Abstract
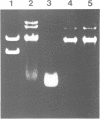
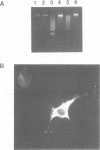
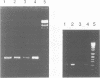
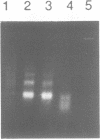
Cell death by apoptosis occurs in a wide range of physiological events including repertoire selection of lymphocytes and during immune responses in vivo. A hallmark of apoptosis is the internucleosomal DNA degradation for which a Ca2+,Mg(2+)-dependent endonuclease has been postulated. This nuclease activity was extracted from both rat thymocyte and lymph node cell nuclei. When incubated with nuclei harbouring only limited amounts of endogenous nuclease activity, the ladder pattern of DNA fragments characteristic of apoptosis was induced. This extractable nucleolytic activity was immunoprecipitated with antibodies specific for rat deoxyribonuclease I (DNase I) and was inhibited by actin in complex with gelsolin segment 1, strongly pointing to the presence of a DNase I-type enzyme in the nuclear extracts. COS cells transiently transfected with the cDNA of rat parotid DNase I expressed the enzyme, and their nuclei were able to degrade their DNA into oligosome-sized fragments. PCR analysis of mRNA isolated from thymus, lymph node cells and kidney yielded a product identical in size to that from rat parotid DNase I. Immunohistochemical staining with antibodies to rat DNase I confirmed the presence of DNase I antigen in thymocytes and lymph node cells. The tissue distribution of DNase I is thus extended to tissues with no digestive function and to cells which are known to be susceptible to apoptosis. We propose that during apoptosis, an endonuclease indistinguishable from DNase I gains access to the nucleus due to the breakdown of the ER and the nuclear membrane.
Full text
PDF

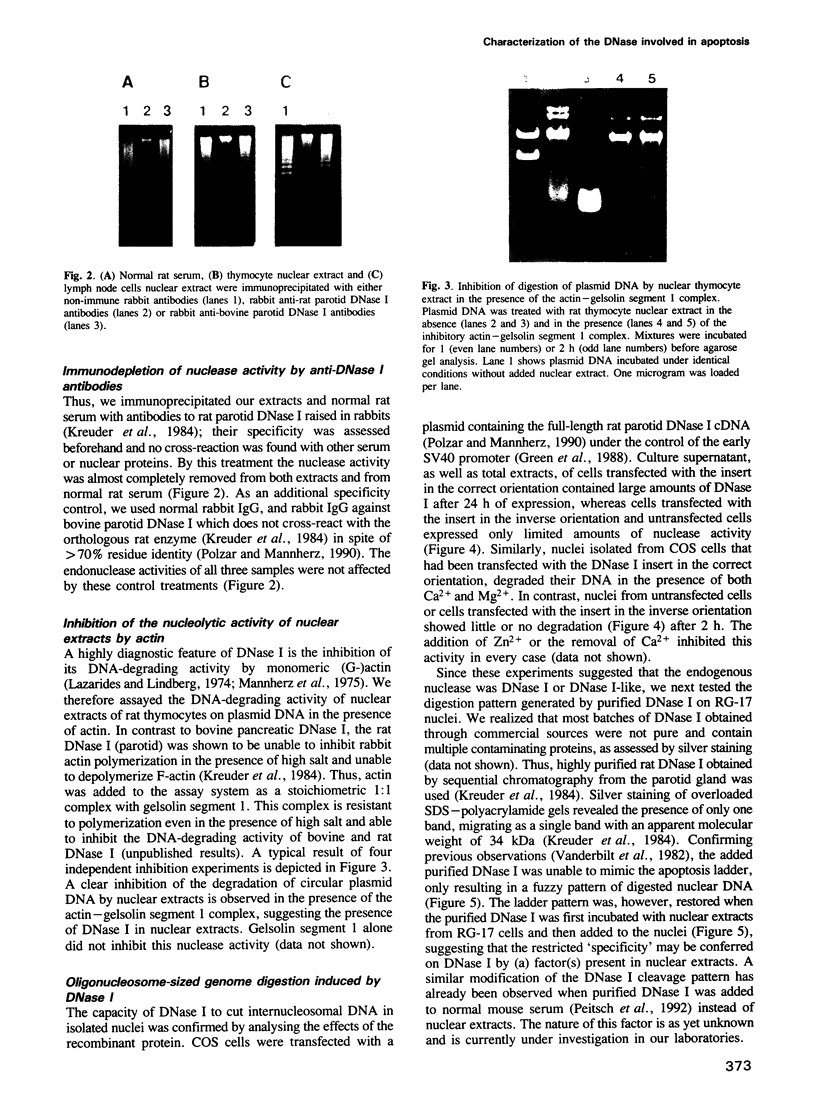

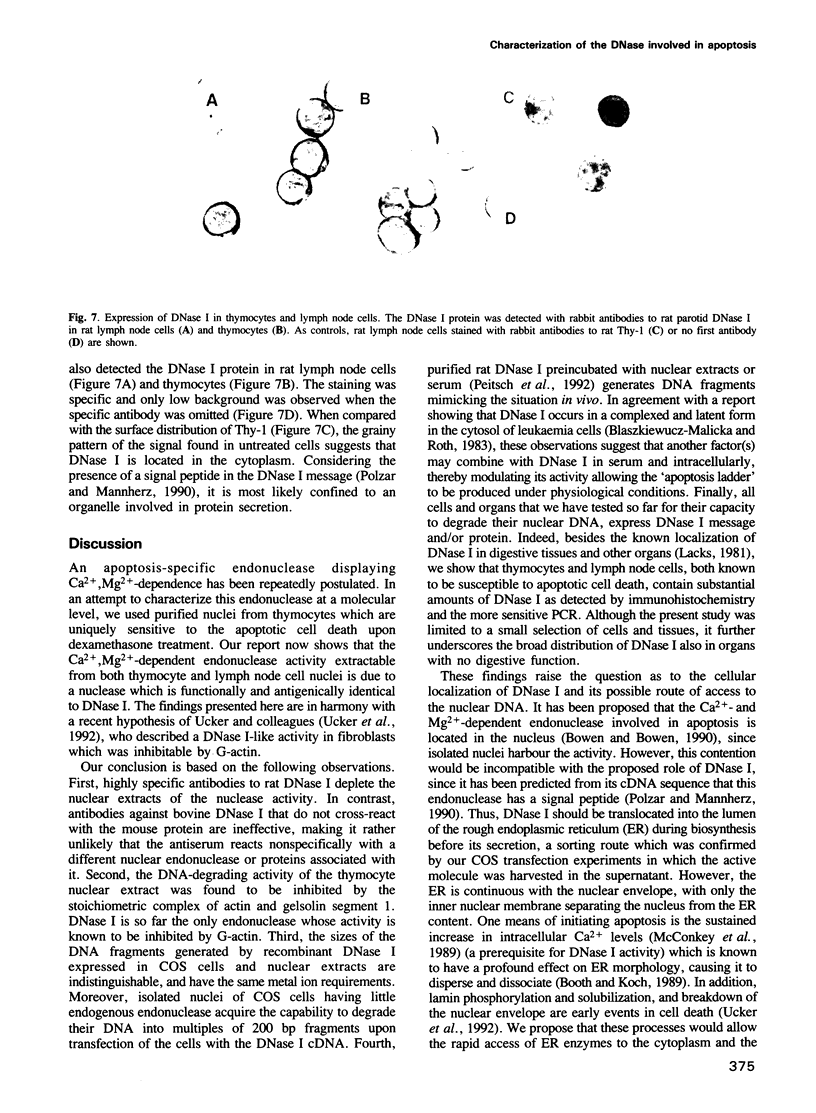


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Litwack G. Glucocorticoid-induced lymphocytolysis is not mediated by an induced endonuclease. J Biol Chem. 1989 Mar 5;264(7):4104–4111. [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Baxter G. D., Smith P. J., Lavin M. F. Molecular changes associated with induction of cell death in a human T-cell leukaemia line: putative nucleases identified as histones. Biochem Biophys Res Commun. 1989 Jul 14;162(1):30–37. doi: 10.1016/0006-291x(89)91957-8. [DOI] [PubMed] [Google Scholar]
