Abstract
Methylation is a versatile reaction involved in the synthesis and modification of biologically active molecules, including RNAs. N6-methyl-threonylcarbamoyl adenosine (m6t6A) is a post-transcriptional modification found at position 37 of tRNAs from bacteria, insect, plants, and mammals. Here, we report that in Escherichia coli, yaeB (renamed as trmO) encodes a tRNA methyltransferase responsible for the N6-methyl group of m6t6A in tRNAThr specific for ACY codons. TrmO has a unique single-sheeted β-barrel structure and does not belong to any known classes of methyltransferases. Recombinant TrmO employs S-adenosyl-L-methionine (AdoMet) as a methyl donor to methylate t6A to form m6t6A in tRNAThr. Therefore, TrmO/YaeB represents a novel category of AdoMet-dependent methyltransferase (Class VIII). In a ΔtrmO strain, m6t6A was converted to cyclic t6A (ct6A), suggesting that t6A is a common precursor for both m6t6A and ct6A. Furthermore, N6-methylation of t6A enhanced the attenuation activity of the thr operon, suggesting that TrmO ensures efficient decoding of ACY. We also identified a human homolog, TRMO, indicating that m6t6A plays a general role in fine-tuning of decoding in organisms from bacteria to mammals.
INTRODUCTION
Methylation is a versatile and ubiquitous reaction involved in the synthesis and modifications of biological molecules including DNA, RNA, proteins, and other compounds. Methylation of DNA and histones is an essential aspect of epigenetic regulation of gene expression (1). Methylation of RNA and proteins plays modulatory roles in the biochemical and biophysical properties of these molecules (2,3). In addition, methylation is also involved in synthesis and/or conversion of various cellular metabolites, including some toxic compounds (4,5).
Methylation of biomolecules is catalyzed by methyltransferase (or methylase). Several families of methyltransferases exist, and they employ a variety of methyl-group donors, including methylcobalamin, methyl- and methylene-tetrahydrofolate, and S-adenosyl-L-methionine (AdoMet) (6). AdoMet-dependent methyltransferases, the largest family, are categorized into seven classes according to their structural motifs (6–8). Methyltransferases in this family frequently bear AdoMet-binding motifs, which have widely divergent amino acid sequences (9), suggesting that novel AdoMet-methyltransferases still remain to be discovered.
tRNAs contain numerous post-transcriptional modifications. The decoding abilities of tRNAs are modulated by modified bases in the anticodon region (10). The first letter of the anticodon (position 34) is subject to various chemical modifications, known as “wobble modifications,” which play an important role in regulating decoding capability. In addition, bulky modifications are frequently introduced at position 37, which is 3′-adjacent to the anticodon. The modified bases at position 37 stabilize codon–anticodon pairing via base-stacking interactions in the decoding center of the ribosome (11,12).
N 6-threonylcarbamoyladenosine (t6A) (Supplementary Figure S1) and its derivatives are universal modified bases present at position 37 of tRNAs responsible for codons starting with A (ANN codons) from all domains of life (13). The bulky structure of t6A supports formation of the canonical U-turn structure of the anticodon loop (14) by preventing U33-A37 base pairing (15). t6A plays a crucial role in maintaining decoding accuracy during protein synthesis, and it is also required for aminoacylation of tRNAs (16), tRNA binding to the A-site codon (17), efficient translocation (18), reading-frame maintenance (19), and prevention of leaky scanning of initiation codons and read-through of stop codons (20). Biogenesis of t6A has been extensively studied. In bacterial systems, the formation of t6A on tRNA was successfully reconstituted in vitro using four essential enzymes, TsaC (YrdC), TsaD (YgjD), TsaB (YeaZ), and TsaE (YjeE), in the presence of the substrates L-threonine, ATP, and bicarbonate (21). In the first step of this reaction, TsaC employs L-threonine, bicarbonate, and ATP to synthesize threonylcarbamoyl-adenylate (TC-AMP), an active intermediate in t6A formation (22). Next, the three other enzymes (TsaD, TsaB, and TsaE) catalyze nucleophilic attack of the N6-amino group of A37 on the carbonyl group of TC-AMP to synthesize t6A, releasing AMP as a leaving group. In eukaryotes and archaea, the TsaC/YrdC homolog Sua5 and several components of the KEOPS/EKC complex, including Kae1, Pcc1, and Bud32, are involved in formation of t6A (19,23–28). On the other hand, in mitochondria, the TsaD/YgjD homolog Qri7 is the sole enzyme responsible for the second step of t6A formation (29,30).
Although the presence of t6A in cellular tRNAs from E. coli and yeast has been well documented for more than four decades, we recently showed that t6A is a hydrolyzed artifact of cyclic t6A (ct6A) (Supplementary Figure S1), a bona fide modified base of E. coli tRNAs (31). ct6A is widely distributed among tRNAs from a certain group of bacteria, fungi, plants, and some protists, whereas t6A is present in tRNAs of mammals, archaea, and other group of bacteria. In E. coli cells, almost all t6A is converted to ct6A via a dehydration reaction catalyzed by TcdA. Thus, ct6A is an additional modification of t6A that enhances tRNA-decoding activity. In addition to ct6A, two other derivatives of t6A exist: 2-methylthio-N6-threonylcarbamoyladenosine (ms2t6A) (Supplementary Figure S1), found in tRNALys from Bacillus subtilis and mammals (32,33), and N6-methyl-N6-threonylcarbamoyladenosine (m6t6A) (Figure 1A), found in tRNAs from bacteria, fly, plants, and rat (Figure 1B) (34,35). The methylthiolation of ms2t6A is required for the accurate decoding of lysine codons. B. subtilis MtaB (36,37) and human Cdkal1 (38) serve as the methylthiotransferases responsible for introducing ms2t6A into tRNA. In addition, variation in Cdkal1 is associated with risk of type 2 diabetes (38). In E. coli, m6t6A is present at position 37 of tRNAThr1(GGU) and tRNAThr3(GGU), both of which decipher ACY codons (39,40) (Figure 1B and Supplementary Figure S2), whereas the isoacceptors tRNAThr2(CGU) and tRNAThr4(UGU) contain ct6A37 (31) (Supplementary Figure S2).
Figure 1.
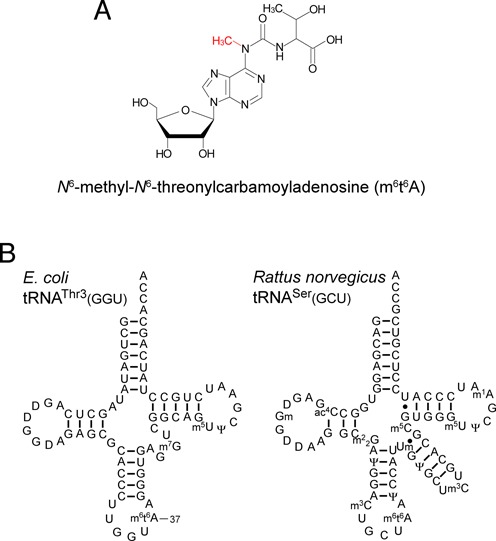
N6-methyl-N6-threonylcarbamoyl adenosine (m6t6A) and tRNAs bearing m6t6A. (A) Chemical structure of m6t6A. The N6-methyl group is shown in red. (B) Secondary structures of E. coli tRNAThr3(GGU) and Rattus norvegicus tRNASer (GCU) with post-transcriptional modifications: dihydrouridine (D), N6-methyl-N6-threonylcarbamoyladenosine (m6t6A), 7-methylguanosine (m7G), 5-methyluridine (m5U), pseudouridine (Ψ), N4-acetylcytidine (ac4C), 2′-O-methylguanosine (Gm), N2, N2-dimethylguanosine (m22G), 3-methylcytidine (m3C), 2′-O-methyluridine (Um), 5-methylcytidine (m5C), and 1-methyladenosine (m1A).
N 6-methylation of m6t6A plays a role in efficient attenuation of the thrABC operon (41). The expression level of thrABC is regulated by translation of a leader peptide encoded by thrL, which contains consecutive ACC codons. The mutant strain tsaA, which lacks N6-methylation of m6t6A37, exhibits relieved attenuation of thrABC (41), probably due to inefficient translation of the ACC codons in ThrL. The tsaA gene is predicted to be located at 4.6 min in the E. coli genome (41). Although the methyl donor responsible for N6-methylation of m6t6A37 was shown to be AdoMet (41), no AdoMet-methyltransferase was identified near 4.6 min in the E. coli genome during the 15 years following that discovery.
We have been screening for genes responsible for RNA modifications using ‘ribonucleome analysis’ (42), a reverse-genetics approach combined with mass spectrometry. The screen has identified a number of genes involved in tRNA modifications (43–48) and rRNA modifications (49,50). In this study, by performing ribonucleome analysis in E. coli, we demonstrate that tsaA is found to be yaeB which is responsible for N6-methylation of m6t6A in tRNAThr. Using AdoMet as a methyl donor, recombinant YaeB methylated the N6 position of t6A to form m6t6A in tRNAThr. Therefore, we renamed YaeB as TrmO (tRNA-methyltransferase O). TrmO, which contains a single-sheeted β-barrel structure and does not belong to any known class of AdoMet methyltransferases, is the founding member of a novel class (VIII) of AdoMet-dependent methyltransferases. We here characterize TrmO in terms of evolutionary distribution, tRNA-decoding ability and substrate specificity. In addition, we identified a human homolog TRMO.
MATERIALS AND METHODS
Strains and medium
E. coli genomic-deletion strains (OCL/R-series) derived from MG1655sp (MG1655 rpsL polA12 Zih::Tn10), each lacking about 20 kbp (∼20 genes), were kindly provided by Dr. Junichi Kato. OCR36 [MG1655 Δ(OCR36–1)::Kan/p36–4] specifically lacked m6t6A. A series of single-deletion strains with the kanamycin-resistance marker (Kmr) were obtained from the Genetic Stock Research Center, National Institute of Genetics, Japan. The ΔtrmO::Cmr strain was constructed by one-step gene disruption (51) utilizing the chloramphenicol-resistance marker (Cmr) from pBT (Invitrogen). The ΔtrmO/ΔtcdA double-deletion strain was constructed by P1 transduction of ΔtcdA::Kmr to the ΔtrmO::Cmr strain. The primers are listed in Supplementary Table S2. E. coli strains were grown in 5 ml of LB medium at 37°C overnight.
RNA mass spectrometry
Total RNA was extracted from the cells by the AGPC method (52) using ISOGEN (Nippon Gene, Japan) or Tripure (Roche). Nucleoside analysis of the extracted RNAs was performed by LC/MS using an LCQ Advantage ion-trap mass spectrometer (Thermo Fisher Scientific) equipped with an ESI source and an HP1100 liquid chromatography system (Agilent Technologies), as described previously (42). RNA fragments of the isolated tRNAs digested by RNases were analyzed by capillary LC/nano ESI-MS as described (31,42,53). In brief, 1 pmol of isolated tRNA was digested with 50 units of RNase T1 (Epicentre) in 20 mM NH4OAc (pH 5.3) at 37 °C for 30 min. The digests were analyzed using an LTQ Orbitrap mass spectrometer (Thermo Scientific) with a nano-electrosprayer connected with a splitless nanoflow HPLC system (DiNa, KYA Technologies).
