Abstract
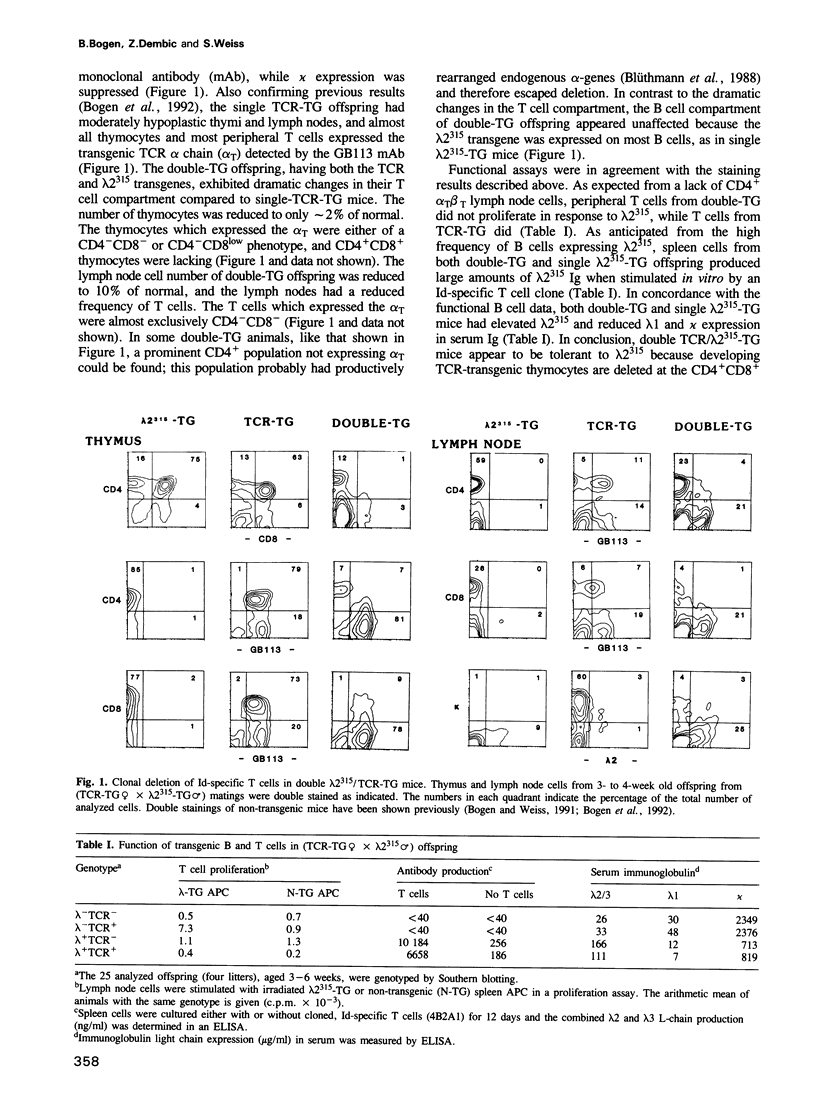
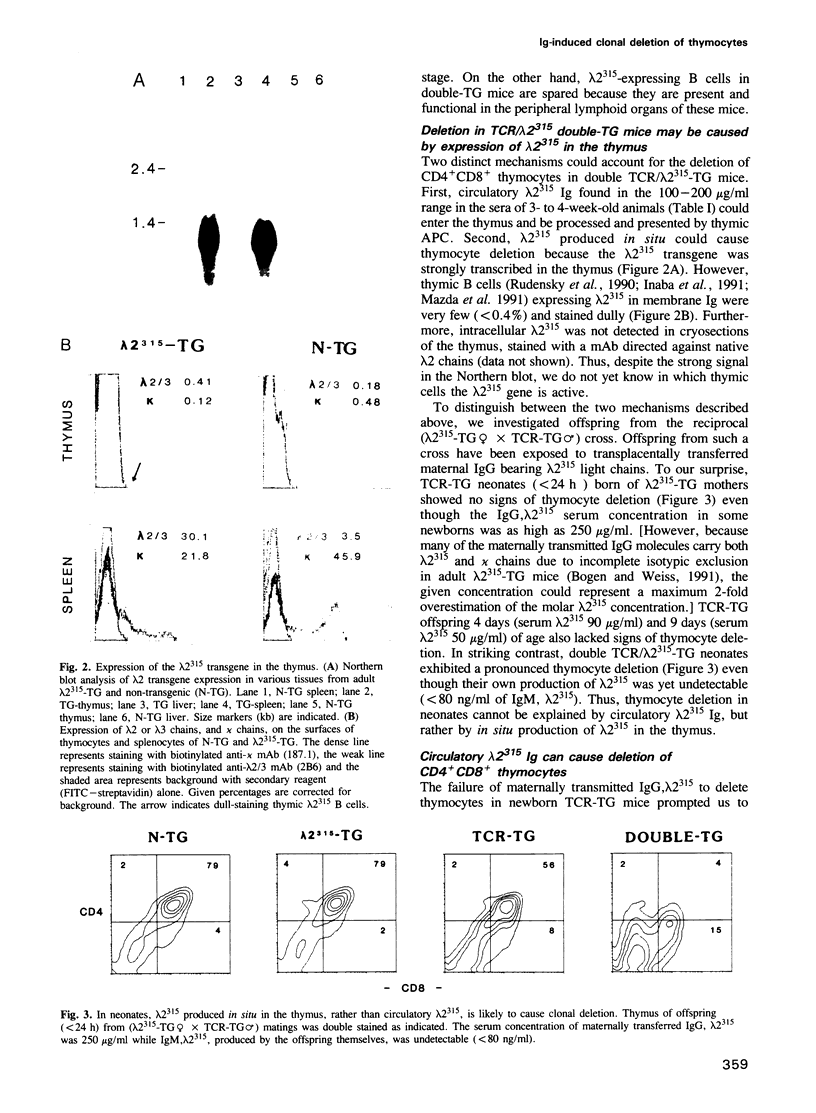
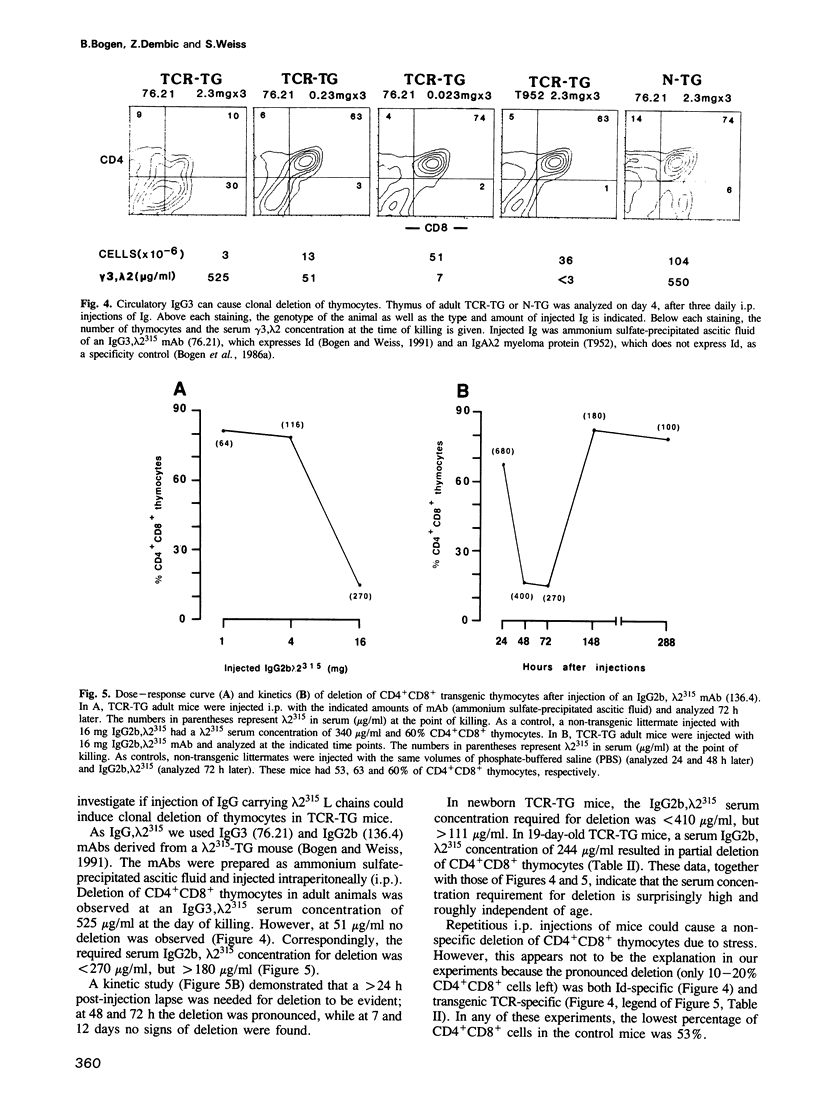
We have investigated whether immunoglobulin can induce clonal deletion of thymocytes by employing two strains of transgenic mice. One strain is transgenic for an alpha/beta T cell receptor (TCR) which recognizes a processed idiotypic peptide of the lambda 2(315) light chain variable region, bound to the I-Ed class II major histocompatibility complex molecule. The other mouse strain is transgenic for the lambda 2(315) gene. Double transgenic offspring from a TCR-transgenic female mated with a lambda 2(315) transgenic male exhibit a pronounced clonal deletion of CD4+CD8+ thymocytes. Analysis of neonates from the reciprocal (lambda 2(315)-transgenic female x TCR-transgenic male) cross suggests that the deletion in double transgenic offspring most likely is caused by lambda 2(315) produced within the thymus rather than by maternally derived IgG, lambda 2(315). Nevertheless, IgG, lambda 2(315) can cause deletion of CD4+CD8+ thymocytes when injected in large amounts intraperitoneally into either adult or neonatal TCR-transgenic mice. Deletion is evident 48 and 72 h after injection, but by day 7 the thymus has already regained its normal appearance. A serum concentration of several hundred microgram/ml is required for deletion to be observed. Therefore, the heterogeneous idiotypes of serum Ig are probably each of too low concentration to cause thymocyte deletion in normal animals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein S., Pritchard-Briscoe H., Anderson T. A., Crosbie J., Gammon G., Loblay R. H., Basten A., Goodnow C. C. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991 Mar 8;251(4998):1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- Arnold B., Dill O., Küblbeck G., Jatsch L., Simon M. M., Tucker J., Hämmerling G. J. Alloreactive immune responses of transgenic mice expressing a foreign transplantation antigen in a soluble form. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2269–2273. doi: 10.1073/pnas.85.7.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüthmann H., Kisielow P., Uematsu Y., Malissen M., Krimpenfort P., Berns A., von Boehmer H., Steinmetz M. T-cell-specific deletion of T-cell receptor transgenes allows functional rearrangement of endogenous alpha- and beta-genes. Nature. 1988 Jul 14;334(6178):156–159. doi: 10.1038/334156a0. [DOI] [PubMed] [Google Scholar]
