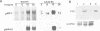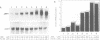Abstract
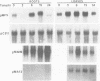
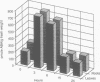
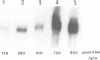
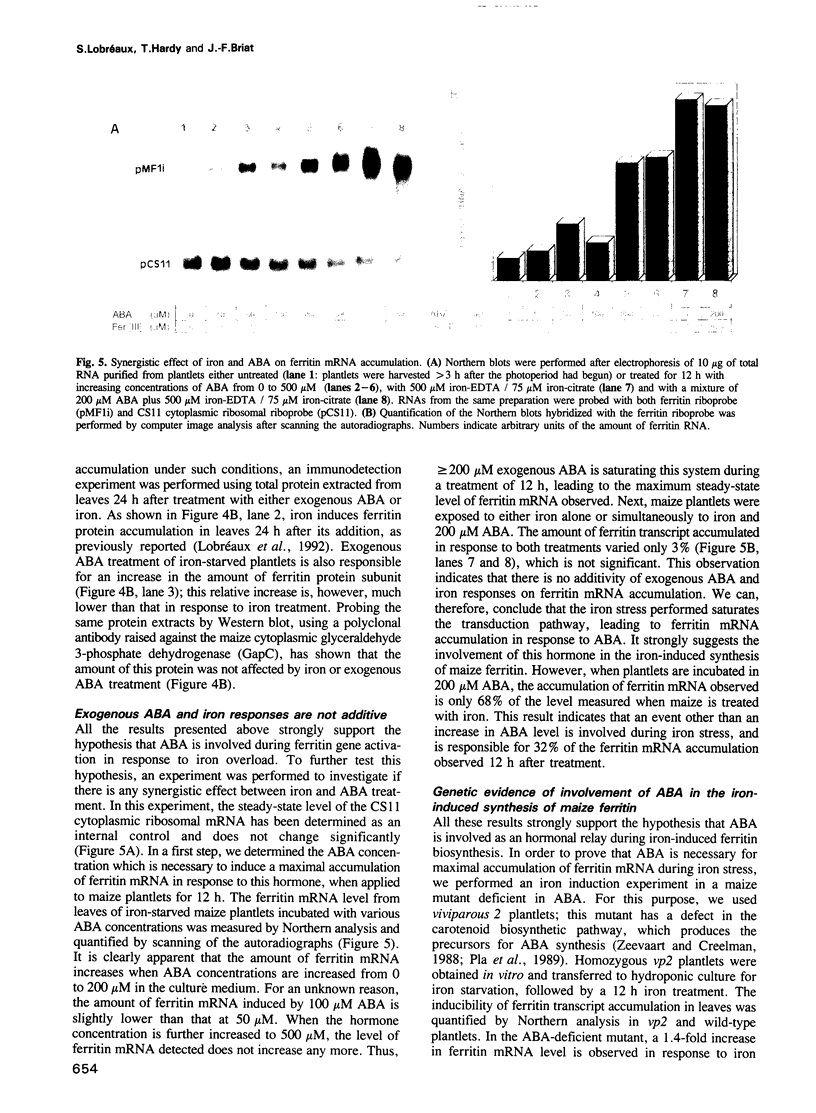
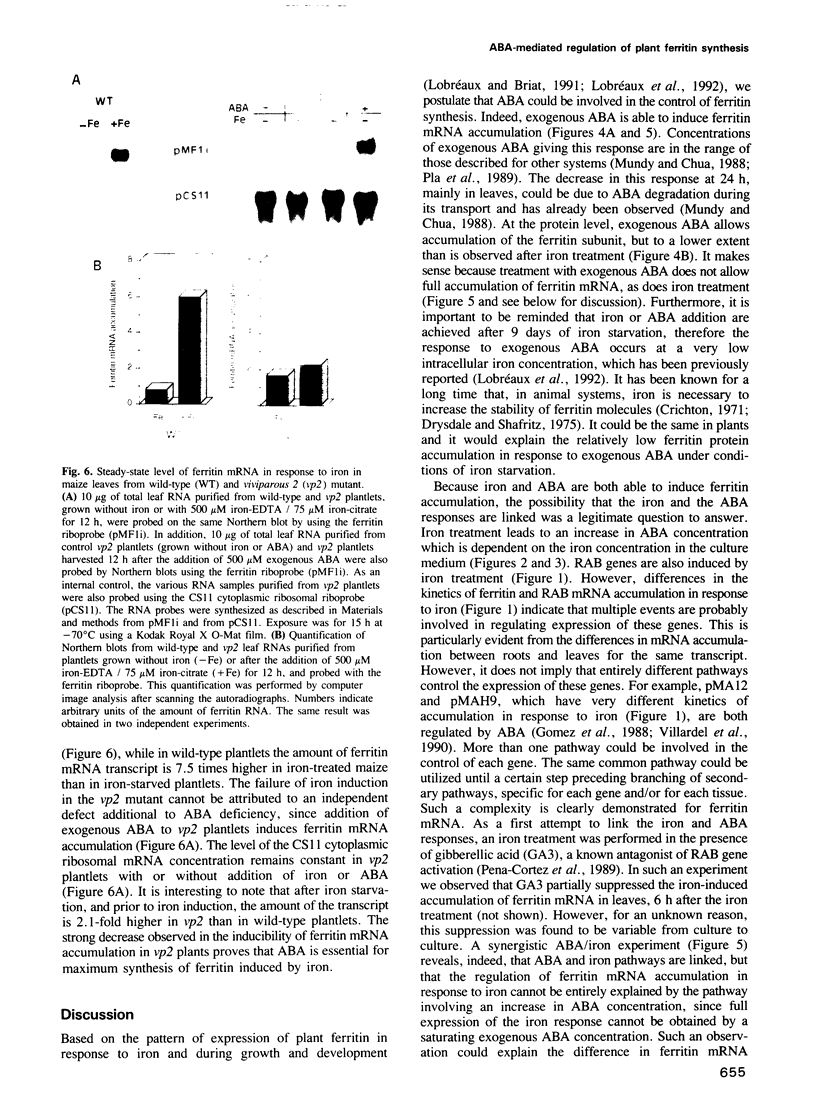
The ubiquitous iron storage protein ferritin has a highly conserved structure in plants and animals, but a distinct cytological location and a different level of control in response to iron excess. Plant ferritins are plastid-localized and transcriptionally regulated in response to iron, while animal ferritins are found in the cytoplasm and have their expression mainly controlled at the translational level. In order to understand the basis of these differences, we developed hydroponic cultures of maize plantlets which allowed an increase in the intracellular iron concentration, leading to a transient accumulation of ferritin mRNA and protein (Lobréaux,S., Massenet,O. and Briat,J.F., 1992, Plant Mol. Biol., 19, 563-575). Here, it is shown that iron induces ferritin and RAB (Responsive to Abscisic Acid) mRNA accumulation relatively with abscisic acid (ABA) accumulation. Ferritin mRNA also accumulates in response to exogenous ABA. Synergistic experiments demonstrate that the ABA and iron responses are linked, although full expression of the ferritin genes cannot be entirely explained by an increase in ABA concentration. Inducibility of ferritin mRNA accumulation by iron is dramatically decreased in the maize ABA-deficient mutant vp2 and can be rescued by addition of exogenous ABA, confirming the involvement