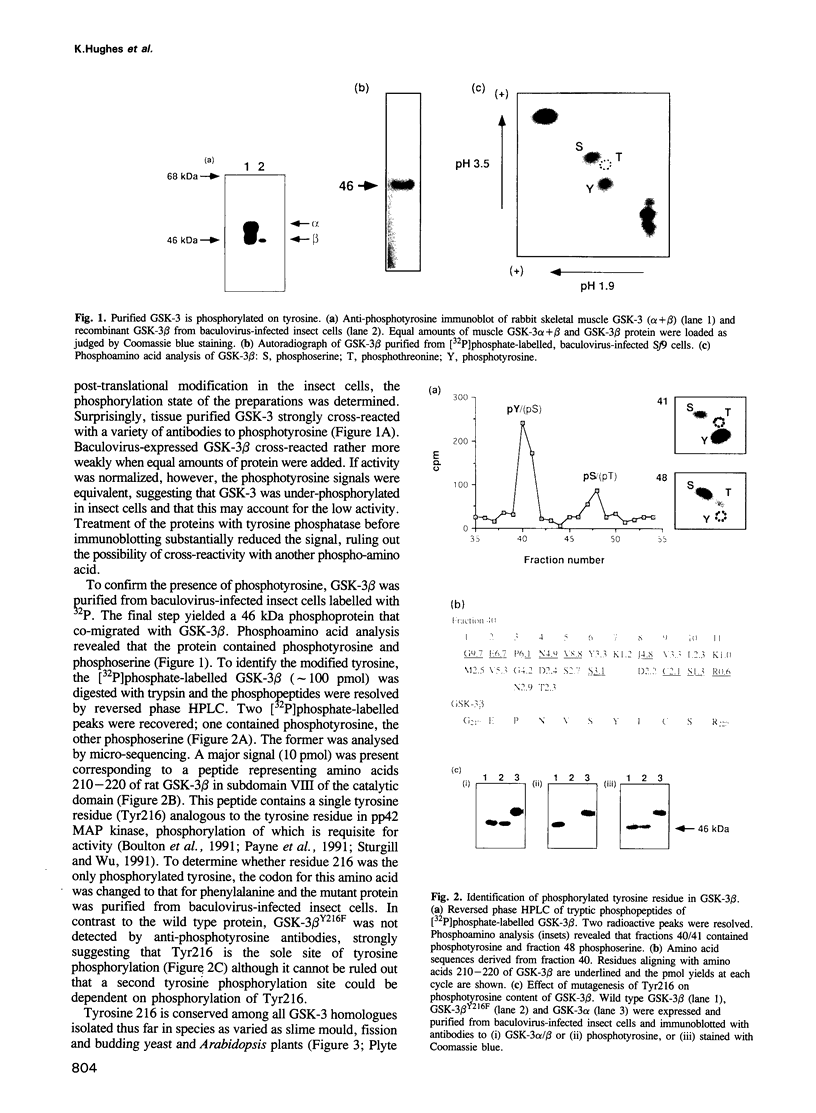
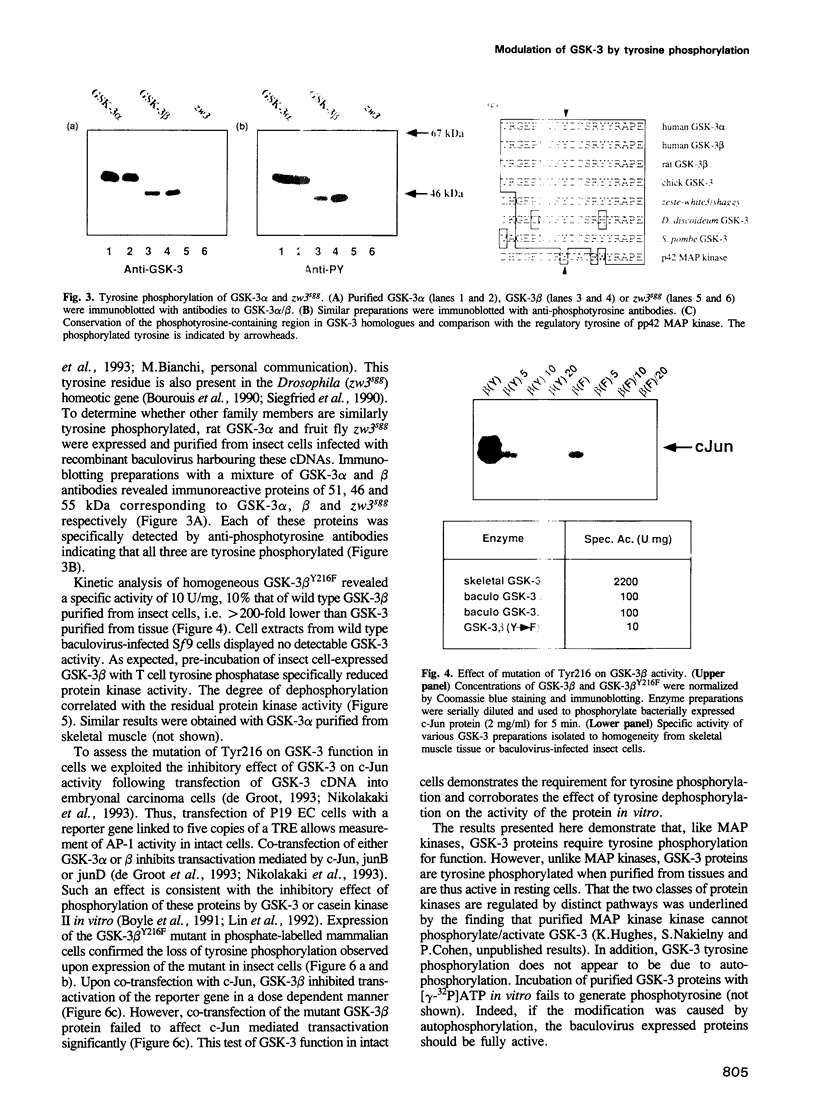
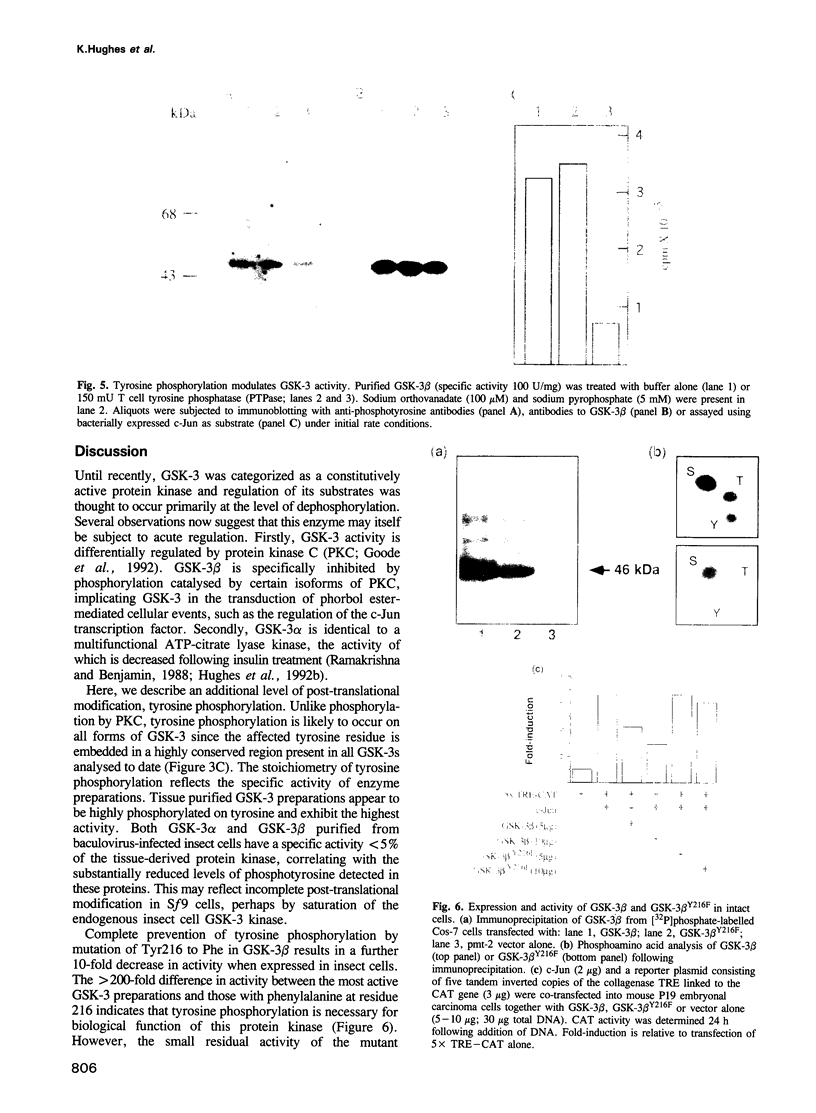
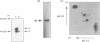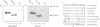Abstract
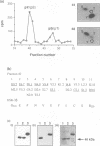
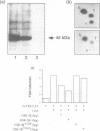
Glycogen synthase kinase-3 (GSK-3) is a protein serine kinase implicated in the cellular response to insulin. The enzyme is the mammalian homologue of the zeste-white3 (shaggy) homeotic gene of Drosophila melanogaster and has been implicated in the regulation of the c-Jun/AP-1 transcription factor. In mammals this protein serine kinase is encoded by two related genes termed GSK-3 alpha and beta. Here, we demonstrate that these two proteins and the fruit fly protein are phosphorylated on tyrosine in vivo. Moreover, GSK-3 beta activity and function are shown to be dependent on tyrosine phosphorylation. The modified tyrosine residue is conserved in all members of the GSK-3 family and is equivalent to that required for activity by mitogen-activated protein (MAP) kinases. However, unlike MAP kinases, GSK-3 is highly phosphorylated on tyrosine and thus active in resting cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binétruy B., Smeal T., Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991 May 9;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Bourouis M., Moore P., Ruel L., Grau Y., Heitzler P., Simpson P. An early embryonic product of the gene shaggy encodes a serine/threonine protein kinase related to the CDC28/cdc2+ subfamily. EMBO J. 1990 Sep;9(9):2877–2884. doi: 10.1002/j.1460-2075.1990.tb07477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Percival K. J. Transformation of yeast spheroplasts without cell fusion. Anal Biochem. 1987 Jun;163(2):391–397. doi: 10.1016/0003-2697(87)90240-5. [DOI] [PubMed] [Google Scholar]