Isolation of individual tRNAs
For each sample, the cell pellet was resuspended in 5 ml RNA extraction buffer [50 mM NaOAc (pH 5.2) and 10 mM Mg(OAc)2 (pH 5.2)], mixed with 5 ml water-saturated phenol, and vigorously stirred for 60 min. The aqueous phase was separated by centrifugation and washed with chloroform. RNA was extracted with an equal volume of Tripure (Roche) and about 0.2 volume of chloroform, and then precipitated with 2-propanol. The RNA pellet was dissolved in deionized water and precipitated again with ethanol. The resultant pellet was rinsed with 80% ethanol and dried. Individual tRNAs were isolated by reciprocal circulating chromatography (RCC) using an automatic RCC device, basically following the previously described method (54). The 5′-terminal ethylcarbamate amino-modified DNA probes, 5′-TGGTGCTGATACCCAGAGTCGAACTGGGGA-3′ for E. coli tRNAThr1(GGU), 5′-TGGTGCTGATAGGCAGATTCGAACTGCCGA-3′ for E. coli tRNAThr3(GGU), and 5′-TGGATTAGCAGTCCATCGCCTTAACCACTCGGCCA-3′ for human tRNASer(GCU) were covalently immobilized on NHS-activated Sepharose 4 Fast Flow (GE Healthcare). DNA resins were packed into the custom-made tips attached to a multichannel head on an RCC device.
Nucleoside preparation
Total nucleosides containing ct6A were usually prepared by neutral one-step digestion of total RNA (31). Total RNA (40 μg) was digested at 37°C for 1.5 h in 20 mM HEPES-KOH (pH 7.1) containing 0.1 U Nuclease P1 (Wako Pure Chemical Industries, Ltd.) and 0.08 U bacterial alkaline phosphatase (BAP) (E. coli C75, Wako Pure Chemical Industries, Ltd.). For the analysis of the trmO mutation, total RNA was completely digested by three-step digestion, as follows. Total RNA (40 μg) was incubated at 37°C for 1 h in 25 mM NH4OAc (pH 5.3) containing 0.1 unit Nuclease P1. Thereafter, 0.1 volume of 1 M ammonium bicarbonate (pH 8.0) with 0.127 units of phosphodiesterase I (PDase I) (Worthington Biochemical Corporation) was added to the mixture, followed by incubation at 37°C for 1 h. Finally, 0.08 U BAP was added, and the sample was incubated at 37°C for 1.5 h. Prior to use, Nuclease P1, BAP and phosphodiesterase I were dialyzed against deionized water and stored at -30°C.
Preparation of t6A-containing tRNA transcript
E. coli tRNAThr3(GGU), tRNAThr4(UGU) and their derivatives were prepared by in vitro transcription using T7 RNA polymerase (55). Recombinant T7 RNA polymerase with N-terminal His-tag was expressed as a soluble form in E. coli, and purified with nickel-cheleting affinity chromatography in our labolatory. DNA templates for in vitro transcription were constructed by PCR using synthetic oligo DNAs (Supplementary Table S2). In vitro transcription was performed at 37°C for 3–6 h in a reaction mixture containing 40 mM Tris-HCl (pH 8.0), 5 mM dithiothreitol, 2 mM spermidine, 24 mM MgCl2, 0.01% Triton X-100, 2 mM NTPs, 10 mM 5′-monophosphoguanosine (GMP), template DNA, and T7 RNA polymerase. Each transcript was purified by electrophoresis on a 10% polyacrylamide gel containing 7 M urea, and then eluted in buffer consisting of 300 mM sodium acetate (pH 5.2), 0.1% SDS, and 1 mM EDTA (pH 8.0).
In vitro reconstitution of t6A was carried out essentially as described (21,56). t6A37 was introduced to each tRNA transcript at 37°C for 3 h in a 200 μl reaction mixture consisting of 25 μg of tRNA transcript, 1.5 μM each of the t6A-modifying enzymes (YrdC, YgjD, YeaZ and YjeE), 1 mM L-threonine, 2 mM ATP, 25 mM NaHCO3, 100 mM HEPES-KOH (pH7.5), 300 mM KCl, 20 mM MgCl2, and 5 mM DTT. The modified tRNA was extracted with ISOGEN (Nippon Gene, Japan) or Tripure (Roche), and then dialyzed against deionized water. The degree of t6A in each tRNA was analyzed by LC/MS RNA fragment analysis, as described above.
Expression and purification of recombinant proteins
An E. coli strain carrying plasmid pCA24N, for expression of soluble recombinant TrmO protein fused to a N-terminal 6×His tag, was obtained from the ASKA clone collection [NBRP (NIG, Japan): E. coli] (57). This strain was cultivated in the presence of 0.1 mM IPTG to induce protein expression, and the expressed protein was purified using Ni-NTA beads (QIAGEN) packed in an open column. The pooled protein was dialyzed against a buffer consisting of 50 mM Tris–HCl (pH 7.5), 1 mM DTT, and 100 mM KCl.
The cDNA encoding the human homolog TRMO, obtained by nested RT-PCR using specific primers (Supplementary Table S2) from total RNA of HeLa cells, was cloned into the NheI and SalI sites of vector pET28a to yield pET28a-TRMO (Novagen). BL21 (DE3) Rosetta was transformed with pET28a-TRMO, and the transformant strain was cultured at 37°C. When OD600 reached 0.7, expression of the recombinant protein was induced with 0.1 mM IPTG at 18°C for 3 h. The harvested cells were lysed by sonication in a buffer containing 25 mM Tris-HCl (pH 8.0), 300 mM NaCl, 10% glycerol, and 0.2 mM phenylmethylsulfonyl fluoride (PMSF). Recombinant TRMO was purified with Ni-NTA beads (QIAGEN). TRMO was eluted with 250 mM imidazole, and then passed through a PD-10 column (GE Healthcare) in cell lysis buffer containing 1 mM DTT.
Gel retardation assay
The gel retardation assay was performed essentially as described (43,45). Recombinant TrmO (15–45 pmol) and in vitro transcribed tRNA (15 pmol) were incubated at 37°C for 30 min in a 10 μl mixture containing 50 mM Tris-HCl (pH 8.0), 5 mM MgCl2, 100 mM KCl, and 2 mM spermine. The tRNA–protein complex was resolved by 4% native polyacrylamide gel electrophoresis with a running buffer consisting of 50 mM Tris and 5 mM Mg(OAc)2 (pH 8.0, adjusted with acetic acid). After electrophoresis, the gel was stained with SYBR gold (Invitrogen) to visualize tRNA, and then stained with Coomassie Brilliant Blue (Nacalai Tesque) to visualize the protein.
In vitro N 6-methylation of t6A by TrmO
For in vitro methylation (as shown in Figure 3B–D), 1 μM tRNAThr3(GGU) with or without t6A was incubated at 37°C for 1 h in a 20 μl reaction mixture containing 50 mM HEPES-KOH (pH 6.7), 100 mM KCl, 5 mM MgCl2, 1 mM DTT, 10 μM TrmO, and 1 mM AdoMet. Modified tRNA extracted with Tripure was digested with RNase and analyzed by LC/MS as described above. For methylation by TRMO (Figure 6E and F), the same conditions were used, except that the reaction volume was 100 μl and the enzyme was 1 μM TRMO. For a tRNA mutation study (Figure 4), 1 μM tRNAs bearing t6A37 were incubated at 37°C for 5 min in a 10 μl reaction mixture containing 50 mM HEPES-KOH (pH 6.7), 100 mM KCl, 5 mM MgCl2, 1 mM DTT, 0.1 μM TrmO, and 25 μM [14C-methyl]AdoMet (1.74 Gbq/mmol, Perkin Elmer). The modified tRNAs were extracted with phenol–chloroform–isoamyl alcohol (Nacalai Tesque) and spotted on filter paper (Whatman 3MM). The filter papers were washed three times with 5% trichloroacetic acid, and then soaked with 100% ethanol. After drying, radioactivity on the filter papers was measured by liquid scintillation counting.
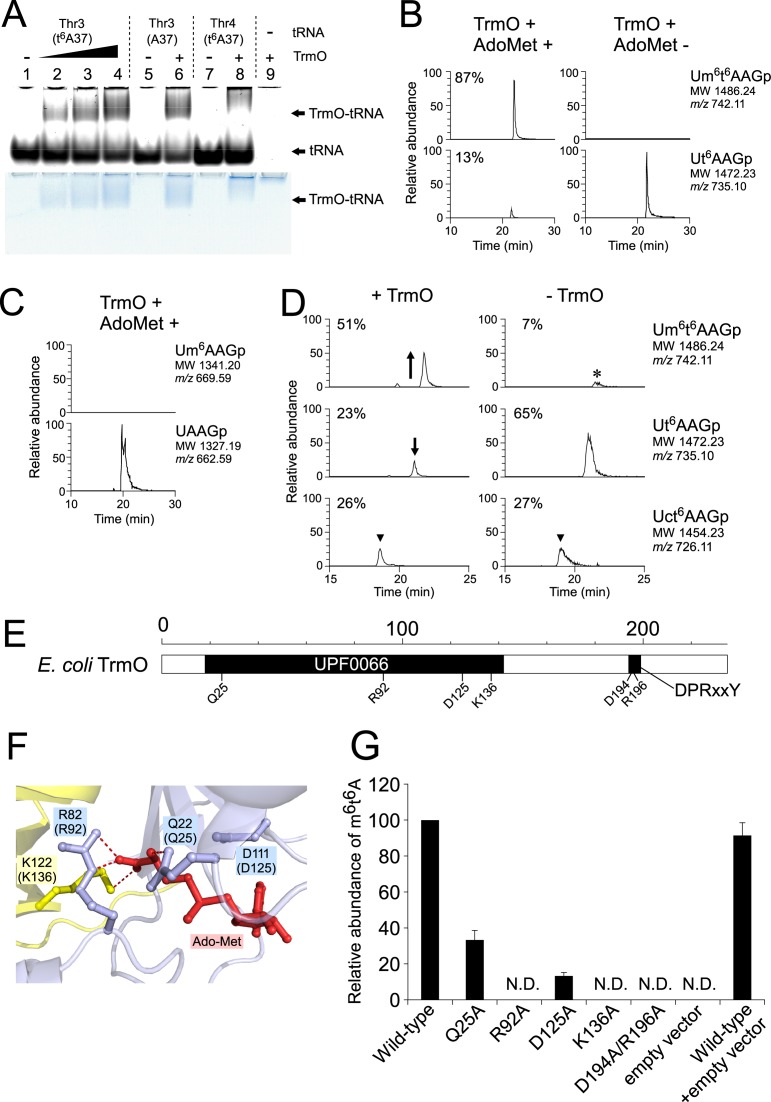
Figure 3.