of ABA in the iron response in plants. Therefore, it is concluded that a major part of the iron-induced biosynthesis of ferritin is achieved through a pathway involving an increase in the level of the plant hormone ABA. The general conclusion of this work is that the synthesis of the same protein in response to the same environmental signal can be controlled by separate and distinct mechanisms in plants and animals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S. C., Arosio P., Bottke W., Briat J. F., von Darl M., Harrison P. M., Laulhère J. P., Levi S., Lobreaux S., Yewdall S. J. Structure, function, and evolution of ferritins. J Inorg Biochem. 1992 Aug 15;47(3-4):161–174. doi: 10.1016/0162-0134(92)84062-r. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987 Aug 25;26(17):5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Bostock R. M., Quatrano R. S. Regulation of Em Gene Expression in Rice : Interaction between Osmotic Stress and Abscisic Acid. Plant Physiol. 1992 Apr;98(4):1356–1363. doi: 10.1104/pp.98.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R. Proteins of iron storage and transport. Adv Protein Chem. 1990;40:281–363. doi: 10.1016/s0065-3233(08)60288-0. [DOI] [PubMed] [Google Scholar]
- Crichton R. R. Studies on the structure of ferritin and apoferritin from horse spleen. II. Chymotrypsin, subtilisin, cathepsin D and pepsin digestion of ferritin and apoferritin. Biochim Biophys Acta. 1971 Jan 19;229(1):75–82. doi: 10.1016/0005-2795(71)90320-5. [DOI] [PubMed] [Google Scholar]