- CRAIG J. W., LARNER J. INFLUENCE OF EPINEPHRINE AND INSULIN ON URIDINE DIPHOSPHATE GLUCOSE-ALPHA-GLUCAN TRANSFERASE AND PHOSPHORYLASE IN MUSCLE. Nature. 1964 Jun 6;202:971–973. doi: 10.1038/202971a0. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in the hormonal control of enzyme activity. Eur J Biochem. 1985 Sep 16;151(3):439–448. doi: 10.1111/j.1432-1033.1985.tb09121.x. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Embi N., Rylatt D. B., Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980 Jun;107(2):519–527. [PubMed] [Google Scholar]
- Goode N., Hughes K., Woodgett J. R., Parker P. J. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem. 1992 Aug 25;267(24):16878–16882. [PubMed] [Google Scholar]
- Hemmings B. A., Yellowlees D., Kernohan J. C., Cohen P. Purification of glycogen synthase kinase 3 from rabbit skeletal muscle. Copurification with the activating factor (FA) of the (Mg-ATP) dependent protein phosphatase. Eur J Biochem. 1981 Oct;119(3):443–451. doi: 10.1111/j.1432-1033.1981.tb05628.x. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Holm C., Meeks-Wagner D. W., Fangman W. L., Botstein D. A rapid, efficient method for isolating DNA from yeast. Gene. 1986;42(2):169–173. doi: 10.1016/0378-1119(86)90293-3. [DOI] [PubMed] [Google Scholar]
- Hughes K., Pulverer B. J., Theocharous P., Woodgett J. R. Baculovirus-mediated expression and characterisation of rat glycogen synthase kinase-3 beta, the mammalian homologue of the Drosophila melanogaster zeste-white 3sgg homeotic gene product. Eur J Biochem. 1992 Jan 15;203(1-2):305–311. doi: 10.1111/j.1432-1033.1992.tb19860.x. [DOI] [PubMed] [Google Scholar]