- Bogen B., Gleditsch L., Weiss S., Dembic Z. Weak positive selection of transgenic T cell receptor-bearing thymocytes: importance of major histocompatibility complex class II, T cell receptor and CD4 surface molecule densities. Eur J Immunol. 1992 Mar;22(3):703–709. doi: 10.1002/eji.1830220313. [DOI] [PubMed] [Google Scholar]
- Bogen B., Lambris J. D. Minimum length of an idiotypic peptide and a model for its binding to a major histocompatibility complex class II molecule. EMBO J. 1989 Jul;8(7):1947–1952. doi: 10.1002/j.1460-2075.1989.tb03599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen B., Malissen B., Haas W. Idiotope-specific T cell clones that recognize syngeneic immunoglobulin fragments in the context of class II molecules. Eur J Immunol. 1986 Nov;16(11):1373–1378. doi: 10.1002/eji.1830161110. [DOI] [PubMed] [Google Scholar]
- Bogen B. Monoclonal antibodies specific for variable and constant domains of murine lambda chains. Scand J Immunol. 1989 Mar;29(3):273–279. doi: 10.1111/j.1365-3083.1989.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Bogen B., Snodgrass R., Briand J. P., Hannestad K. Synthetic peptides and beta-chain gene rearrangements reveal a diversified T cell repertoire for a lambda light chain third hypervariable region. Eur J Immunol. 1986 Nov;16(11):1379–1384. doi: 10.1002/eji.1830161111. [DOI] [PubMed] [Google Scholar]
- Bogen B., Weiss S. A rearranged lambda 2 light gene chain retards but does not exclude kappa and lambda 1 expression. Eur J Immunol. 1991 Oct;21(10):2391–2395. doi: 10.1002/eji.1830211015. [DOI] [PubMed] [Google Scholar]
- Burkly L. C., Lo D., Flavell R. A. Tolerance in transgenic mice expressing major histocompatibility molecules extrathymically on pancreatic cells. Science. 1990 Jun 15;248(4961):1364–1368. doi: 10.1126/science.1694042. [DOI] [PubMed] [Google Scholar]