- Booth C., Koch G. L. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989 Nov 17;59(4):729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Chitrabamrung S., Bannett J. S., Rubin R. L., Tan E. M. A radial diffusion assay for plasma and serum deoxyribonuclease I. Rheumatol Int. 1981;1(2):49–53. doi: 10.1007/BF00541152. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Compton M. M., Cidlowski J. A. Identification of a glucocorticoid-induced nuclease in thymocytes. A potential "lysis gene" product. J Biol Chem. 1987 Jun 15;262(17):8288–8292. [PubMed] [Google Scholar]
- Compton M. M. Development of an apoptosis endonuclease assay. DNA Cell Biol. 1991 Mar;10(2):133–141. doi: 10.1089/dna.1991.10.133. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke R. C., Cohen J. J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986 Fall;5(4):289–299. [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogg D., Hahn S., Erb P. CD4+ T cell-mediated killing of major histocompatibility complex class II-positive antigen-presenting cells (APC). III. CD4+ cytotoxic T cells induce apoptosis of APC. Eur J Immunol. 1992 Jan;22(1):267–272. doi: 10.1002/eji.1830220139. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Bowen I. D. The fine structural localization of acid phosphatase in pore cells of embryonic and newly hatched Deroceras reticulatum (Pulmonata: Stylommatophora). Cell Tissue Res. 1979 Dec;204(2):253–265. doi: 10.1007/BF00234637. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991 Jan 17;349(6306):245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- Kishi K., Yasuda T., Ikehara Y., Sawazaki K., Sato W., Iida R. Human serum deoxyribonuclease I (DNase I) polymorphism: pattern similarities among isozymes from serum, urine, kidney, liver, and pancreas. Am J Hum Genet. 1990 Jul;47(1):121–126. [PMC free article] [PubMed] [Google Scholar]
- Kreuder V., Dieckhoff J., Sittig M., Mannherz H. G. Isolation, characterisation and crystallization of deoxyribonuclease I from bovine and rat parotid gland and its interaction with rabbit skeletal muscle actin. Eur J Biochem. 1984 Mar 1;139(2):389–400. doi: 10.1111/j.1432-1033.1984.tb08018.x. [DOI] [PubMed] [Google Scholar]