TrmO is an AdoMet-dependent methyltransferase responsible for m6t6A formation. (A) Physical interaction of TrmO and tRNAs by gel-retardation assay. The polyacrylamide gel was stained with SYBR gold (upper panel) and Coomassie brilliant blue (lower panel). Lanes 1–4 represent tRNAThr3(GGU) (t6A37) with 0, 15, 30, and 45 pmol TrmO; lanes 5 and 6 represent tRNAThr3(GGU) (A37) without or with TrmO (45 pmol). Lanes 7 and 8 represent tRNAThr4(UGU) (t6A37) without or with TrmO (45 pmol). Lane 9 represents TrmO (45 pmol) only. All conditions contained 15 pmol tRNA. (B) In vitro methylation by TrmO. E. coli tRNAThr3(GGU) transcript bearing t6A37 was incubated in the presence of recombinant TrmO with (left panels) or without (right panels) AdoMet. Upper and lower panels: mass chromatograms showing doubly-charged negative ions of m6t6A-containing tetramer (Um6t6AAGp; MW 1486.24, m/z 742.11) and t6A-containing tetramer (Ut6AAGp; MW 1472.23, m/z 735.10), respectively. (C) The same experiment as in B, using E. coli tRNAThr3(GGU) transcript bearing A37 (without t6A). No methylation took place in this tRNA, even in the presence of both TrmO and AdoMet. (D) The same experiment as in B, using E. coli tRNAThr3(GGU) isolated from the ΔtrmO strain. The peak marked with an asterisk represents the m6t6A-containing fragment derived from carryover of wild-type E. coli tRNAThr3(GGU) bound to the oligo DNA probe. (E) Schematic domain structure of E. coli TrmO. Scale denotes amino acid numbering. (F) Close-up view of the AdoMet-binding site in the crystal structure of A. fulgidus AF0241. Bound AdoMet is shown as a red stick. Four amino acid residues that interact with AdoMet are colored. Note that only K122 (yellow) is a residue from the other subunit. The amino acid numbers of E. coli TrmO are shown in parentheses. Possible hydrogen bonds are shown as dash lines. (G) Mutation study of trmO. The ΔtrmO strain was transformed with plasmid-encoded trmO wild type or mutants. The wild-type strain (BW25113) was also transformed with the empty vector (pHSG415r). The height of the mass chromatogram for the proton adduct of m6t6A (m/z 427) in each transformant was divided by that of m2A (m/z 282), and the relative ratio was normalized to the result of ΔtrmO complemented with wild-type trmO. The bar graph show the average value of three experiments, and error bars indicate the SD values. N.D., not detected.
Figure 6.
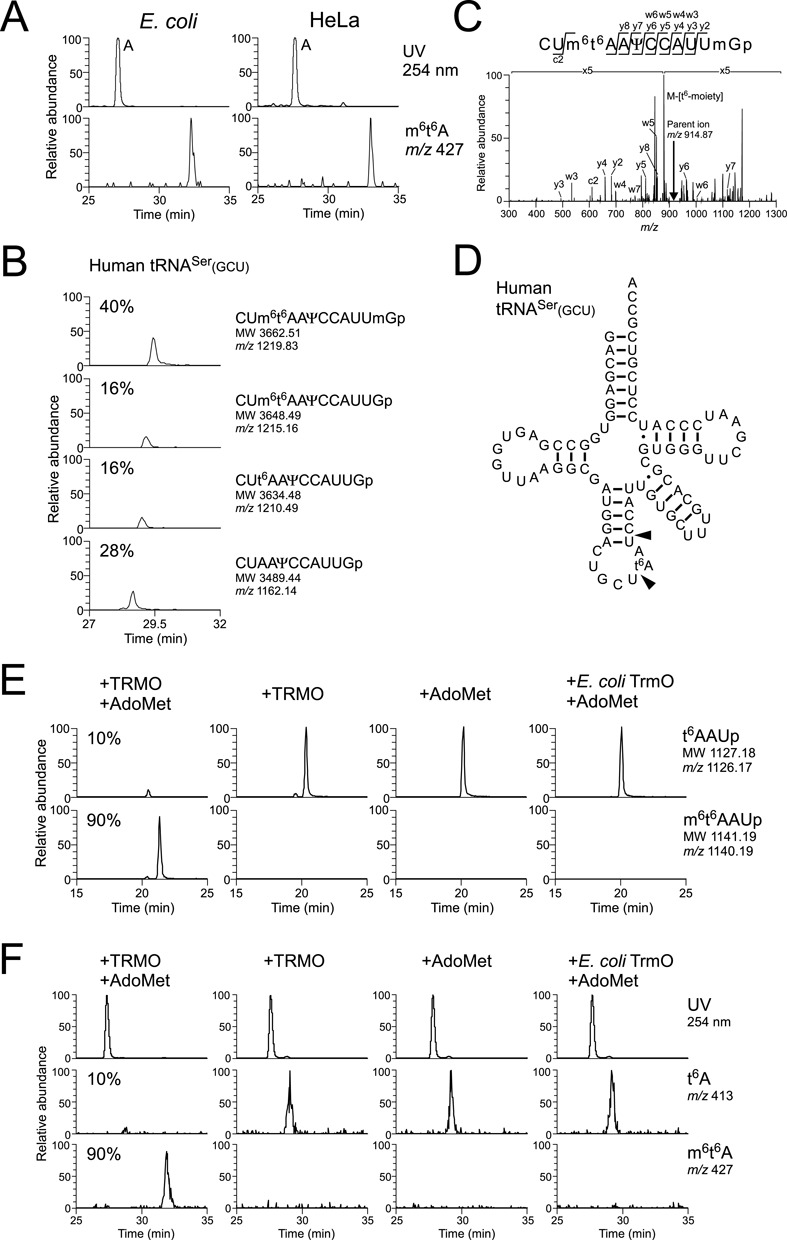
Human homolog TRMO catalyzes m6t6A formation in tRNASer(GCU). (A) LC/MS nucleoside analysis of wild-type E. coli (left panels) and HeLa cells (right panels). Upper panels show UV traces at 254 nm, and lower panels show mass chromatograms for the proton adduct of m6t6A (m/z 427). (B) LC/MS analysis of RNase T1 digested human cytoplasmic tRNASer(GCU). All panels are mass chromatograms for triply-charged negative ions. From upper to bottom panels, m6t6A37- and Um43-containing fragment (CUm6t6AAΨCCAUUmGp; MW 3662.51, m/z 1219.83), m6t6A-containing fragment (CUm6t6AAΨCCAUUGp; MW 3648.49, m/z 1215.16), t6A-containing fragment (CUt6AAΨCCAUUGp; MW 3634.48, m/z 1210.49) and unmodified fragment (CUAAΨCCAUUGp; MW 3489.44, m/z 1162.14). Ψ39 and Um44 are predicted from the sequence of rat tRNASer(GCU). (C) CID spectrum of m6t6A- and Um-containing fragment of human cytoplasmic tRNASer(GCU). (D) Transcript of human cytoplasmic tRNASer(GCU) bearing t6A37 used for in vitro reconstitution of m6t6A37. Arrowheads indicate cleavage sites by RNase A. (E) In vitro methylation by recombinant TRMO. Human cytoplasmic tRNASer(GCU) transcript bearing t6A37 was incubated with TRMO and AdoMet (left most panels), TRMO only (left middle panels), AdoMet only (right middle panels) or E. coli TrmO and AdoMet (right most panels). Upper and lower panels show mass chromatograms for singly charged negative ions of t6A-containing trimer (t6AAUp; MW 1127.18, m/z 1126.17) and m6t6A-containing trimer (m6t6AAUp; MW 1141.19, m/z 1140.19), respectively. (F) LC/MS nucleoside analysis of human cytoplasmic tRNASer(GCU) transcript bearing t6A37, which was incubated with TRMO and AdoMet (left most panels), TRMO only (left middle panels), AdoMet only (right middle panels), or E. coli TrmO and AdoMet (right most panels). Upper panels show UV trace at 254 nm. Middle and lower panels show mass chromatograms for the proton adduct of t6A (m/z 413) and m6t6A (m/z 427), respectively.
Figure 4.
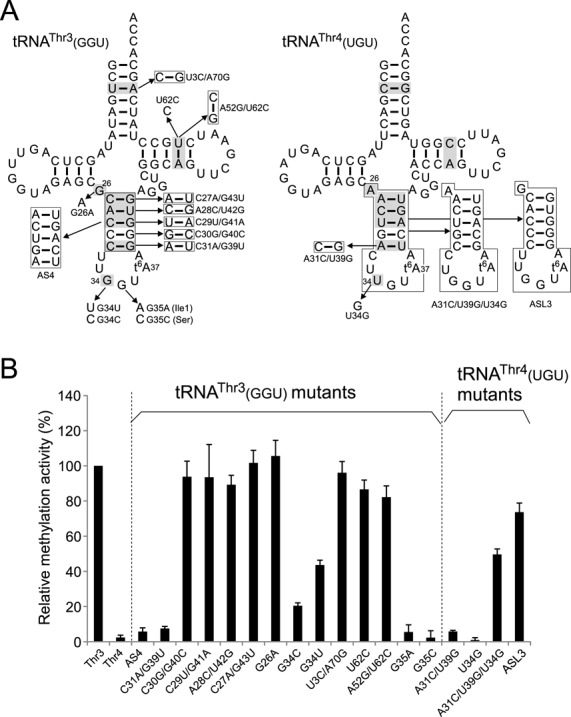
Mutation study to elucidate the mechanism of tRNA discrimination by TrmO. (A) Variants of E. coli tRNAThr3(GGU) (left-hand side) and tRNAThr4(UGU) (right-hand side) used in this study. The numbering system of tRNA is based on the tRNA compilation (79). Bases common to tRNAThr1,3 but different in tRNAThr2,4 are highlighted in gray in each tRNA. (B) Relative methylation activities of TrmO for tRNA variants normalized by the activity of wide-type tRNAThr3(GGU) (Thr3). Radioactivity of the [14C] methyl group incorporated into each tRNA variant over the course of a 5 min reaction was measured. The averaged values of three independent experiments, with SD values, are shown.
Plasmid complementation
The E. coli trmO gene with 5′ and 3′ flanking regions, probably including its original promoter and terminator, was amplified by PCR using specific primers (Supplementary Table S2). The PCR product was cloned into the EcoRI and XhoI sites of pHSG415r, which contains a pSC101 origin, to yield pHSG-TrmO. Mutations were introduced into pHSG-TrmO by site-directed mutagenesis using PrimeSTAR HS DNA polymerase (TaKaRa) using specific primers (Supplementary Table S2). Each mutant was checked by Sanger sequencing. The ΔtrmO strain was transformed with each plasmid, and the resultant transformants were cultivated to mid-log phase. Total RNA from each construct was digested to nucleosides and analyzed by LC/MS, as described above. The height of the mass chromatogram for the proton adduct of m6t6A (m/z 427) was divided by the height of the mass chromatogram for m2A (m/z 282), and the relative ratio was normalized to the result from the ΔtrmO complemented with wild-type pHSG-TrmO.
Luciferase assay
The luciferase assay was performed as described previously (50). A reporter containing the thrL attenuator followed by firefly luciferase gene (ThrL-Luc), and a control reporter without the attenuator (Luc), were constructed as follows. The attenuation site of the thr operon including the promoter, leader peptide (thrL), and terminator was amplified by PCR using specific primers (Supplementary Table S2). The ORF of firefly luciferase was also amplified using specific primers (Supplementary Table S2). These PCR products and linearized pBR322 digested with EcoRI and BamHI were ligated using an In-Fusion HD Cloning Kit (TaKaRa). The control reporter without the attenuator (Luc) was generated by deleting the attenuation site using specific primers (Supplementary Table S2) and the PrimeSTAR HS DNA polymerase (TaKaRa). The sequences of the resultant plasmids were verified by Sanger sequencing.
