Abstract
Background
The aesthetics of the human nose is highly dependent on the complex structure of the lower lateral cartilages (LLC). Understanding optimum shape and mechanical properties of the LLC is pivotal to achieving satisfactory results in nasal tip rhinoplasty.
Objective
The authors introduce an ex vivo animal model to replicate the shape and mechanics of human nasal LLC as a tool for research and surgical education.
Methods
Seven fresh pig heads were obtained from a local butcher shop. Nasal cartilage was harvested in a replicable manner and fashioned into appropriate shapes and dimensions based on the authors' human cadaver studies. Sutures were placed to approximate the cartilage pairs into appropriate human anatomical position.
Results
The porcine cartilage model replicated analogous structures, including the medial crura and the lateral crura, with appropriate cephalic orientation and domal angles. The anterior-posterior dimensions of the medial crura, intermediate crura, and lateral crura were 4 mm, 6 mm, and 10 mm, respectively. Cartilage thickness was approximately 1 mm throughout the specimen. Cephalic orientation of the lateral crura was sculpted to 45°. The average angle of divergence was 54° and varied according to the physiological shape of the porcine nasal vault (range, 43-74°). Average interdomal distance was 13.3 mm (range, 9-18 mm), and average domal width was 6.2 mm (range, 5-7 mm).
Conclusions
This novel porcine model mimics human LLC and is inexpensive, easy to construct, and highly replicable. This model can be used as a valuable educational resource for training novice surgeons in the principles of nasal tip rhinoplasty. Additionally, our construct has broad applications in studying LLC geometry and mechanics.
Keywords: porcine model, lower lateral cartilage, nasal tip rhinoplasty, lateral crura
The cartilaginous portion of the human nose is an intricate structure composed of a septum and paired upper lateral cartilages (ULC) and lower lateral cartilages (LLC). Understanding shape characteristics and mechanical properties of the paired LLC is essential to achieving satisfactory results in nasal tip surgery. To correct nasal tip deformities, the rhinoplasty surgeon can rely on a wealth of proven techniques for manipulating the LLC and effecting a lasting change in the aesthetics of the nose. These maneuvers include various alar cartilage suturing techniques, cephalic trim, lateral crural steal, and strut grafting. For the novice surgeon, proficiency in these challenging tasks comes with experience gained on live patients. Recently, computer and animal models of rhinoplasty have been described.1-7 However, to date there are no surgical models that specifically simulate the experience of restructuring the LLC and nasal tip.
This article presents a simple and inexpensive animal model with food-grade pork to replicate the cartilaginous structure of the human nasal tip. Relying on basic surgical instruments, these life-size models can be fashioned in a highly replicable manner to imitate the shape and mechanical characteristics of LLC. Such a model can provide a resource for practicing nasal tip rhinoplasty techniques without the involvement of live patients, human cadavers, or expensive laboratory animals.
Methods
Human Cadaveric Measurements
Measurements of human cadaveric LLC were obtained with calipers. These measurements included anteroposterior dimensions of the medial crura, intermediate crura, and lateral crura, as well as cartilage thickness and the angle of cephalic orientation. A virtual three-dimensional (3D) model was devised with an average of the collected measurements in Solidworks 2010 (Dassault Systèmes, Vélizy, France) (Figure 1). This computer-generated model provided a guide for constructing the porcine replicas.
Figure 1.
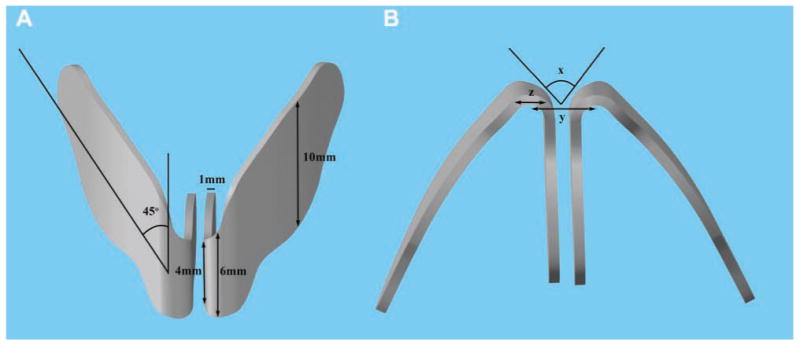
Virtual three-dimensional model of human lower lateral cartilage. (A) Frontal view. (B) Base view. Dimensions of interest: anteroposterior dimensions at the medial crura (4 mm), intermediate crura (6 mm), and lateral crura (10 mm), as well as (x) angle of divergence, (y) interdomal distance, and (z) domal width.