- Didierjean L., Frendo P., Burkard G. Stress responses in maize: sequence analysis of cDNAs encoding glycine-rich proteins. Plant Mol Biol. 1992 Feb;18(4):847–849. doi: 10.1007/BF00020034. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W., Shafritz D. A. In vitro stimulation of apoferritin synthesis by iron. Biochim Biophys Acta. 1975 Feb 24;383(1):97–105. doi: 10.1016/0005-2787(75)90250-6. [DOI] [PubMed] [Google Scholar]
- Fürst P., Hu S., Hackett R., Hamer D. Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell. 1988 Nov 18;55(4):705–717. doi: 10.1016/0092-8674(88)90229-2. [DOI] [PubMed] [Google Scholar]
- Gómez J., Sánchez-Martínez D., Stiefel V., Rigau J., Puigdomènech P., Pagès M. A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature. 1988 Jul 21;334(6179):262–264. doi: 10.1038/334262a0. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Harford J. B. cis-trans models for post-transcriptional gene regulation. Science. 1989 Nov 17;246(4932):870–872. doi: 10.1126/science.2683086. [DOI] [PubMed] [Google Scholar]
- Lebrun M., Freyssinet G. Nucleotide sequence and characterization of a maize cytoplasmic ribosomal protein S11 cDNA. Plant Mol Biol. 1991 Aug;17(2):265–268. doi: 10.1007/BF00039502. [DOI] [PubMed] [Google Scholar]
- Lescure A. M., Proudhon D., Pesey H., Ragland M., Theil E. C., Briat J. F. Ferritin gene transcription is regulated by iron in soybean cell cultures. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8222–8226. doi: 10.1073/pnas.88.18.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobreaux S., Briat J. F. Ferritin accumulation and degradation in different organs of pea (Pisum sativum) during development. Biochem J. 1991 Mar 1;274(Pt 2):601–606. doi: 10.1042/bj2740601. [DOI] [PMC free article] [PubMed] [Google Scholar]