- Cibotti R., Kanellopoulos J. M., Cabaniols J. P., Halle-Panenko O., Kosmatopoulos K., Sercarz E., Kourilsky P. Tolerance to a self-protein involves its immunodominant but does not involve its subdominant determinants. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):416–420. doi: 10.1073/pnas.89.1.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Fowlkes B. J., Schwartz R. H., Pardoll D. M. Deletion of self-reactive thymocytes occurs at a CD4+8+ precursor stage. Nature. 1988 Aug 18;334(6183):620–623. doi: 10.1038/334620a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C. Transgenic mice and analysis of B-cell tolerance. Annu Rev Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Hagman J., Lo D., Doglio L. T., Hackett J., Jr, Rudin C. M., Haasch D., Brinster R., Storb U. Inhibition of immunoglobulin gene rearrangement by the expression of a lambda 2 transgene. J Exp Med. 1989 Jun 1;169(6):1911–1929. doi: 10.1084/jem.169.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M., Inaba K., Hosono M., Kumamoto T., Ishida T., Muramatsu S., Masuda T., Ikehara S. Distinct mechanisms of neonatal tolerance induced by dendritic cells and thymic B cells. J Exp Med. 1991 Mar 1;173(3):549–559. doi: 10.1084/jem.173.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Jones L. A., Chin L. T., Longo D. L., Kruisbeek A. M. Peripheral clonal elimination of functional T cells. Science. 1990 Dec 21;250(4988):1726–1729. doi: 10.1126/science.2125368. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Kung J. T., Sharrow S. O., Sieckmann D. G., Lieberman R., Paul W. E. A mouse IgM allotypic determinant (Igh-6.5) recognized by a monoclonal rat antibody. J Immunol. 1981 Sep;127(3):873–876. [PubMed] [Google Scholar]
- Lassila O., Vainio O., Matzinger P. Can B cells turn on virgin T cells? Nature. 1988 Jul 21;334(6179):253–255. doi: 10.1038/334253a0. [DOI] [PubMed] [Google Scholar]
- Lin R. H., Stockinger B. T cell immunity or tolerance as a consequence of self antigen presentation. Eur J Immunol. 1989 Jan;19(1):105–110. doi: 10.1002/eji.1830190117. [DOI] [PubMed] [Google Scholar]
- Lorenz R. G., Allen P. M. Thymic cortical epithelial cells can present self-antigens in vivo. Nature. 1989 Feb 9;337(6207):560–562. doi: 10.1038/337560a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Hengartner H., Pedrazzini T. Intrathymic deletion of self-reactive cells prevented by neonatal anti-CD4 antibody treatment. Nature. 1988 Sep 8;335(6186):174–176. doi: 10.1038/335174a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Mazda O., Watanabe Y., Gyotoku J., Katsura Y. Requirement of dendritic cells and B cells in the clonal deletion of Mls-reactive T cells in the thymus. J Exp Med. 1991 Mar 1;173(3):539–547. doi: 10.1084/jem.173.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Morahan G. Peripheral T cell tolerance. Annu Rev Immunol. 1992;10:51–69. doi: 10.1146/annurev.iy.10.040192.000411. [DOI] [PubMed] [Google Scholar]