Preparation of the Porcine Model
Seven food-grade fresh pig heads were obtained from a local meat shop. The pig heads were individually placed on a cutting board and, with a #10 surgical blade, a midsagittal incision was made at the glabella, extending rostrally to the base of the snout. The incision was then carried laterally into the gingivolabial sulci. The subperichondrial and subperiosteal soft tissue envelope was sharply degloved, exposing the nasal cartilage and the bony skeleton. With a hammer and chisel, osteotomies were performed starting at the nasal aperture and extending toward the frontal bone. The nasal bones were fractured off to reveal the underlying cartilage (Figure 2A,B). The distal cartilaginous snout was transected in a coronal plane approximately 4 cm from the tip, revealing septum and flared crura bilaterally (Figure 2C). A second coronal transcartilaginous incision was made 10 cm posterior to the initial cut. A final osteotomy was performed to release the cartilaginous septum off the maxillary crest. The mucoperichondrium was then carefully dissected off the cartilaginous framework (Figure 2D). The cephalic orientation was marked at a 45° angle to the coronal plane with a marking pen. The desired anteroposterior dimensions of the crura were marked according to the 3D model; the medial crura, intermediate crura, and lateral crura were 4 mm, 6 mm, and 10 mm, respectively. Incisions were made along marked lines with a #15 surgical blade, extending vertically along the septum. The septum was then bisected and the specimens were sharply sculpted to 1-mm thickness. The cartilaginous pairs were sutured back together with 5-0 clear nylon sutures, to replicate nasal tip anatomy (Figure 3).
Figure 2.
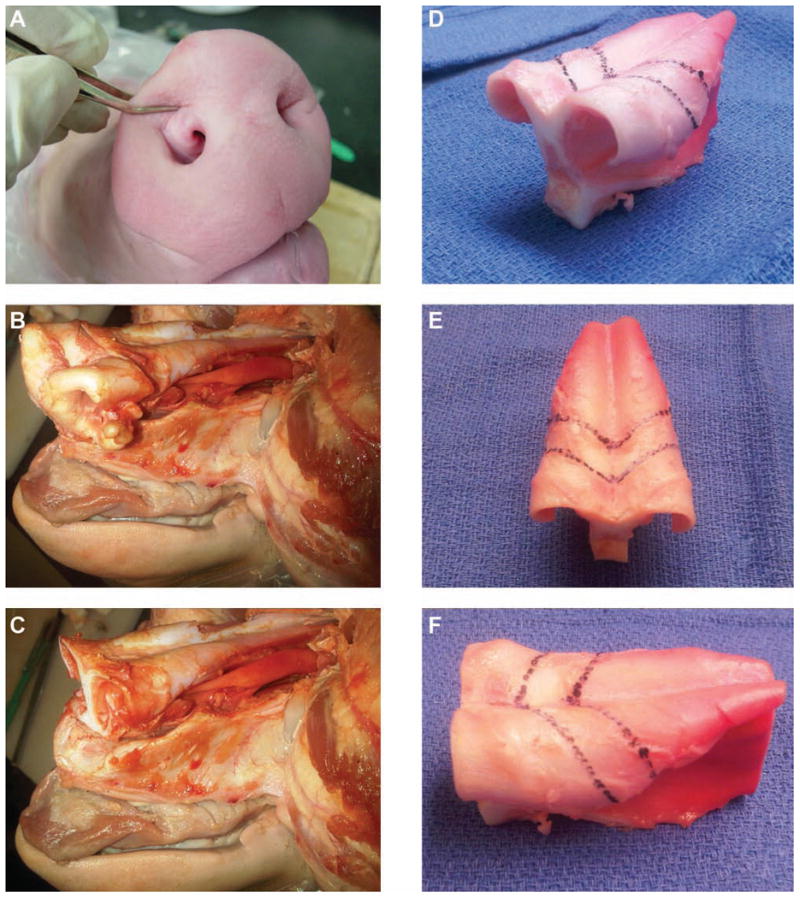
Porcine nasal cartilage harvest technique. (A) The anterior soft tissue is pinched to demonstrate the underlying cartilaginous framework. (B) The cartilaginous framework is exposed after soft tissue degloving and osteotomies to remove the nasal bones. (C) Coronal transection and removal of the snout is completed. (D-F) Incision lines are drawn on the dorsal surface of the harvested cartilage according to previously obtained human anatomic dimensions.