E. coli wild-type (BW25113), ΔtrmO, ΔtcdA and ΔtrmO/ΔtcdA strains were transformed with each of the reporters. Each transformant was cultivated in 2 ml LB liquid medium at 37°C. When the cultures reached 0.4–0.6 OD600, 0.5 ml of the culture was harvested and resuspended in 100 μl lysis buffer [50 mM HEPES-KOH (pH 7.6), 100 mM KCl, 10 mM MgCl2, 7 mM β-mercaptoethanol, and 400 μg/ml lysozyme]. Cell lysates were prepared by the freeze-thaw method and cleared by centrifugation (15 min; 15,000 rpm, 4°C). Cleared lysates (5 μl) were analyzed with a GLOMAX96 Microplate Luminometer (Promega) using the Dual-Luciferase Reporter Assay System (Promega). The efficiency of attenuation was measured by the chemiluminescence of firefly luciferase, normalized to the OD600 of the culture.
Phylogenetic analysis and taxonomic distribution of yaeB
The number of yaeB homologs in each of the 955 taxa present in the SEED database (58) were retrieved from the “COG1720” subsystem. All taxa with a yaeB homolog are listed in Supplementary Table S1. Taxonomic distribution of yaeB homologs, pruned to the level of orders, is shown in Supplementary Figure S4. The data were formatted to contain only NCBI taxonomic identification numbers and the number of occurrences of yaeB in each taxon. The amino acid sequences of all COG1720 proteins were downloaded from SEED and converted to the nexus format using the University of Florida's High Performance Cluster (UFHPC) instance of Galaxy (galaxy.hpc.ufl.edu) (59–61). Alignments were trimmed and refined using Se-Al (tree.bio.ed.ac.uk/software/seal). The 19 eukaryotic members of COG1720 did not produce quality alignments and were excluded from further analysis. Additionally, proteins from 11 archaea and 70 bacteria were truncated or were missing conserved residues (Gly7, Pro74, Asn75, Asp107 for Archaea, and Gln17, Pro89, Asn90, Asp121 for Bacteria), and were also excluded from further analysis. Alignments used in phylogenetic analysis are included in supplementary material and include 216 bacterial and 60 archaeal taxa. Phylogenetic analysis was performed using MrBayes 3.2.1 (62) on the University of Florida High Performance Cluster (hpc.ufl.edu) using the Dayhoff-6 amino acid categories and inferred a tree with the CAT+Γ model to account for evolutionary rate site variations. MrBayes was run for 1 000 000 or 2 000 000 (for combined bacterial and archaeal tree) MCMC iterations with a 10% burnin. Trees were sampled every 1000 iterations, and a consensus tree was generated by MrBayes using 50% majority rule. Convergences of the runs were checked with Tracer (tree.bio.ed.ac.uk/software/tracer). Consensus trees were visualized using iTOL (itol.embl.de) with posterior probabilities displayed on the branches.
RESULTS
Identification of yaeB gene responsible for N6-methylation of t6A37
To identify genes responsible for tRNA/rRNA modifications, we have performed screens employing a combination of reverse genetics and mass spectrometry, a strategy we call ‘ribonucleome analysis’ (42). In this type of screen, we use LC/MS to analyze modified nucleosides in total RNA from a series of genome-deletion strains. In the initial screen, which analyzed 130 genomic-deletion strains covering approximately 50% of E. coli open reading frames (ORFs), we demonstrated that m6t6A was specifically absent in a particular genomic-deletion strain, OCR36, which covers 4.72–5.25 min of the E. coli genome (Figure 2A). The deleted region of OCR36 encodes 23 ORFs, including ten uncharacterized genes, three rRNA genes, and four tRNA genes. However, this region did not contain any known class of AdoMet-dependent methyltransferases. We analyzed strains bearing single-gene deletions in each of ten uncharacterized genes and found that m6t6A was specifically absent in the ΔyaeB strain (Figure 2A), suggesting that yaeB is involved in m6t6A formation. Based on this finding, yaeB was found to be the gene altered in the tsaA mutant (41). In parallel, we identified yaeB in the tsaA mutant by genetic mapping (Supplementary result).
Figure 2.
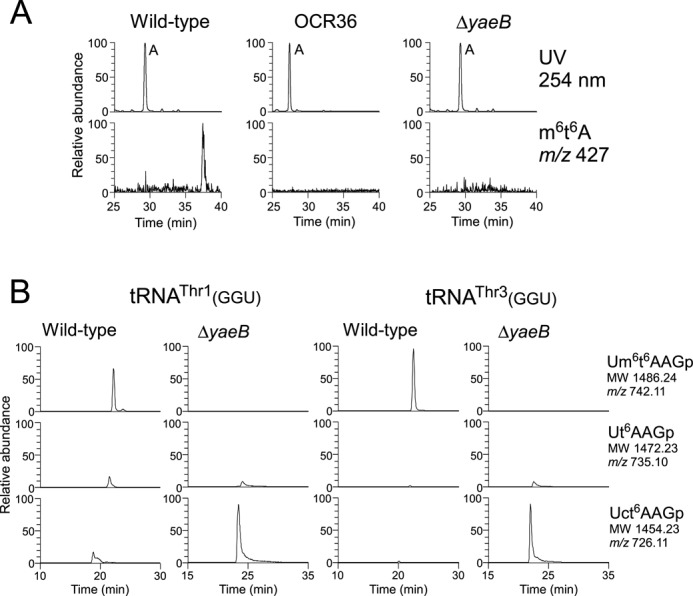
E. coli yaeB is responsible for m6t6A formation. (A) yaeB is the gene altered in the tsaA mutant. LC/MS analysis of total nucleosides extracted from wild type (left panels), OCR36 (middle panels), and ΔyaeB (right panels).Upper and lower panels show UV traces at 254 nm and mass chromatograms for the proton adduct of m6t6A (m/z 427). (B) Mass chromatograms of the RNA fragments containing position 37 of tRNAThr1(GGU) (left panels) and tRNAThr3(GGU) (right panels) isolated from E. coli wild type and ΔyaeB. Top, middle, and bottom panels show doubly charged negative ions of m6t6A-containing tetramer (Um6t6AAGp; MW 1486.24, m/z 742.11), t6A-containing tetramer (Ut6AAGp; MW 1472.23, m/z 735.10), and ct6A-containing tetramer (Uct6AAGp; MW 1454.23, m/z 726.11), respectively.
Next, we isolated tRNAThr1(GGU) and tRNAThr3(GGU) from the wild-type and ΔyaeB strains, and analyzed the RNase T1-digested fragments by LC/MS. The m6t6A-containing tetramer (Um6t6AAGp) was clearly present in both tRNAs isolated from the wild-type strain (Figure 2B). In tRNAs from ΔyaeB, however, no m6t6A-containing tetramer was detected; instead, we observed a ct6A-containing tetramer (Uct6AAGp), as well as a small fraction of t6A-containing tetramer (Ut6AAGp) (Figure 2B). The results indicate that in the absence of yaeB, tRNAThr1(GGU) and tRNAThr3(GGU) can serve as substrates for TcdA, which catalyzes dehydration of t6A to form ct6A(31). Therefore, t6A is a common precursor for m6t6A and ct6A.
TrmO is an AdoMet-dependent methyltransferase responsible for m6t6A formation.
According to the NCBI protein database, yaeB contains the uncharacterized protein domain UPF0066. Homologs of yaeB are widely distributed in bacteria, archaea, and eukaryotes. Crystal structures of three homologs, AF0241 (Archaeoglobus fulgidus)(63), RPA0152 (Rhodopseudomonas palustris), and HI0510 (Haemophilus influenzae), have been solved and deposited in PDB (Supplementary Figure S3). All of these homologs have single-sheeted, anti-parallel β-barrel structures and form homodimers. Intriguingly, two of these structures (of homologs AF0241 and RPA0152) contained endogenous AdoMet molecules, strongly suggesting that YaeB is an AdoMet-dependent methyltransferase.
To determine whether YaeB is the tRNA methyltransferase physiologically responsible for N6-methylation of m6t6A, we recombinantly expressed YaeB and purified it to homogeneity. To generate substrates, we prepared tRNA transcripts by in vitro transcription, and introduced t6A at position 37 by in vitro enzymatic reaction (21,56) (see Materials and Methods). At the outset, we examined the ability of YaeB to interact with tRNAs in gel-mobility shift experiments. A tRNA–protein complex was clearly observed for tRNAThr3(GGU) containing t6A37, and a larger amount of this complex was formed in the presence of higher levels of recombinant YaeB (Figure 3A), demonstrating that YaeB directly recognizes tRNAThr3(GGU). Because the efficiency of complex formation was not altered when unmodified tRNAThr3(GGU) was used for this experiment, we concluded that YaeB does not require t6A37 to interact with tRNAThr3(GGU). When tRNAThr4(UGU) containing t6A37 was mixed with recombinant YaeB, the tRNA–protein complex appeared to be unstable, yielding a smeared band. These results indicate that YaeB preferentially binds to tRNAThr3(GGU).
Next, we performed in vitro reconstitution of m6t6A. The tRNAThr3(GGU) bearing t6A37 was incubated with recombinant YaeB in the presence or absence of AdoMet. After the reaction, we analyzed RNase T1 digests of the substrate tRNAThr3(GGU) by LC/MS. In the presence of AdoMet, m6t6A-containing tetramer (Um6t6AAGp) could be clearly detected (Figure 3B), whereas no methylation occurred in the absence of AdoMet (Figure 3B). This result demonstrated that YaeB is an AdoMet-dependent methyltransferase responsible for the N6-methylation of t6A at position 37. To reflect this function, we renamed the protein TrmO, according to the preferred nomenclature. When tRNAThr3(GGU) without t6A37 was used as the substrate, no methylation was observed (Figure 3C), indicating that the N6-threonylcarbamoyl moiety of t6A37 is definitively required for N6-methylation by TrmO, even though t6A37 is not required for recognition of the tRNA by this enzyme (Figure 3A).
We next examined the in vitro N6-methylation of native tRNAThr3(GGU) isolated from ΔtrmO. The isolated tRNAThr3(GGU) that we used for in vitro reaction had both t6A and ct6A at position 37 due to spontaneous hydrolysis of ct6A to t6A during handling of tRNA. Consequently, m6t6A was formed in response to a reduction in t6A (Figure 3D), whereas ct6A did not change upon in vitro methylation (Figure 3D), indicating that TrmO does not methylate ct6A. Thus, TrmO methylates t6A to form m6t6A before cyclization of t6A catalyzed by TcdA.
Distribution of TrmO and AdoMet-binding site in the β-barrel–type RNA methyltransferase
To elucidate the evolutionary distribution of TrmO homologs, we utilized the genome sequences available in SEED (58). Of the 955 genomes in that database, 318 genomes from all three domains of life contained at least one gene encoding a protein annotated as YaeB (Supplementary Table S1 and http://tinyurl.com/m6t6A37). A summary tree, pruned to the level of taxonomic orders (Figure S4), illustrates that yaeB is distributed across all three domains. Bacterial yaeB is widely distributed in proteobacteria, especially γ-proteobacteria, all species in Vibrionales, and some species in Firmicutes, but is not present in Lactobacillales or Mycoplasma spp. In eukaryotes, yaeB homologs are found in vertebrates, insects, and plants, but not in nematodes or fungi. In archaeal phyla, yaeB homologs are present in both Euryarchaeota and Crenarchaeota. Multiple alignment of TrmO homologs from representative species revealed a high degree of conservation in the N-terminal region (UPF0066) (Figure 3E and Supplementary Figure S5). The mammalian homologs contain large internal insertions (Supplementary Figure S5).