- Murphy K. M., Heimberger A. B., Loh D. Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990 Dec 21;250(4988):1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis P., Stet R. J., Wagenaar J. P., Wubbena A. S., Kampinga J., Karrenbeld A. The transcapsular route: a new way for (self-) antigens to by-pass the blood-thymus barrier? Immunol Today. 1988 Dec;9(12):372–375. doi: 10.1016/0167-5699(88)91236-4. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Oyen O., Frøysa A., Sandberg M., Eskild W., Joseph D., Hansson V., Jahnsen T. Cellular localization and age-dependent changes in mRNA for cyclic adenosine 3',5'-monophosphate-dependent protein kinases in rat testis. Biol Reprod. 1987 Nov;37(4):947–956. doi: 10.1095/biolreprod37.4.947. [DOI] [PubMed] [Google Scholar]
- Pircher H., Bürki K., Lang R., Hengartner H., Zinkernagel R. M. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989 Nov 30;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Fowlkes B. J. Clonal deletion versus clonal anergy: the role of the thymus in inducing self tolerance. Science. 1990 Jun 15;248(4961):1342–1348. doi: 10.1126/science.1972593. [DOI] [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991 Mar 8;251(4998):1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Rudensky A. Y., Mazel S. M., Yurin V. L. Presentation of endogenous immunoglobulin determinant to immunoglobulin-recognizing T cell clones by the thymic cells. Eur J Immunol. 1990 Oct;20(10):2235–2239. doi: 10.1002/eji.1830201012. [DOI] [PubMed] [Google Scholar]
- Sanz I. Multiple mechanisms participate in the generation of diversity of human H chain CDR3 regions. J Immunol. 1991 Sep 1;147(5):1720–1729. [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Schönrich G., Kalinke U., Momburg F., Malissen M., Schmitt-Verhulst A. M., Malissen B., Hämmerling G. J., Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991 Apr 19;65(2):293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- Spain L. M., Berg L. J. Developmental regulation of thymocyte susceptibility to deletion by "self"-peptide. J Exp Med. 1992 Jul 1;176(1):213–223. doi: 10.1084/jem.176.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Gao E. K., Webb S. R. T cell reactivity to MHC molecules: immunity versus tolerance. Science. 1990 Jun 15;248(4961):1357–1363. doi: 10.1126/science.1694041. [DOI] [PubMed] [Google Scholar]