- Lacks S. A. Deoxyribonuclease I in mammalian tissues. Specificity of inhibition by actin. J Biol Chem. 1981 Mar 25;256(6):2644–2648. [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J. D., Hewitt R. R. The relationship between human serum and human pancreatic DNase I. J Biol Chem. 1979 Dec 25;254(24):12588–12594. [PubMed] [Google Scholar]
- MacDonald H. R., Baschieri S., Lees R. K. Clonal expansion precedes anergy and death of V beta 8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. Eur J Immunol. 1991 Aug;21(8):1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Glasebrook A. L., Schneider R., Lees R. K., Pircher H., Pedrazzini T., Kanagawa O., Nicolas J. F., Howe R. C., Zinkernagel R. M. T-cell reactivity and tolerance to Mlsa-encoded antigens. Immunol Rev. 1989 Feb;107:89–108. doi: 10.1111/j.1600-065x.1989.tb00004.x. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K. Programmed death of autoreactive thymocytes. Nature. 1990 Feb 15;343(6259):642–644. doi: 10.1038/343642a0. [DOI] [PubMed] [Google Scholar]
- Malicka-Blaszkiewicz M., Roth J. S. Evidence for the presence of DNase-actin complex in L1210 leukemia cells. FEBS Lett. 1983 Mar 7;153(1):235–239. doi: 10.1016/0014-5793(83)80155-0. [DOI] [PubMed] [Google Scholar]
- Mannherz H. G., Leigh J. B., Leberman R., Pfrang H. A specific 1:1 G-actin:DNAase i complex formed by the action of DNAase I on F-actin. FEBS Lett. 1975 Dec 1;60(1):34–38. doi: 10.1016/0014-5793(75)80412-1. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Nicotera P., Hartzell P., Bellomo G., Wyllie A. H., Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989 Feb 15;269(1):365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- Miyauchi K., Ogawa M., Shibata T., Matsuda K., Mori T., Ito K., Minamiura N., Yamamoto T. Development of a radioimmunoassay for human deoxyribonuclease I. Clin Chim Acta. 1986 Jan 30;154(2):115–123. doi: 10.1016/0009-8981(86)90003-3. [DOI] [PubMed] [Google Scholar]
- Newell M. K., Haughn L. J., Maroun C. R., Julius M. H. Death of mature T cells by separate ligation of CD4 and the T-cell receptor for antigen. Nature. 1990 Sep 20;347(6290):286–289. doi: 10.1038/347286a0. [DOI] [PubMed] [Google Scholar]