According to the crystal structures of Archaeoglobus fulgidus YaeB in complex with AdoMet (Figure 3F and Supplementary Figure S3)(63), Gln22 (Gln25 in E. coli) and Asp111 (Asp125 in E. coli) recognize the α-amino group of AdoMet, whereas Arg82 (Arg92 in E. coli) and Lys122 (Lys136 in E. coli) make hydrogen bonds with the carboxy group of AdoMet. These residues are highly conserved among TrmO homologs (Supplementary Figure S5). To confirm importance of these conserved residues at the AdoMet-binding site, each of the residues was mutated to Ala in a plasmid-encoded E. coli trmO. The mutant plasmids were introduced into the ΔtrmO strain, and total nucleosides extracted from each transformant were analyzed by LC/MS (Figure 3G). Wild-type trmO fully rescued m6t6A formation in ΔtrmO, whereas the R92A and K136A mutants did not, and m6t6A formation was also dramatically reduced in the Q25A and D125A mutants. The results suggest that these residues play important roles in AdoMet binding, and that Arg92 and Lys136 in particular are critical residues in TrmO.
Among the γ-proteobacteria, of which E. coli is a member, the crystal structure of Haemophilus influenzae YaeB homolog has been solved (Figure S3). In addition to the N-terminal β-barrel methyltransferase domain, H. influenzae YaeB has an additional C-terminal domain containing the conserved sequence motif, DPRxxY (Figure 3E and Supplementary Figure S5). This domain is specific to YaeB homologs from γ- and β-proteobacteria and mammals (Supplementary Figure S5). Mutation of the conserved motif in the C-terminal domain (D194A/R196A) abolished m6t6A formation (Figure 3G), indicating that the DPRxxY motif is required for the N6-methylation reaction.
TrmO discriminates tRNAsThr for ACY codons from other isoacceptors
Among the four tRNAThr isoacceptors in E. coli, m6t6A37 is only present in tRNAThr1(GGU) and tRNAThr3(GGU) (Supplementary Figure S2), both of which have GGU anticodons responsible for ACY codons. TrmO discriminates these ACY-specific tRNAsThr from other isoacceptors. To determine the elements embedded in these tRNAs that are recognized by TrmO, we performed mutation studies using in vitro transcribed tRNAs with enzymatically introduced t6A37. By comparing the four isoacceptors, we identified the bases that are common to tRNAThr1(GGU) and tRNAThr3(GGU), but different in the other two isoacceptors (Figure 4A and Supplementary Figure S2); U3-A71, G26, the anticodon stem, G34 (wobble position), and A52-U62. In a control experiment, TrmO efficiently methylated tRNAThr3(GGU), but did not recognize tRNAThr4(UGU) (Figure 4B). Next, we tested a series of tRNAThr3(GGU) variants in which these bases were replaced by the corresponding bases of tRNAThr4(UGU). A variant AS4, in which the anticodon stem was swapped with that of tRNAThr4(UGU), lost the ability to be methylated by TrmO (Figure 4B). In addition, we replaced each of base pairs in the anticodon stem (Figure 4B). When C31-G39 was replaced by A31-U39, little methylation was observed, although modification of other base pairs in the anticodon stem did not influence methylation activity, indicating that TrmO recognizes the bottom base pair (C31-G39) in the anticodon stem of tRNAThr1,3. When G34 was replaced with C or U, a significant reduction in methylation was observed (Figure 4B), indicating that the wobble base G34 acts as another determinant for TrmO. Other differences between tRNAThr1,3 and tRNAThr2,4 did not affect methylation activity. To confirm that C31-G39 and G34 in tRNAThr1,3 act as positive determinants for N6-methylation by TrmO, we examined tRNAThr4(UGU) variants transplanted with these determinants (Figure 4B). TrmO did not methylate the variant A31C/U39G, in which A31-U39 was replaced by C31-G39, nor the variant U34G, whose wobble U base was replaced by G34. On the other hand, when both determinants were introduced simultaneously (A31C/U39G/U34G), this mutant tRNAThr4(UGU) was methylated by TrmO. In addition, when the entire anticodon stem-loop of tRNAThr4(UGU) was replaced by that of tRNAThr3(GGU) (ASL3), methylation activity increased slightly. From these results, we conclude that C31-G39 and G34 are major determinants of m6t6A formation, and that other elements in the anticodon stem-loop slightly contribute to efficient methylation by TrmO.
Other ct6A-containing tRNAs, tRNAIle1(GAU) and tRNASer3(GCU), harbor C31-G39 and G34. To determine why these tRNAs cannot serve as substrates of TrmO, we extended the mutation study. The second base of the anticodon (position 35) of these tRNAs differs from that of tRNAThr1,3: tRNAIle1(GAU) and tRNASer3(GCU) have A35 and C35, respectively; therefore, we constructed tRNAThr3(GGU) variants with either the G35A or G35C mutation. Neither variant was significantly methylated (Figure 4B), showing that TrmO can distinguish tRNAThr1,3 from tRNAIle1(GAU) or tRNASer3(GCU) by recognizing G35.
N 6-methylation of t6A enhances the decoding ability of tRNAThr
The thr operon is attenuated by efficient translation of the leader peptide encoded by thrL (64). Björk and colleagues showed that attenuation of the thr operon was alleviated in the tsaA mutant (41). Because the ThrL peptide contains consecutive ACC codons (Figure 5A), reduction in the decoding ability of tRNAThr1,3 causes ribosomes to get stuck on this mRNA, thereby preventing transcription termination by unwinding the terminator helix in the 5′ region of the structural genes of the operon. In the tsaA mutant, tRNAThr1,3 lacking N6-methylation at position 37 relieved attenuation of the thr operon reporter construct, suggesting that a lack of N6-methylation decreases the decoding ability of tRNAThr1,3. However, according to the results described above (Figure 2B), m6t6A of tRNAThr1,3 in the tsaA mutant should be converted to ct6A, not to t6A. Therefore, Qian et al. (41) compared decoding efficiencies of m6t6A37 versus ct6A37 in tRNAThr1,3. To confirm the effect of N6-methylation of m6t6A on the decoding ability of tRNAThr1,3, we constructed a reporter consisting of the thrL attenuator followed by the firefly luciferase gene (ThrL-Luc) and a control reporter without the attenuator (Luc) (Figure 5B). Each of these reporters was introduced into four E. coli strains (WT, ΔtrmO, ΔtcdA and ΔtrmO/ΔtcdA), and the luciferase activities were measured. In the presence of the thrL attenuator, ΔtrmO, in which m6t6A was converted to ct6A, exhibited a 1.5-fold increase in luciferase activity relative to WT (Figure 5C). This result is consistent with the previous report (41). Based on this result, we conclude that m6t6A facilitates ACC decoding more than ct6A. Furthremore, the ΔtrmO/ΔtcdA double-deletion strain, in which m6t6A was converted to t6A, exhibited a 2-fold increase in luciferase activity relative to ΔtcdA (Figure 5C). This result clearly demonstrated that the decoding activity of tRNAThr1,3 is reinforced by N6-methylation of m6t6A. In addition, comparison of the results from ΔtrmO and ΔtrmO/ΔtcdA revealed that ct6A confers more efficient decoding of ACC codons than t6A.
Figure 5.
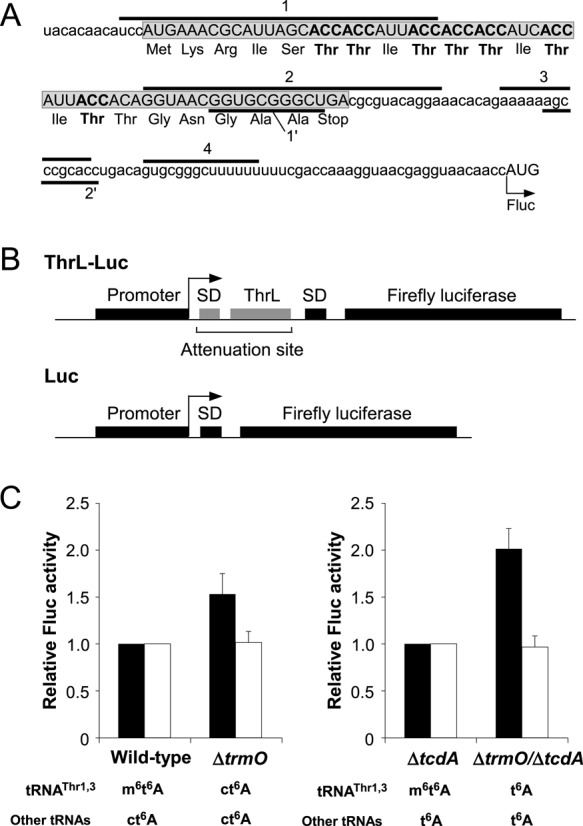
N6-methylation of t6A enhances the decoding ability of tRNAThr. (A) mRNA sequence of the attenuation site in the thr operon. The amino acid sequence denotes the ThrL leader peptide (boxed and shaded ORF). ACC codons and Thr residues are shown in bold. Segments 1 and 2 potentially form hairpin-like structures. Segments 3 and 4 form a stable terminator hairpin that halts transcription. Segments 1′ and 2′ also form a stable duplex that acts as an anti-terminator by preventing the formation of the terminator hairpin mediated by segments 3 and 4. When the ThrL leader peptide is actively translated, the terminator hairpin formed by segments 3 and 4 is stabilized, and the transcription of the downstream gene (in this case, firefly luciferase) is attenuated. On the other hand, when the consecutive ACC codons in the ThrL leader are not translated efficiently, the stable duplex formed by segments 1′ and 2′ prevents terminator hairpin formation, leading to efficient transcription of the downstream gene. (B) Schematic depiction of the reporter constructs for ThrL-Luc and Luc. (C) Relative attenuation activity of the ThrL-Luc reporter in ΔtrmO. Relative firefly luciferase (Fluc) activity of ThrL-Luc (black bars) or Luc (white bars) reporter was normalized to the OD600 of the culture. Relative Fluc activities of ΔtrmO and ΔtrmO/ΔtcdA were normalized to those of wild-type and ΔtcdA, respectively. The averaged values of four independent experiments, with SD values, are shown. The expected modification status at position 37 of tRNAThr1,3 and other tRNAs is indicated under the data for each strain.
The human homolog TRMO is responsible for m6t6A formation of tRNASer
m6t6A is present in rat cytoplasmic tRNASer(GCU) responsible for the AGY codons (34)(Figure 1B). We also detected the m6t6A nucleoside (m/z 427) in total RNA from HeLa cells (Figure 6A), clearly confirming that m6t6A is actually present in human cells. Then, we isolated tRNASer(GCU) responsible for AGY codon from total RNA of HeLa cell, analyzed RNase T1-digested RNA fragments by LC/MS. As expected, m6t6A-containing fragments [CUm6t6AAΨCCAUU(m)Gp] along with t6A-containing fragment (CUt6AAΨCCAUUGp) and unmodified fragment (CUAAΨCCAUUGp) were clearly observed (Figure 6B). m6t6A37 was found in 56% of tRNASer(GCU), while the remaining tRNAs have t6A37 (16%) and A37 (16%). The presence of m6t6A at position 37 was confirmed by collision-induced dissociation (CID) (Figure 6C).