- Swat W., Ignatowicz L., von Boehmer H., Kisielow P. Clonal deletion of immature CD4+8+ thymocytes in suspension culture by extrathymic antigen-presenting cells. Nature. 1991 May 9;351(6322):150–153. doi: 10.1038/351150a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Vasquez N. J., Kaye J., Hedrick S. M. In vivo and in vitro clonal deletion of double-positive thymocytes. J Exp Med. 1992 May 1;175(5):1307–1316. doi: 10.1084/jem.175.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Weiss S., Bogen B. B-lymphoma cells process and present their endogenous immunoglobulin to major histocompatibility complex-restricted T cells. Proc Natl Acad Sci U S A. 1989 Jan;86(1):282–286. doi: 10.1073/pnas.86.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S., Bogen B. MHC class II-restricted presentation of intracellular antigen. Cell. 1991 Feb 22;64(4):767–776. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]
- Weiss S., Lehmann K., Cohn M. Monoclonal antibodies to murine immunoglobulin isotypes. Hybridoma. 1983;2(1):49–54. doi: 10.1089/hyb.1983.2.49. [DOI] [PubMed] [Google Scholar]
- Weiss S., Wu G. E. Somatic point mutations in unrearranged immunoglobulin gene segments encoding the variable region of lambda light chains. EMBO J. 1987 Apr;6(4):927–932. doi: 10.1002/j.1460-2075.1987.tb04840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley P. J., Poindexter N. J., Landon C., Kapp J. A. A peripheral mechanism preserves self-tolerance to a secreted protein in transgenic mice. J Immunol. 1990 Sep 1;145(5):1376–1381. [PubMed] [Google Scholar]
- Winchester G., Sunshine G. H., Nardi N., Mitchison N. A. Antigen-presenting cells do not discriminate between self and nonself. Immunogenetics. 1984;19(6):487–491. doi: 10.1007/BF00403439. [DOI] [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Kisielow P. Self-nonself discrimination by T cells. Science. 1990 Jun 15;248(4961):1369–1373. doi: 10.1126/science.1972594. [DOI] [PubMed] [Google Scholar]