Figure 3.
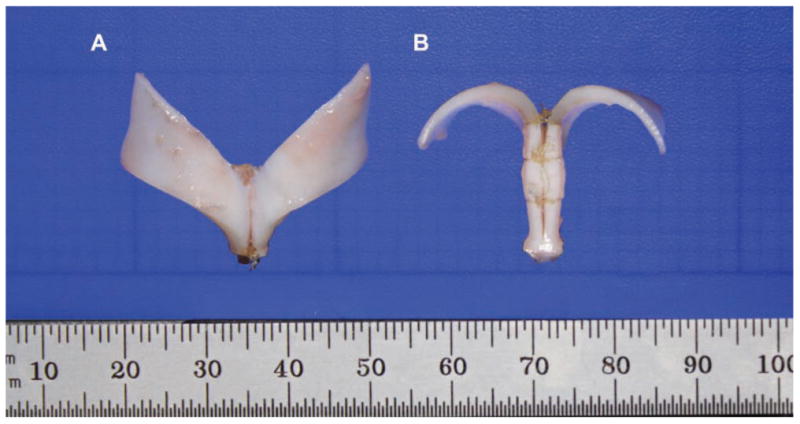
The completed model is shown, with nylon sutures securing the bisected septum. (A) Frontal view. (B) Base view.
Imaging and Measurements
A Nikon digital camera (Coolpix 990; Nikon, Inc., Melville, New York) was used to photograph the specimens. The mounted camera setup and position were not disturbed throughout the entire study. The camera was set to macro mode with automatic focus before photographs were obtained (Figure 3). The images were imported into Adobe Photoshop CS3 (Adobe Systems, Inc., San Jose, California), and the angle of divergence, interdomal distances, and the right and left domal widths were measured according to descriptions by Rohrich and Adams.8 After measurements, each model was rehydrated for 30 minutes in phosphate-buffered saline and stored at 4°C. The tissue was mounted onto a work bench with an adjustable clamp for educational and research use within one week of storage.
Results
The dimensions of the porcine model of LLC were based on human cadaveric measurements (Figure 1). The average anteroposterior dimensions of the human medial, intermediate, and lateral crura were 4 mm, 6 mm, and 10 mm, respectively. The angle of cephalic orientation was approximately 45°. Cartilage thickness was approximately 1 mm, with some tapering toward the lateral ends.
A total of seven individual porcine cartilage models were fashioned to the above specifications. The average angle of divergence was 54° (range, 43-74°). Average interdomal distance was 13.3 mm (range, 9-18 mm), and average domal width was 6.2 mm (range, 5-7 mm) (Table 1). Specimens were amenable to 5-0 nylon sutures and did not exhibit any cracking. Malleability and stiffness qualitatively approximated that of human LLC. As long as the specimens remained well hydrated, cartilage stiffness did not change for at least one week while refrigerated.
Table 1. Nasal Tip Anatomic Measurements of Porcine Models.
| Domal Width, mm | ||||
|---|---|---|---|---|
| Specimen | Angle of Divergence,° | Interdomal Distance, mm | Right | Left |
| 1 | 49 | 9 | 6 | 5 |
| 2 | 53 | 10 | 7 | 6 |
| 3 | 43 | 11 | 7 | 6 |
| 4 | 49 | 16 | 6 | 7 |
| 5 | 74 | 15 | 6 | 5 |
| 6 | 49 | 14 | 6 | 7 |
| 7 | 64 | 18 | 7 | 6 |
| Average | 54 | 13.3 | 6.4 | 6.0 |
Discussion
In this article, we have introduced a novel ex vivo model for nasal tip rhinoplasty that is inexpensive and highly replicable. These life-size models of LLC are simple to construct and are fashioned to the specifications obtained from human cadaveric measurements. The anteroposterior width of the LLC, the cephalic orientation of the lateral crura, and the cartilage thickness were fixed dimensions among all specimens. Variation will exist in certain parameters (angle of divergence, interdomal distance, domal width) based on the natural curvature of the nasal vault from which the cartilage was harvested. Porcine models will demonstrate variation much like the variations seen between pointy, boxy, and bulbous nasal tips in humans. The overall geometry of our models fits a human Type III boxy nasal tip, as described by Rohrich and Adams,8 making this an ideal educational model for practicing the requisite skills to manage this condition.