- Hughes K., Ramakrishna S., Benjamin W. B., Woodgett J. R. Identification of multifunctional ATP-citrate lyase kinase as the alpha-isoform of glycogen synthase kinase-3. Biochem J. 1992 Nov 15;288(Pt 1):309–314. doi: 10.1042/bj2880309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Frost J., Deng T., Smeal T., al-Alawi N., Kikkawa U., Hunter T., Brenner D., Karin M. Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell. 1992 Sep 4;70(5):777–789. doi: 10.1016/0092-8674(92)90311-y. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Caudwell F. B., Cohen P. Glycogen synthase from rabbit skeletal muscle; effect of insulin on the state of phosphorylation of the seven phosphoserine residues in vivo. Eur J Biochem. 1983 Jan 17;130(1):227–234. doi: 10.1111/j.1432-1033.1983.tb07140.x. [DOI] [PubMed] [Google Scholar]
- Patel G., Nasmyth K., Jones N. A new method for the isolation of recombinant baculovirus. Nucleic Acids Res. 1992 Jan 11;20(1):97–104. doi: 10.1093/nar/20.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L., Ang S. G., Gibson B. W., Williams D. H., Holmes C. F., Caudwell F. B., Pitcher J., Cohen P. Analysis of the in vivo phosphorylation state of rabbit skeletal muscle glycogen synthase by fast-atom-bombardment mass spectrometry. Eur J Biochem. 1988 Aug 15;175(3):497–510. doi: 10.1111/j.1432-1033.1988.tb14222.x. [DOI] [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Cyclic nucleotide-independent protein kinase from rat liver. Purification and characterization of a multifunctional protein kinase. J Biol Chem. 1985 Oct 5;260(22):12280–12286. [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Insulin action rapidly decreases multifunctional protein kinase activity in rat adipose tissue. J Biol Chem. 1988 Sep 5;263(25):12677–12681. [PubMed] [Google Scholar]
- Ramakrishna S., Murthy K. S., Benjamin W. B. Effect of insulin on ATP-citrate lyase phosphorylation: regulation of peptide A and peptide B phosphorylations. Biochemistry. 1989 Jan 24;28(2):856–860. doi: 10.1021/bi00428a067. [DOI] [PubMed] [Google Scholar]