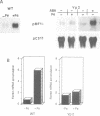
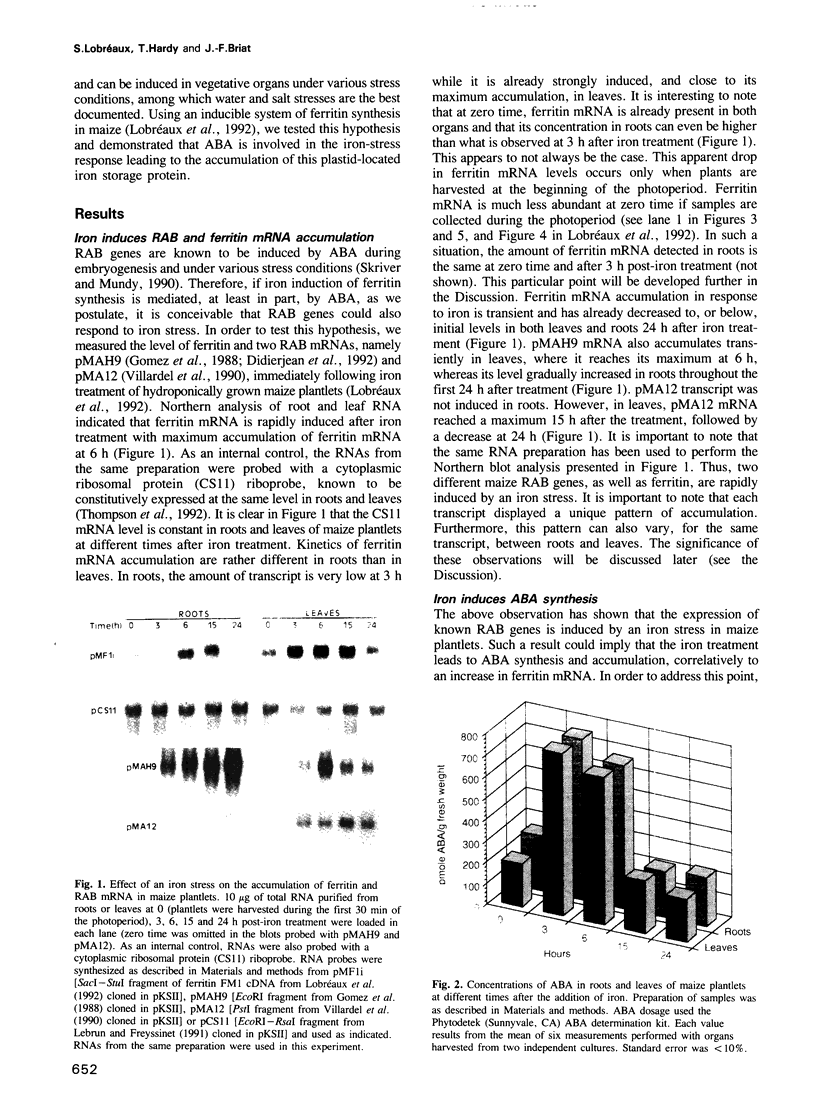
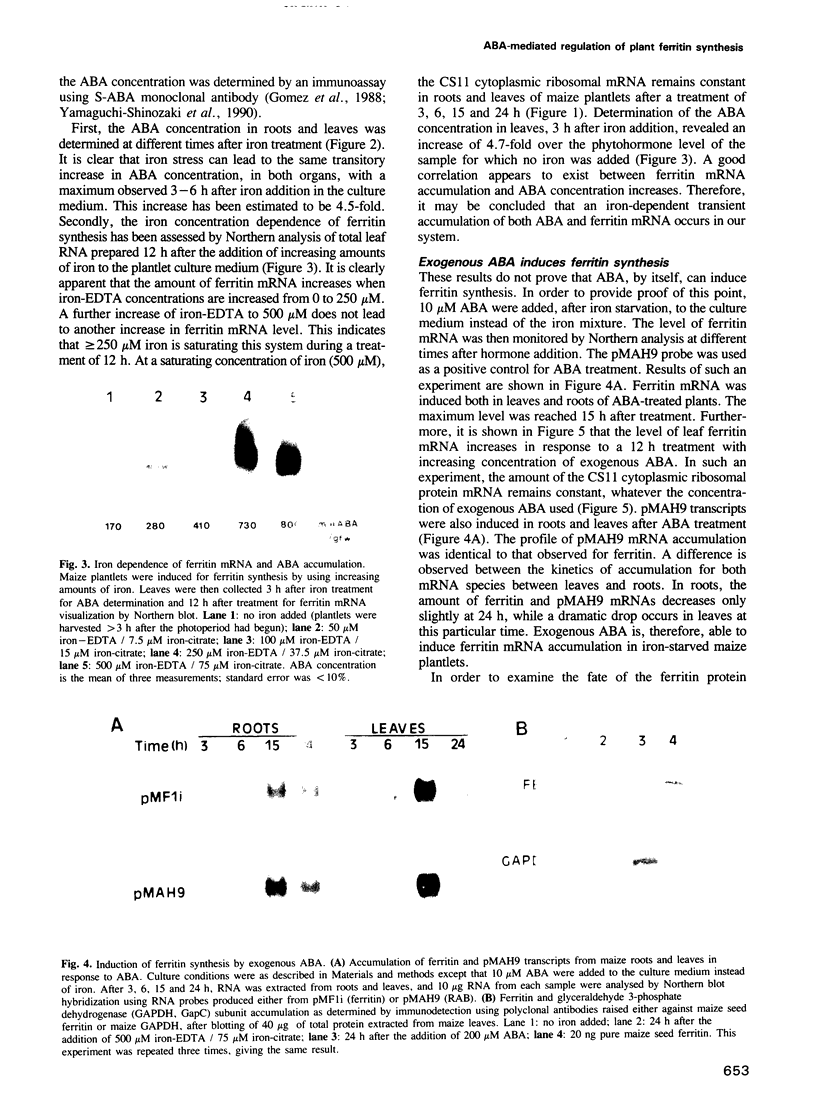
- Lobreaux S., Massenet O., Briat J. F. Iron induces ferritin synthesis in maize plantlets. Plant Mol Biol. 1992 Jul;19(4):563–575. doi: 10.1007/BF00026783. [DOI] [PubMed] [Google Scholar]
- Mundy J., Chua N. H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988 Aug;7(8):2279–2286. doi: 10.1002/j.1460-2075.1988.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Cortes H., Willmitzer L., Sanchez-Serrano J. J. Abscisic Acid Mediates Wound Induction but Not Developmental-Specific Expression of the Proteinase Inhibitor II Gene Family. Plant Cell. 1991 Sep;3(9):963–972. doi: 10.1105/tpc.3.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla M., Goday A., Vilardell J., Gómez J., Pagès M. Differential regulation of ABA-induced 23-25 kDa proteins in embryo and vegetative tissues of the viviparous mutants of maize. Plant Mol Biol. 1989 Oct;13(4):385–394. doi: 10.1007/BF00015550. [DOI] [PubMed] [Google Scholar]
- Proudhon D., Briat J. F., Lescure A. M. Iron induction of ferritin synthesis in soybean cell suspensions. Plant Physiol. 1989 Jun;90(2):586–590. doi: 10.1104/pp.90.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pēna-Cortés H., Sánchez-Serrano J. J., Mertens R., Willmitzer L., Prat S. Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland M., Briat J. F., Gagnon J., Laulhere J. P., Massenet O., Theil E. C. Evidence for conservation of ferritin sequences among plants and animals and for a transit peptide in soybean. J Biol Chem. 1990 Oct 25;265(30):18339–18344. [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence M. J., Henzl M. T., Lammers P. J. The structure of a Phaseolus vulgaris cDNA encoding the iron storage protein ferritin. Plant Mol Biol. 1991 Sep;17(3):499–504. doi: 10.1007/BF00040644. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem. 1990 Mar 25;265(9):4771–4774. [PubMed] [Google Scholar]
- Thompson M. D., Jacks C. M., Lenvik T. R., Gantt J. S. Characterization of rps17, rp19 and rpl15: three nucleus-encoded plastid ribosomal protein genes. Plant Mol Biol. 1992 Mar;18(5):931–944. doi: 10.1007/BF00019207. [DOI] [PubMed] [Google Scholar]
- Vilardell J., Goday A., Freire M. A., Torrent M., Martínez M. C., Torné J. M., Pagès M. Gene sequence, developmental expression, and protein phosphorylation of RAB-17 in maize. Plant Mol Biol. 1990 Mar;14(3):423–432. doi: 10.1007/BF00028778. [DOI] [PubMed] [Google Scholar]
- van der Mark F., Bienfait F., van den Ende H. Variable amounts of translatable ferritin mRNA in bean leaves with various iron contents. Biochem Biophys Res Commun. 1983 Sep 15;115(2):463–469. doi: 10.1016/s0006-291x(83)80167-3. [DOI] [PubMed] [Google Scholar]
- van der Mark F., van den Briel W., Huisman H. G. Phytoferritin is synthesized in vitro as a high-molecular-weight precursor. Studies on the synthesis and the uptake in vitro of the precursors of ferritin and ferredoxin by intact chloroplasts. Biochem J. 1983 Sep 15;214(3):943–950. doi: 10.1042/bj2140943. [DOI] [PMC free article] [PubMed] [Google Scholar]