To identify the human enzyme responsible for N6-methylation of m6t6A, we searched for homologs of E. coli TrmO, and retrieved C9orf156 as a plausible human homolog (Supplementary Figure S5). In the NCBI database, C9orf156 was misannotated as nef-associated protein 1 (NAP1), which should be annotated as ACOT8. C9orf156 shares 41% sequence identity with E. coli TrmO. To determine whether this gene is responsible for N6-methylation of m6t6A in human tRNA, we recombinantly expressed C9orf156 in E. coli and purified it to homogeneity. To generate the substrate, we prepared human tRNASer(GCU) transcript containing t6A37 introduced in vitro using E. coli t6A-modifying enzymes (Figure 6D). As shown in Figure 6E, m6t6A-containing trimer (m6t6AAUp) was clearly detected only in the presence of both recombinant C9orf156 and AdoMet. We also confirmed m6t6A formation by LC/MS nucleoside analysis (Figure 6F). Based on this protein's homology to E. coli TrmO and its similar biochemical function, we named it TRMO. Additionally, we investigated whether E. coli TrmO could use human tRNASer(GCU) as a substrate, but detected no methylation (Figure 6E and F), possibly because human tRNASer(GCU) has A31-U39 and C35, which should function as anti-determinants for E. coli TrmO. These results indicated that the substrate recognition mechanism of human TRMO differs from that of E. coli TrmO.
DISCUSSION
To date, seven classes of AdoMet-dependent methyltransferases have been described (7,8,65). TrmO/YaeB has a single-sheeted β-barrel structure encoded by the conserved UPF0066 domain, and does not belong to any known classes of AdoMet-dependent methyltransferases. Class V AdoMet-dependent methyltransferase also has an anti-parallel β-barrel structure composed of four β-sheets in a SET domain (Supplementary Figure S6)(66–68). The class V methyltransferases, which contain the SET domain, participate in Lys-methylation of histones, a process involved in chromatin remodeling (1,66). The conserved motifs in the SET domain constitute a pseudoknot structure (69), which configures the AdoMet binding pocket with a site for the target Lys residue. However, TrmO/YaeB does not have any of the motifs or pseudoknot structures conserved in the SET domain (Supplementary Figures S3 and S6A). In addition, the β-barrel of TrmO/YaeB is composed of a single sheet of six consecutive β-sheets (Supplementary Figure S6B), whereas the β-barrel of SET domain is composed of four β-sheets (Supplementary Figure S6A). Therefore, TrmO belongs to a novel class of AdoMet methyltransferase, designated as class VIII.
In E. coli, YfiC (TrmM or TrmN6) is another N6-methyltransferase responsible for m6A formation at position 37 in tRNAVal (UAC) (70). Although there was a possibility that YfiC/TrmM redundantly N6-methylates t6A in tRNAThr1,3, we did not observe any m6t6A in ΔtrmO strain (Figure 2AB), showing that YfiC/TrmM is not involved in m6t6A formation. YfiC/TrmM harbors a motif termed Methyltransferase_26 which contains a catalytic site of TaqI DNA N6 adenosine methyltransferase, categorized in class I AdoMet-dependent methyltransferase (8,71,72). Therefore, TrmO and YfiC/TrmM have evolved from distinct ancestors, albeit both enzymes catalyze N6-methylation of A37 of tRNAs.
In the three YaeB homologs for which crystal structures are available, the AdoMet binding pockets are almost identical (Supplementary Figure S7). AdoMet binds to the upper part of the β-barrel with surrounding extra loops (Supplementary Figures S3 and S6B). In our mutational study of E. coli TrmO, we examined four residues (Gln25, Arg92, Asp111, and Lys136) that are likely to be involved in AdoMet binding, according to the crystal structure of A. fulgidus YaeB (AF0241). Both Arg92 and Lys136, which we demonstrated to be critical for N6-methylation of t6A, recognize the carboxyl group of AdoMet. Intriguingly, the side chain of Lys122 (Lys136 in E. coli) in A. fulgidus YaeB extends from the other monomer and interacts with the carboxyl group of AdoMet (Figure 3F and Supplementary Figure S7)(63). This intersubunit composition of the AdoMet-binding pocket is a unique feature of class VIII AdoMet methyltransferases.
In bacteria and eukaryotes, TrmO has an additional C-terminal domain containing the conserved DPRxxY motif. We found that Asp194 and Arg196 in this motif of E. coli TrmO are necessary for N6-methylation. Many RNA methyltransferases have RNA-binding domains, including THUMP (49,73), the OB-fold (74), and the PUA domain (75), in addition to the catalytic domains in complex with AdoMet. Thus, we hypothesize that the C-terminal domain of TrmO plays a role in tRNA binding.
According to phylogenetic analysis, yaeB homologs are widely distributed in Archaea. Several archaea contain multiple homologs of yaeB, e.g., Methanoculleus marisnigri contains seven yaeB paralogs (Supplementary Table S1). However, no archaeal YaeB has a C-terminal domain containing the DPRxxY motif that is conserved in bacterial and mammalian TrmO homologs. In addition, m6t6A has never been detected in any archaeal tRNAs analyzed so far (34). Halobacterium salinarum is one of the few archaea in which the tRNA modifications have been characterized, but m6t6A was not detected in tRNAThr responsible for ACY codons (76), even though a YaeB homolog is present in Halobacterium salinarum strain NRC-1 (Supplementary Table S1). Thus, archaeal YaeB might not be involved in m6t6A formation, but is a class VIII AdoMet methyltransferase that might target another RNA or protein for methylation. However, we cannot exclude the possibility that m6t6A is present in other unanalyzed tRNAs from archaeal species whose genomes encode YaeB homologs. A Bayesian analysis showed that a branch of bacterial YaeB is intermingled with the archaeal branch (Supplementary Figure S8). Although very few bacteria contain multiple YaeB paralogs (Supplementary Table S1), Shewanella halifaxensis HAW-EB4 and Desulfotalea psychrophila LSv54 each have two paralogs of YaeB that are phylogenetically distant, and one paralog from each species is grouped within the archaeal branch.
In this study, we showed that m6t6A has a stronger ability to attenuate the thrL operon than ct6A and t6A, indicating that N6-methylation of t6A reinforces the decoding ability of tRNAThr1,3. In addition, ct6A conferred more efficient ACC decoding than t6A. Previously, we showed that cyclization of t6A is an additional modification that supports the decoding efficiency of tRNALys (31). Taken together with the results in this study, ct6A supports the decoding efficiency of other tRNAs in general. To discuss the molecular basis of decoding ability by N6-methylation of t6A, we constructed a structural model of m6t6A at the ribosomal A-site, based on the crystal structure of the anticodon stem-loop with t6A recognizing the AAG codon (11) (Supplementary Figure S9A and B). The N6-methyl group of m6t6A37 occupied the space between the carbonyl oxygen (O4) of U36 and the exocyclic amine (N6) of A38 (Supplementary Figure S9B). The distances from the N6-methyl group to O4 of U36 and to N6 of A38 were 3.3 Å and 3.2 Å, respectively. These distances are close enough to form van der Waals interactions, which would strengthen the stacking ability of m6t6A37 with U36 and A38. In solution structure, t6A37 destabilizes the anticodon stacking by promoting U36 bulges (77), implying that the N6-methyl group might have a function in stabilizing the anticodon by preventing the fluctuation of the uracil base of U36 at the ribosomal A-site. On the other hand, ct6A37 strengthens recognition of the first adenosine base of ANN codons, due to increased stacking and the additional hydrogen bond (Supplementary Figure S9C) (Miyauchi et al., 2013). We hypothesize that ct6A and m6t6A increase the decoding abilities of tRNAs via distinct mechanisms.
Biogenesis of m6t6A is depicted in Figure 7. There are two t6A derivatives in E. coli tRNAs: 11 species of tRNAs responsible for ANN codons have ct6A37, whereas tRNAsThr1,3 have m6t6A37. Initially, t6A is formed at position 37 on tRNAs by four enzymes, TsaB, TsaC, TsaD and TsaE, using L-threonine, bicarbonate, and ATP as substrates (21). Thereafter, TrmO methylates t6A in tRNAThr1,3 to form m6t6A, using AdoMet as the methyl donor. TrmO specifically recognizes the bottom base pair (C31–G39) of the anticodon stem and G34 as positive determinants to select these tRNAs. TrmO cannot employ ct6A as a substrate to form m6t6A. On the other hand, TcdA catalyzes ATP-dependent dehydration of t6A to form ct6A in 11 tRNAs responsible for ANN codons (31). This reaction is activated by the cysteine desulfurase CsdA and the sulfur-transfer protein CsdE (31), indicating that the sulfur-relay system is involved in efficient ct6A formation. In the absence of TrmO, t6A in tRNAThr1,3 is also converted to ct6A. This observation suggests that t6A is a common precursor of ct6A and m6t6A. In addition, TcdA has the ability to recognize all 13 tRNA species bearing t6A37, including tRNAsThr1,3, and form ct6A in these species. In other words, tRNAThr1,3 do not have any anti-determinants for TcdA. Hence, TrmO competes against TcdA for recognition of tRNAThr1,3 as a target for m6t6A formation. If t6A dehydration and ct6A hydrolysis are in equilibrium in the cell, TrmO might shift the equilibrium toward t6A by catching tRNAThr1,3 bearing t6A, leading to the accumulation of m6t6A.
Figure 7.
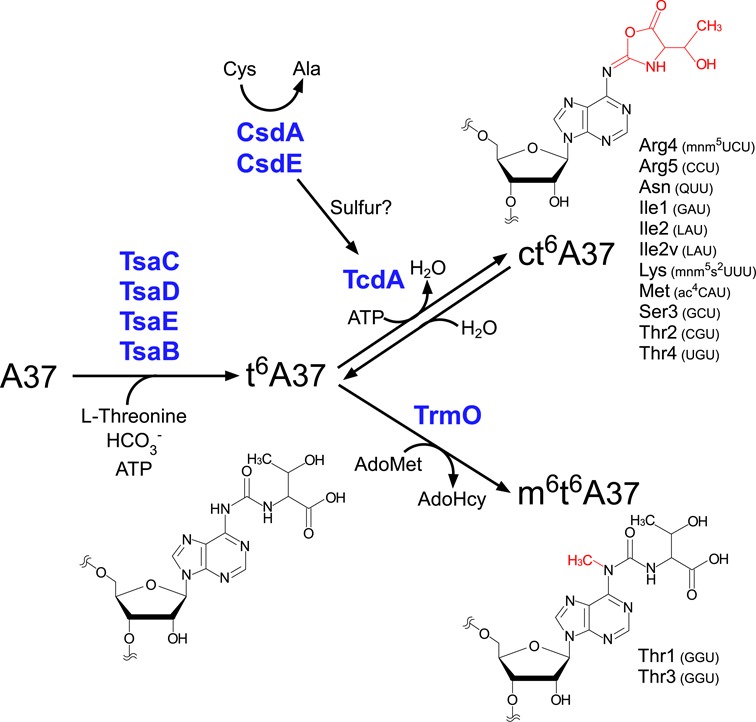
Biosynthesis of t6A derivatives. In E. coli, A37 of all 13 tRNAs responsible for ANN codons is modified to t6A by four enzymes (YgjD, YjeE, YeaZ and YrdC), which use L-threonine, bicarbonate, and ATP as substrates. For 11 tRNAs (i.e., excluding tRNAThr1,3), t6A37 is further dehydrated to ct6A by TcdA in the presence of ATP. This cyclization reaction is activated by cysteine desulfurase CsdA and sulfur acceptor protein CsdE. For tRNAThr1,3, t6A37 is methylated to form m6t6A37; this reaction is catalyzed by TrmO using AdoMet as a methyl donor.