- Rylatt D. B., Aitken A., Bilham T., Condon G. D., Embi N., Cohen P. Glycogen synthase from rabbit skeletal muscle. Amino acid sequence at the sites phosphorylated by glycogen synthase kinase-3, and extension of the N-terminal sequence containing the site phosphorylated by phosphorylase kinase. Eur J Biochem. 1980 Jun;107(2):529–537. [PubMed] [Google Scholar]
- Siegfried E., Perkins L. A., Capaci T. M., Perrimon N. Putative protein kinase product of the Drosophila segment-polarity gene zeste-white3. Nature. 1990 Jun 28;345(6278):825–829. doi: 10.1038/345825a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sturgill T. W., Wu J. Recent progress in characterization of protein kinase cascades for phosphorylation of ribosomal protein S6. Biochim Biophys Acta. 1991 May 17;1092(3):350–357. doi: 10.1016/s0167-4889(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Vandenheede J. R., Yang S. D., Goris J., Merlevede W. ATP x Mg-dependent protein phosphatase from rabbit skeletal muscle. II. Purification of the activating factor and its characterization as a bifunctional protein also displaying synthase kinase activity. J Biol Chem. 1980 Dec 25;255(24):11768–11774. [PubMed] [Google Scholar]
- Woodgett J. R. A common denominator linking glycogen metabolism, nuclear oncogenes and development. Trends Biochem Sci. 1991 May;16(5):177–181. doi: 10.1016/0968-0004(91)90071-3. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Cohen P. Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5). Biochim Biophys Acta. 1984 Aug 14;788(3):339–347. doi: 10.1016/0167-4838(84)90047-5. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990 Aug;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R. Use of peptide substrates for affinity purification of protein-serine kinases. Anal Biochem. 1989 Aug 1;180(2):237–241. doi: 10.1016/0003-2697(89)90423-5. [DOI] [PubMed] [Google Scholar]