased antioxidant levels in tissue. Apart from that, GTE is also responsible for the significant recovery in terms of heart rate and developed tension. The combination group of GTE (500 and 100 mg/kg) and HCTZ demonstrated better results compared to HCTZ alone. Comparatively, high dose of GTE (500 mg/kg) was found to be more beneficial to reduce HCTZ induced myocardial toxicity. Histopathological study showed that high and low doses of GTE and their combination with HCTZ demonstrated a significant reduction in the histological score by reducing inflammation and restoring myocardial cell integrity, which supports the other findings of this study.
CONCLUSION
It can be concluded that GTE exhibited dose-dependent protection against ischemia-reperfusion induced myocardial injury. Apart from that, combination of GTE reduced significant myocardial side-effects associated with HCTZ. This observation can be very important for ischemic hypertensive patients, where HCTZ cannot be given as monotherapy due to potential myocardial side-effects. Future studies can be carried out to establish the fact clinically.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem. 2002;277:34933–40. doi: 10.1074/jbc.M204672200. [DOI] [PubMed] [Google Scholar]
- 2.Fugh-Berman A, Ernst E. Herb-drug interactions: Review and assessment of report reliability. Br J Clin Pharmacol. 2001;52:587–95. doi: 10.1046/j.0306-5251.2001.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoes AW, Grobbee DE, Peet TM, Lubsen J. Do non-potassium-sparing diuretics increase the risk of sudden cardiac death in hypertensive patients. Recent evidence? Drugs. 1994;47:711–33. doi: 10.2165/00003495-199447050-00002. [DOI] [PubMed] [Google Scholar]
- 4.Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355:134–8. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 5.Asdaq SM, Inamdar MN. The potential for interaction of hydrochlorothiazide with garlic in rats. Chem Biol Interact. 2009;181:472–9. doi: 10.1016/j.cbi.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea: A review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 7.Brown MD. Green tea (Camellia sinensis) extract and its possible role in the prevention of cancer. Altern Med Rev. 1999;4:360–70. [PubMed] [Google Scholar]
- 8.Papparella I, Ceolotto G, Montemurro D, Antonello M, Garbisa S, Rossi G, et al. Green tea attenuates angiotensin II-induced cardiac hypertrophy in rats by modulating reactive oxygen species production and the Src/epidermal growth factor receptor/Akt signaling pathway. J Nutr. 2008;138:1596–601. doi: 10.1093/jn/138.9.1596. [DOI] [PubMed] [Google Scholar]
- 9.Antonello M, Montemurro D, Bolognesi M, Di Pascoli M, Piva A, Grego F, et al. Prevention of hypertension, cardiovascular damage and endothelial dysfunction with green tea extracts. Am J Hypertens. 2007;20:1321–8. doi: 10.1016/j.amjhyper.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa K, Ninomiya M, Okubo T, Aoi N, Juneja LR, Kim M, et al. Tea catechin supplementation increases antioxidant capacity and prevents phospholipid hydroperoxidation in plasma of humans. J Agric Food Chem. 1999;47:3967–73. doi: 10.1021/jf981195l. [DOI] [PubMed] [Google Scholar]
- 11.Yanagi S, Matsumura K, Marui A, Morishima M, Hyon SH, Ikeda T, et al. Oral pretreatment with a green tea polyphenol for cardioprotection against ischemia-reperfusion injury in an isolated rat heart model. J Thorac Cardiovasc Surg. 2011;141:511–7. doi: 10.1016/j.jtcvs.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Bajerska J, Wozniewicz M, Jeszka J, Drzymala-Czyz S, Walkowiak J. Green tea aqueous extract reduces visceral fat and decreases protein availability in rats fed with a high-fat diet. Nutr Res. 2011;31:157–64. doi: 10.1016/j.nutres.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Finar IL. Organic Chemistry. 4th ed. London: ELBS; 1993. p. 518. [Google Scholar]
- 14.Mukherjee PK. Quality Control of Herbal Drugs: An Approach to Evaluation of Botanicals. 1st ed. New Delhi: Business Horizons; 2002. [Google Scholar]
- 15.Inamdar MN, Venkataraman BV, Aleem MA. A simple and improved perfusion apparatus for isolated hearts. Indian J Pharmacol. 1994;26:262–5. [Google Scholar]
- 16.Chakraborty M, Asdaq SM. Interaction of Semecarpus anacardium L. with propranolol against isoproterenol induced myocardial damage in rats. Indian J Exp Biol. 2011;49:200–6. [PubMed] [Google Scholar]
- 17.Mouhieddine S, Tresallet N, Boucher F, de Leiris J. Ultrastructural basis of the free-radical scavenging effect of indapamide in experimental myocardial ischemia and reperfusion. J Cardiovasc Pharmacol. 1993;22(Suppl 6):S47–52. [PubMed] [Google Scholar]
- 18.James TN, Keyes JW. The Etiology of Myocardial Infarction. Boston, Mass: Little, Brown and Co; 1963. Studies of the dying myocardial cell; pp. 189–205. [Google Scholar]
- 19.Jennings RB, Schaper J, Hill ML, Steenbergen C, Jr, Reimer KA. Effect of reperfusion late in the phase of reversible ischemic injury. Changes in cell volume, electrolytes, metabolites, and ultrastructure. Circ Res. 1985;56:262–78. doi: 10.1161/01.res.56.2.262. [DOI] [PubMed] [Google Scholar]
- 20.Asdaq SM, Inamdar MN. Pharmacodynamic interaction of garlic with hydrochlorothiazide in rats. Indian J Physiol Pharmacol. 2009;53:127–36. [PubMed] [Google Scholar]
- 21.Hernández Figueroa TT, Rodríguez-Rodríguez E, Sánchez-Muniz FJ. The green tea, a good choice for cardiovascular disease prevention? Arch Latinoam Nutr. 2004;54:380–94. [PubMed] [Google Scholar]
- 22.Asdaq SM, Inamdar MN, Asad M. Pharmacodynamic interaction of garlic with propranolol in ischemia-reperfusion induced myocardial damage. Pak J Pharm Sci. 2010;23:42–7. [PubMed] [Google Scholar]