Qualitatively, this cartilage model exhibits similar physical properties to human LLC, making it ideal for surgical education pertaining to alar cartilage suturing techniques, cephalic trim, lateral crural steal, and lateral crural overlay, among other rhinoplasty maneuvers. In addition to quantitatively measuring the geometric and mechanical properties of the nasal tip, potential research utilities include experimentation of nonsurgical cartilage reshaping techniques with laser and electromechanical energy.9,10
Presence of ample residual septal cartilage tissue in the porcine nose also allows for construction and utilization of various cartilaginous grafts to be used in conjunction with this model. The major drawbacks are the model's ex vivo nature and the lack of a soft tissue envelope, which is important in creating pockets for cartilage grafts and observing the tissue healing response. A previously-proposed rabbit in vivo model may address some of these issues.2 However, the prohibitive cost of live animal models would prevent that from becoming a widely applicable educational tool. On the other hand, isolating the cartilaginous framework of the nasal tip in our proposed model may in fact be advantageous for tip mechanics research.
Conclusions
This article introduces a novel animal model to simulate the human LLC and nasal tip that is inexpensive, easy to construct, and highly replicable. Utilizing food-grade pork and basic surgical instruments, these life-size cartilage models can be fashioned in a reproducible manner to imitate the shape and mechanical characteristics of LLC. Such a model can be a valuable educational resource for training novice surgeons in the principles of nasal tip rhinoplasty without the involvement of live humans, human cadavers, or expensive laboratory animals. Additionally, our construct has broad research applications in the study of LLC geometry and mechanics.
Acknowledgments
Funding: The authors received no financial support for the research and authorship of this article.
Footnotes
Disclosures: The authors declared no potential conflicts of interest with respect to the authorship and publication of this article.
References
- 1.Coan BS, Neff E, Mukundan S, Jr, Marcus JR. Validation of a cadaveric model for comprehensive physiologic and anatomic evaluation of rhinoplastic techniques. Plast Reconstr Surg. 2009;124(6):2107–2117. doi: 10.1097/PRS.0b013e3181bf7e3a. [DOI] [PubMed] [Google Scholar]
- 2.Cöloglu H, Uysal A, Koçer U, Kankaya Y, Oruç M, Uysal S. Rhinoplasty model in rabbit. Plast Reconstr Surg. 2006;117(6):1851–1859. doi: 10.1097/01.prs.0000221875.24467.2d. [DOI] [PubMed] [Google Scholar]
- 3.Kaiser ML, Karam AM, Sepehr A, Wong H, Liaw LH, Vokes DE, Wong BJ. Cartilage regeneration in the rabbit nasal septum. Laryngoscope. 2006;116(10):1730–1734. doi: 10.1097/01.mlg.0000231430.81255.75. [DOI] [PubMed] [Google Scholar]
- 4.Lopez MA, Shah AR, Westine JG, O'Grady K, Toriumi DM. Analysis of the physical properties of costal cartilage in a porcine model. Arch Facial Plast Surg. 2007;9(1):35–39. doi: 10.1001/archfaci.9.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Ozkul T, Ozkul MH. Computer simulation tool for rhinoplasty planning. Comput Biol Med. 2004;34(8):697–718. doi: 10.1016/j.compbiomed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Vartanian AJ, Holcomb J, Ai Z, Rasmussen M, Tardy ME, Thomas JR. The virtual nose: a 3-dimensional virtual reality model of the human nose. Arch Facial Plast Surg. 2004;6(5):328–333. doi: 10.1001/archfaci.6.5.328. [DOI] [PubMed] [Google Scholar]
- 7.Zabaneh G, Lederer R, Grosvenor A, Wilkes G. Rhinoplasty: a hands-on training module. Plast Reconstr Surg. 2009;124(3):952–954. doi: 10.1097/PRS.0b013e3181b17bf5. [DOI] [PubMed] [Google Scholar]
- 8.Rohrich RJ, Adams WP., Jr The boxy nasal tip: classification and management based on alar cartilage suturing techniques. Plast Reconstr Surg. 2001;107(7):1849–1863. discussion 1864-1868. [PubMed] [Google Scholar]
- 9.Ho KH, Diaz Valdes SH, Protsenko DE, Aguilar G, Wong BJ. Electromechanical reshaping of septal cartilage. Laryngoscope. 2003;113(11):1916–1921. doi: 10.1097/00005537-200311000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Leclère FM, Petropoulos I, Buys B, Mordon S. Laser assisted septal cartilage reshaping (LASCR): a prospective study in 12 patients. Lasers Surg Med. 2010;42(8):693–698. doi: 10.1002/lsm.20958. [DOI] [PubMed] [Google Scholar]