- Oefner C., Suck D. Crystallographic refinement and structure of DNase I at 2 A resolution. J Mol Biol. 1986 Dec 5;192(3):605–632. doi: 10.1016/0022-2836(86)90280-9. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Peitsch M. C., Hesterkamp T., Polzar B., Mannherz H. G., Tschopp J. Functional characterisation of serum DNase I in MRL-lpr/lpr mice. Biochem Biophys Res Commun. 1992 Jul 31;186(2):739–745. doi: 10.1016/0006-291x(92)90808-x. [DOI] [PubMed] [Google Scholar]
- Polzar B., Mannherz H. G. Nucleotide sequence of a full length cDNA clone encoding the deoxyribonuclease I from the rat parotid gland. Nucleic Acids Res. 1990 Dec 11;18(23):7151–7151. doi: 10.1093/nar/18.23.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. A., Liu T. Y., Stein W. H., Moore S. Properties of chromatographically purified bovine pancreatic deoxyribonuclease. J Biol Chem. 1969 Feb 10;244(3):917–923. [PubMed] [Google Scholar]
- Price P. A., Stein W. H., Moore S. Effect of divalent cations on the reduction and re-formation of the disulfide bonds of deoxyribonuclease. J Biol Chem. 1969 Feb 10;244(3):929–932. [PubMed] [Google Scholar]
- Russell J. H., Dobos C. B. Mechanisms of immune lysis. II. CTL-induced nuclear disintegration of the target begins within minutes of cell contact. J Immunol. 1980 Sep;125(3):1256–1261. [PubMed] [Google Scholar]
- Shi Y. F., Sahai B. M., Green D. R. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989 Jun 22;339(6226):625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Ucker D. S., Obermiller P. S., Eckhart W., Apgar J. R., Berger N. A., Meyers J. Genome digestion is a dispensable consequence of physiological cell death mediated by cytotoxic T lymphocytes. Mol Cell Biol. 1992 Jul;12(7):3060–3069. doi: 10.1128/mcb.12.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderbilt J. N., Bloom K. S., Anderson J. N. Endogenous nuclease. Properties and effects on transcribed genes in chromatin. J Biol Chem. 1982 Nov 10;257(21):13009–13017. [PubMed] [Google Scholar]
- Way M., Gooch J., Pope B., Weeds A. G. Expression of human plasma gelsolin in Escherichia coli and dissection of actin binding sites by segmental deletion mutagenesis. J Cell Biol. 1989 Aug;109(2):593–605. doi: 10.1083/jcb.109.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]