In human cells, we determined that TRMO (C9orf156) is responsible for this methylation. Because little information on this gene is available, the physiological role of m6t6A in human tRNAs remains unknown. According to their different substrate specificities, bacterial TrmO and mammalian TRMO should have evolved differently in their respective species to employ specific tRNAs as substrates, optimizing the translational efficiency and fidelity in the physiological context of each organism. In contrast to bacterial tRNAs, mammalian tRNAs do not possess ct6A37, suggesting that N6-methylation of t6A has a profound effect on the decoding ability of tRNASer(GCU). In this study, we showed that m6t6A is partially introduced in tRNASer(GCU) in HeLa cell (Figure 6B), implying the frequency of m6t6A is regulated. In the human proteome, Ser-rich sequences can be found in RS domains in SR proteins, which are key factors in RNA splicing (78). Thus, it is tempting to speculate that m6t6A in tRNASer(GCU) can modulate the translational efficiency of AGY-encoded Ser clusters in SR proteins, suggesting an intriguing regulatory mechanism of translational efficiency mediated by the frequency of m6t6A.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
Acknowledgments
We are grateful to the members of the Suzuki laboratory, in particular Takeo Suzuki and Yuriko Sakaguchi, for technical support and many insightful discussions. The authors acknowledge Bret Boyd for help with phylogenetic analysis, and the University of Florida Research Computing for providing computational resources and support that have contributed to the research results reported in this publication (http://researchcomputing.ufl.edu).
FUNDING
This work was supported by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, and Culture of Japan (to T. S.), and by a JSPS Fellowship for Japanese Junior Scientists (to S.K. and Y.I.).
Conflict of interest statement. None declared.
REFERENCES
- 1.Cedar H., Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat. Rev. Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 2.Motorin Y., Helm M. RNA nucleotide methylation. Wiley Interdiscip. Rev. RNA. 2011;2:611–631. doi: 10.1002/wrna.79. [DOI] [PubMed] [Google Scholar]
- 3.Paik W.K., Paik D.C., Kim S. Historical review: the field of protein methylation. Trends Biochem. Sci. 2007;32:146–152. doi: 10.1016/j.tibs.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Herbert R.B. The Biosynthesis of Secondary Metabolites. London, UK: Chapman & Hall; 1989. [Google Scholar]
- 5.Stolz J.F., Basu P., Santini J.M., Oremland R.S. Arsenic and selenium in microbial metabolism. Annu. Rev. Microbiol. 2006;60:107–130. doi: 10.1146/annurev.micro.60.080805.142053. [DOI] [PubMed] [Google Scholar]
- 6.Kozbial P.Z., Mushegian A.R. Natural history of S-adenosylmethionine-binding proteins. BMC Struct. Biol. 2005;5:19. doi: 10.1186/1472-6807-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaminska K.H., Purta E., Hansen L.H., Bujnicki J.M., Vester B., Long K.S. Insights into the structure, function and evolution of the radical-SAM 23S rRNA methyltransferase Cfr that confers antibiotic resistance in bacteria. Nucleic Acids Res. 2010;38:1652–1663. doi: 10.1093/nar/gkp1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schubert H.L., Blumenthal R.M., Cheng X. Many paths to methyltransfer: a chronicle of convergence. Trends Biochem. Sci. 2003;28:329–335. doi: 10.1016/S0968-0004(03)00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin J.L., McMillan F.M. SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold. Curr. Opin. Struct. Biol. 2002;12:783–793. doi: 10.1016/s0959-440x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T. Biosynthesis and function of tRNA wobble modifications. In: Grosjean H., editor. Fine-tuning of RNA functions by Modification and Editing. New York: Springer-Verlag; 2005. pp. 24–69. [Google Scholar]
- 11.Murphy F.V., Ramakrishnan V., Malkiewicz A., Agris P.F. The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat. Struct. Mol. Biol. 2004;11:1186–1191. doi: 10.1038/nsmb861. [DOI] [PubMed] [Google Scholar]
- 12.Agris P.F., Vendeix F.A., Graham W.D. tRNA's wobble decoding of the genome: 40 years of modification. J. Mol. Biol. 2007;366:1–13. doi: 10.1016/j.jmb.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 13.Grosjean H. Nucleic Acids are not boring long polymers of only four types of nucleotides: A guided tour. In: Grosjean H., editor. DNA and RNA Modification Enzymes: Structure, Mechanism, Function and Evolution. Austin: Landes Bioscience; 2009. pp. 1–18. [Google Scholar]
- 14.Sundaram M., Durant P.C., Davis D.R. Hypermodified nucleosides in the anticodon of tRNA(Lys) stabilize a canonical U-turn structure. Biochemistry (Mosc). 2000;39:12575–12584. doi: 10.1021/bi005120y. [DOI] [PubMed] [Google Scholar]
- 15.Stuart J.W., Gdaniec Z., Guenther R., Marszalek M., Sochacka E., Malkiewicz A., Agris P.F. Functional anticodon architecture of human tRNALys3 includes disruption of intraloop hydrogen bonding by the naturally occurring amino acid modification, t6A. Biochemistry (Mosc). 2000;39:13396–13404. doi: 10.1021/bi0013039. [DOI] [PubMed] [Google Scholar]
- 16.Niimi T., Nureki O., Yokogawa T., Hayashi N., Nishikawa K., Watanabe K., Yokoyama S. Recognition of the Anticodon Loop of tRNAIle 1 by Isoleucyl-tRNA Synthetase from Escherichia coli. Nucleosides Nucleotides. 1994;13:1231–1237. [Google Scholar]
- 17.Yarian C., Townsend H., Czestkowski W., Sochacka E., Malkiewicz A.J., Guenther R., Miskiewicz A., Agris P.F. Accurate translation of the genetic code depends on tRNA modified nucleosides. J. Biol. Chem. 2002;277:16391–16395. doi: 10.1074/jbc.M200253200. [DOI] [PubMed] [Google Scholar]
- 18.Phelps S.S., Malkiewicz A., Agris P.F., Joseph S. Modified nucleotides in tRNA(Lys) and tRNA(Val) are important for translocation. J. Mol. Biol. 2004;338:439–444. doi: 10.1016/j.jmb.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 19.El Yacoubi B., Hatin I., Deutsch C., Kahveci T., Rousset J.P., Iwata-Reuyl D., Murzin A.G., de Crécy-Lagard V. A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J. 2011;30:882–893. doi: 10.1038/emboj.2010.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin C.A., Ellis S.R., True H.L. The Sua5 protein is essential for normal translational regulation in yeast. Mol. Cell. Biol. 2010;30:354–363. doi: 10.1128/MCB.00754-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deutsch C., El Yacoubi B., de Crécy-Lagard V., Iwata-Reuyl D. Biosynthesis of threonylcarbamoyl adenosine (t6A), a universal tRNA nucleoside. J. Biol. Chem. 2012;287:13666–13673. doi: 10.1074/jbc.M112.344028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauhon C.T. Mechanism of N6-threonylcarbamoyladenonsine (t6A) biosynthesis: isolation and characterization of the intermediate threonylcarbamoyl-AMP. Biochemistry (Mosc). 2012;51:8950–8963. doi: 10.1021/bi301233d. [DOI] [PubMed] [Google Scholar]
- 23.El Yacoubi B., Lyons B., Cruz Y., Reddy R., Nordin B., Agnelli F., Williamson J.R., Schimmel P., Swairjo M.A., de Crécy-Lagard V. The universal YrdC/Sua5 family is required for the formation of threonylcarbamoyladenosine in tRNA. Nucleic Acids Res. 2009;37:2894–2909. doi: 10.1093/nar/gkp152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrochia L., Crozat E., Hecker A., Zhang W., Bareille J., Collinet B., van Tilbeurgh H., Forterre P., Basta T. In vitro biosynthesis of a universal t6A tRNA modification in Archaea and Eukarya. Nucleic Acids Res. 2013;41:1953–1964. doi: 10.1093/nar/gks1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrochia L., Guetta D., Hecker A., Forterre P., Basta T. Functional assignment of KEOPS/EKC complex subunits in the biosynthesis of the universal t6A tRNA modification. Nucleic Acids Res. 2013;41:9484–9499. doi: 10.1093/nar/gkt720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daugeron M.C., Lenstra T.L., Frizzarin M., El Yacoubi B., Liu X., Baudin-Baillieu A., Lijnzaad P., Decourty L., Saveanu C., Jacquier A., et al. Gcn4 misregulation reveals a direct role for the evolutionary conserved EKC/KEOPS in the t6A modification of tRNAs. Nucleic Acids Res. 2011;39:6148–6160. doi: 10.1093/nar/gkr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan M., Mehta P., Yu Y., Prugar E., Koonin E.V., Karzai A.W., Sternglanz R. The highly conserved KEOPS/EKC complex is essential for a universal tRNA modification, t6A. EMBO J. 2011;30:873–881. doi: 10.1038/emboj.2010.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naor A., Thiaville P.C., Altman-Price N., Cohen-Or I., Allers T., de Crécy-Lagard V., Gophna U. A genetic investigation of the KEOPS complex in halophilic Archaea. PLoS One. 2012;7:e43013. doi: 10.1371/journal.pone.0043013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberto J., Breuil N., Hecker A., Farina F., Brochier-Armanet C., Culetto E., Forterre P. Qri7/OSGEPL, the mitochondrial version of the universal Kae1/YgjD protein, is essential for mitochondrial genome maintenance. Nucleic Acids Res. 2009;37:5343–5352. doi: 10.1093/nar/gkp557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan L.C., Mao D.Y., Neculai D., Strecker J., Chiovitti D., Kurinov I., Poda G., Thevakumaran N., Yuan F., Szilard R.K., et al. Reconstitution and characterization of eukaryotic N6-threonylcarbamoylation of tRNA using a minimal enzyme system. Nucleic Acids Res. 2013;41:6332–6346. doi: 10.1093/nar/gkt322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyauchi K., Kimura S., Suzuki T. A cyclic form of N6-threonylcarbamoyladenosine as a widely distributed tRNA hypermodification. Nat. Chem. Biol. 2013;9:105–111. doi: 10.1038/nchembio.1137. [DOI] [PubMed] [Google Scholar]
- 32.Yamada Y., Ishikura H. The presence of N-[(9-β-D-ribofuranosyl-2-methylthiopurin-6-yl)carbamoyl]threonine in lysine tRNA1 from Bacillus subtilis. J. Biochem. 1981;98:1589–1591. doi: 10.1093/oxfordjournals.jbchem.a133353. [DOI] [PubMed] [Google Scholar]
- 33.Yamaizumi Z., Nishimura S., Limburg K., Raba M., Gross H.J., Crain P.F., McCloskey J.A. Structure Elucidation by High-Resolution Mass-Spectrometry of a Highly Modified Nucleoside from Mammalian Transfer-Rna - N-[(9-β-D-Ribofuranosyl-2-Methylthiopurin-6-yl)Carbamoyl]Threonine. J. Am. Chem. Soc. 1979;101:2224–2225. [Google Scholar]
- 34.Juhling F., Morl M., Hartmann R.K., Sprinzl M., Stadler P.F., Putz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura-Harada F., Minden D.L.V., McCloskey J.A., Nishimura S. N-[(9-β-D-Ribofuranosylpurin-6-yl)-N-methylcarbamoyl]threonine, a modified nucleoside isolated from Escherichia coli threonine transfer ribonucleic acid. Biochemistry (Mosc). 1972;11:3910–3915. doi: 10.1021/bi00771a012. [DOI] [PubMed] [Google Scholar]
- 36.Anton B.P., Russell S.P., Vertrees J., Kasif S., Raleigh E.A., Limbach P.A., Roberts R.J. Functional characterization of the YmcB and YqeV tRNA methylthiotransferases of Bacillus subtilis. Nucleic Acids Res. 2010;38:6195–6205. doi: 10.1093/nar/gkq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arragain S., Handelman S.K., Forouhar F., Wei F.Y., Tomizawa K., Hunt J.F., Douki T., Fontecave M., Mulliez E., Atta M. Identification of eukaryotic and prokaryotic methylthiotransferase for biosynthesis of 2-methylthio-N6-threonylcarbamoyladenosine in tRNA. J. Biol. Chem. 2010;285:28425–28433. doi: 10.1074/jbc.M110.106831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei F.Y., Suzuki T., Watanabe S., Kimura S., Kaitsuka T., Fujimura A., Matsui H., Atta M., Michiue H., Fontecave M., et al. Deficit of tRNA(Lys) modification by Cdkal1 causes the development of type 2 diabetes in mice. J. Clin. Invest. 2011;121:3598–3608. doi: 10.1172/JCI58056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke L., Carbon J. The nucleotide sequence of a threonine transfer ribonucleic acid from Escherichia coli. J. Biol. Chem. 1974;249:6874–6885. [PubMed] [Google Scholar]
- 40.Komine Y., Inokuchi H. Nucleotide sequence of tRNA(Thr1) of Escherichia coli and of the gene (thrV) that encodes it. Nucleic Acids Res. 1992;20:4089. doi: 10.1093/nar/20.15.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian Q., Curran J.F., Björk G.R. The methyl group of the N6-methyl-N6-threonylcarbamoyladenosine in tRNA of Escherichia coli modestly improves the efficiency of the tRNA. J. Bacteriol. 1998;180:1808–1813. doi: 10.1128/jb.180.7.1808-1813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki T., Ikeuchi Y., Noma A., Suzuki T., Sakaguchi Y. Mass spectrometric identification and characterization of RNA-modifying enzymes. Methods Enzymol. 2007;425:211–229. doi: 10.1016/S0076-6879(07)25009-8. [DOI] [PubMed] [Google Scholar]
- 43.Ikeuchi Y., Kitahara K., Suzuki T. The RNA acetyltransferase driven by ATP hydrolysis synthesizes N4-acetylcytidine of tRNA anticodon. EMBO J. 2008;27:2194–2203. doi: 10.1038/emboj.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikeuchi Y., Shigi N., Kato J., Nishimura A., Suzuki T. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol. Cell. 2006;21:97–108. doi: 10.1016/j.molcel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Soma A., Ikeuchi Y., Kanemasa S., Kobayashi K., Ogasawara N., Ote T., Kato J., Watanabe K., Sekine Y., Suzuki T. An RNA-modifying enzyme that governs both the codon and amino acid specificities of isoleucine tRNA. Mol. Cell. 2003;12:689–698. doi: 10.1016/s1097-2765(03)00346-0. [DOI] [PubMed] [Google Scholar]
- 46.Noma A., Kirino Y., Ikeuchi Y., Suzuki T. Biosynthesis of wybutosine, a hyper-modified nucleoside in eukaryotic phenylalanine tRNA. EMBO J. 2006;25:2142–2154. doi: 10.1038/sj.emboj.7601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noma A., Sakaguchi Y., Suzuki T. Mechanistic characterization of the sulfur-relay system for eukaryotic 2-thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Res. 2009;37:1335–1352. doi: 10.1093/nar/gkn1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noma A., Yi S., Katoh T., Takai Y., Suzuki T. Actin-binding protein ABP140 is a methyltransferase for 3-methylcytidine at position 32 of tRNAs in Saccharomyces cerevisiae. RNA. 2011;17:1111–1119. doi: 10.1261/rna.2653411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimura S., Ikeuchi Y., Kitahara K., Sakaguchi Y., Suzuki T. Base methylations in the double-stranded RNA by a fused methyltransferase bearing unwinding activity. Nucleic Acids Res. 2012;40:4071–4085. doi: 10.1093/nar/gkr1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura S., Suzuki T. Fine-tuning of the ribosomal decoding center by conserved methyl-modifications in the Escherichia coli 16S rRNA. Nucleic Acids Res. 2010;38:1341–1352. doi: 10.1093/nar/gkp1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Datsenko K.A., Wanner B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chomczynski P., Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat. Protoc. 2006;1:581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 53.Ikeuchi Y., Kimura S., Numata T., Nakamura D., Yokogawa T., Ogata T., Wada T., Suzuki T. Agmatine-conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea. Nat. Chem. Biol. 2010;6:277–282. doi: 10.1038/nchembio.323. [DOI] [PubMed] [Google Scholar]
- 54.Miyauchi K., Ohara T., Suzuki T. Automated parallel isolation of multiple species of non-coding RNAs by the reciprocal circulating chromatography method. Nucleic Acids Res. 2007;35:e24. doi: 10.1093/nar/gkl1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sampson J.R., Uhlenbeck O.C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc. Natl. Acad. Sci. U.S.A. 1988;85:1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taniguchi T., Miyauchi K., Nakane D., Miyata M., Muto A., Nishimura S., Suzuki T. Decoding system for the AUA codon by tRNAIle with the UAU anticodon in Mycoplasma mobile. Nucleic Acids Res. 2013;41:2621–2631. doi: 10.1093/nar/gks1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitagawa M., Ara T., Arifuzzaman M., Ioka-Nakamichi T., Inamoto E., Toyonaga H., Mori H. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 2005;12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 58.Overbeek R., Begley T., Butler R.M., Choudhuri J.V., Chuang H.Y., Cohoon M., de Crécy-Lagard V., Diaz N., Disz T., Edwards R., et al. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 2005;33:5691–5702. doi: 10.1093/nar/gki866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goecks J., Nekrutenko A., Taylor J. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 2010;11:R86. doi: 10.1186/gb-2010-11-8-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blankenberg D., Von Kuster G., Coraor N., Ananda G., Lazarus R., Mangan M., Nekrutenko A., Taylor J. Galaxy: a web-based genome analysis tool for experimentalists. Curr. Protoc. Mol. Biol. 2010;10:11–21. doi: 10.1002/0471142727.mb1910s89. Chapter 19. Unit 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giardine B., Riemer C., Hardison R.C., Burhans R., Elnitski L., Shah P., Zhang Y., Blankenberg D., Albert I., Taylor J., et al. Galaxy: a platform for interactive large-scale genome analysis. Genome Res. 2005;15:1451–1455. doi: 10.1101/gr.4086505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 63.Forouhar F., Kuzin A., Seetharaman J., Lee I., Zhou W., Abashidze M., Chen Y., Yong W., Janjua H., Fang Y., et al. Functional insights from structural genomics. J. Struct. Funct. Genomics. 2007;8:37–44. doi: 10.1007/s10969-007-9018-3. [DOI] [PubMed] [Google Scholar]
- 64.Kolter R., Yanofsky C. Attenuation in amino acid biosynthetic operons. Annu. Rev. Genet. 1982;16:113–134. doi: 10.1146/annurev.ge.16.120182.000553. [DOI] [PubMed] [Google Scholar]
- 65.Yang J., Kulkarni K., Manolaridis I., Zhang Z., Dodd R.B., Mas-Droux C., Barford D. Mechanism of isoprenylcysteine carboxyl methylation from the crystal structure of the integral membrane methyltransferase ICMT. Mol. Cell. 2011;44:997–1004. doi: 10.1016/j.molcel.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 66.Qian C., Zhou M.M. SET domain protein lysine methyltransferases: Structure, specificity and catalysis. Cell. Mol. Life Sci. 2006;63:2755–2763. doi: 10.1007/s00018-006-6274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson J.R., Jing C., Walker P.A., Martin S.R., Howell S.A., Blackburn G.M., Gamblin S.J., Xiao B. Crystal structure and functional analysis of the histone methyltransferase SET7/9. Cell. 2002;111:105–115. doi: 10.1016/s0092-8674(02)00964-9. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X., Tamaru H., Khan S.I., Horton J.R., Keefe L.J., Selker E.U., Cheng X. Structure of the Neurospora SET domain protein DIM-5, a histone H3 lysine methyltransferase. Cell. 2002;111:117–127. doi: 10.1016/s0092-8674(02)00999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor W.R., Xiao B., Gamblin S.J., Lin K. A knot or not a knot? SETting the record ‘straight’ on proteins. Comput. Biol. Chem. 2003;27:11–15. doi: 10.1016/s1476-9271(02)00099-3. [DOI] [PubMed] [Google Scholar]
- 70.Golovina A.Y., Sergiev P.V., Golovin A.V., Serebryakova M.V., Demina I., Govorun V.M., Dontsova O.A. The yfiC gene of E. coli encodes an adenine-N6 methyltransferase that specifically modifies A37 of tRNA1Val(cmo5UAC) RNA. 2009;15:1134–1141. doi: 10.1261/rna.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Finn R.D., Bateman A., Clements J., Coggill P., Eberhardt R.Y., Eddy S.R., Heger A., Hetherington K., Holm L., Mistry J., et al. Pfam: the protein families database. Nucleic Acids Res. 2014;42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schluckebier G., Kozak M., Bleimling N., Weinhold E., Saenger W. Differential binding of S-adenosylmethionine S-adenosylhomocysteine and Sinefungin to the adenine-specific DNA methyltransferase M. TaqI. J. Mol. Biol. 1997;265:56–67. doi: 10.1006/jmbi.1996.0711. [DOI] [PubMed] [Google Scholar]
- 73.Neumann P., Lakomek K., Naumann P.T., Erwin W.M., Lauhon C.T., Ficner R. Crystal structure of a 4-thiouridine synthetase-RNA complex reveals specificity of tRNA U8 modification. Nucleic Acids Res. 2014;42:6685–6673. doi: 10.1093/nar/gku249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee T.T., Agarwalla S., Stroud R.M. A unique RNA Fold in the RumA-RNA-cofactor ternary complex contributes to substrate selectivity and enzymatic function. Cell. 2005;120:599–611. doi: 10.1016/j.cell.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 75.Andersen N.M., Douthwaite S. YebU is a m5C methyltransferase specific for 16 S rRNA nucleotide 1407. J. Mol. Biol. 2006;359:777–786. doi: 10.1016/j.jmb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 76.Nicoghosian K., Xian-Rong G., Cedergren R. Halobacterium cutirubrum tRNA sequences. FEBS Lett. 1985;193:255–260. [Google Scholar]
- 77.Durant P.C., Bajji A.C., Sundaram M., Kumar R.K., Davis D.R. Structural effects of hypermodified nucleosides in the Escherichia coli and human tRNALys anticodon loop: the effect of nucleosides s2U, mcm5U, mcm5s2U, mnm5s2U, t6A, and ms2t6A. Biochemistry (Mosc). 2005;44:8078–8089. doi: 10.1021/bi050343f. [DOI] [PubMed] [Google Scholar]
- 78.Long J.C., Caceres J.F. The SR protein family of splicing factors: master regulators of gene expression. Biochem. J. 2009;417:15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- 79.Sprinzl M., Vassilenko K.S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 2005;33:D139–D140. doi: 10.1093/nar/gki012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.