Abstract
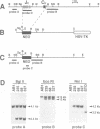
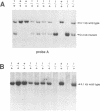
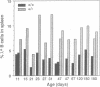
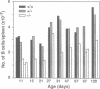
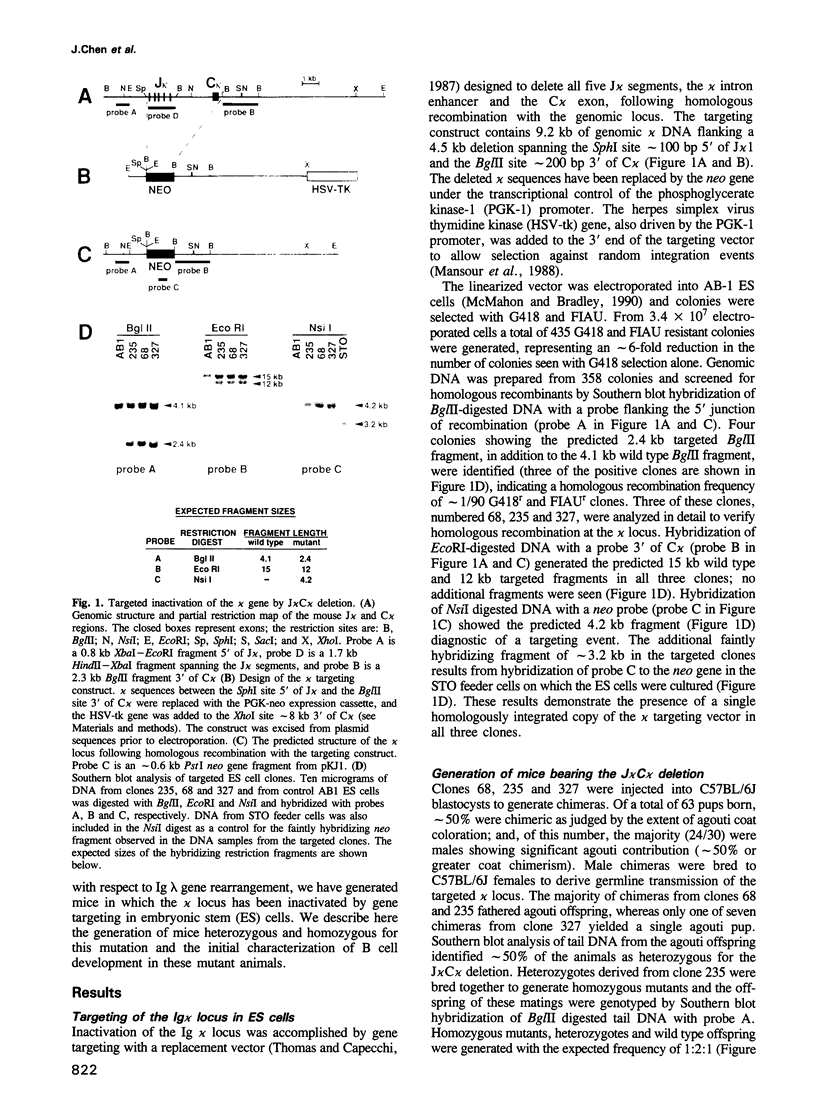
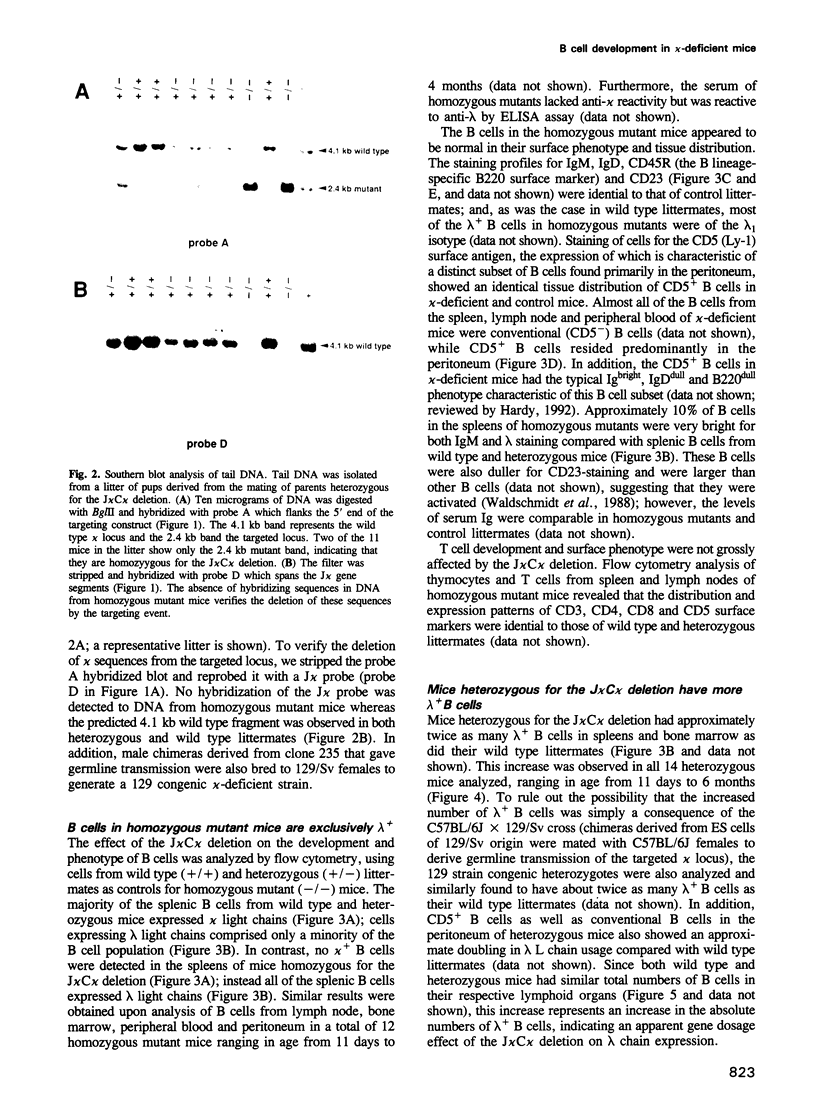
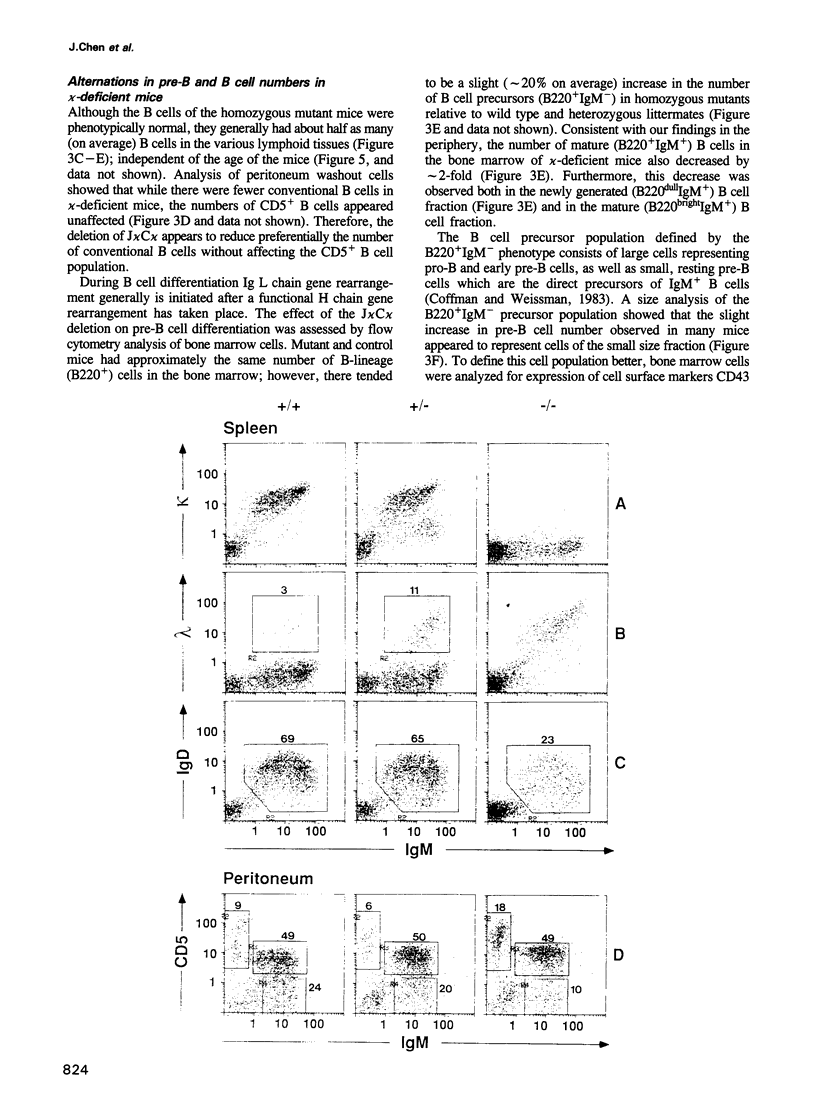
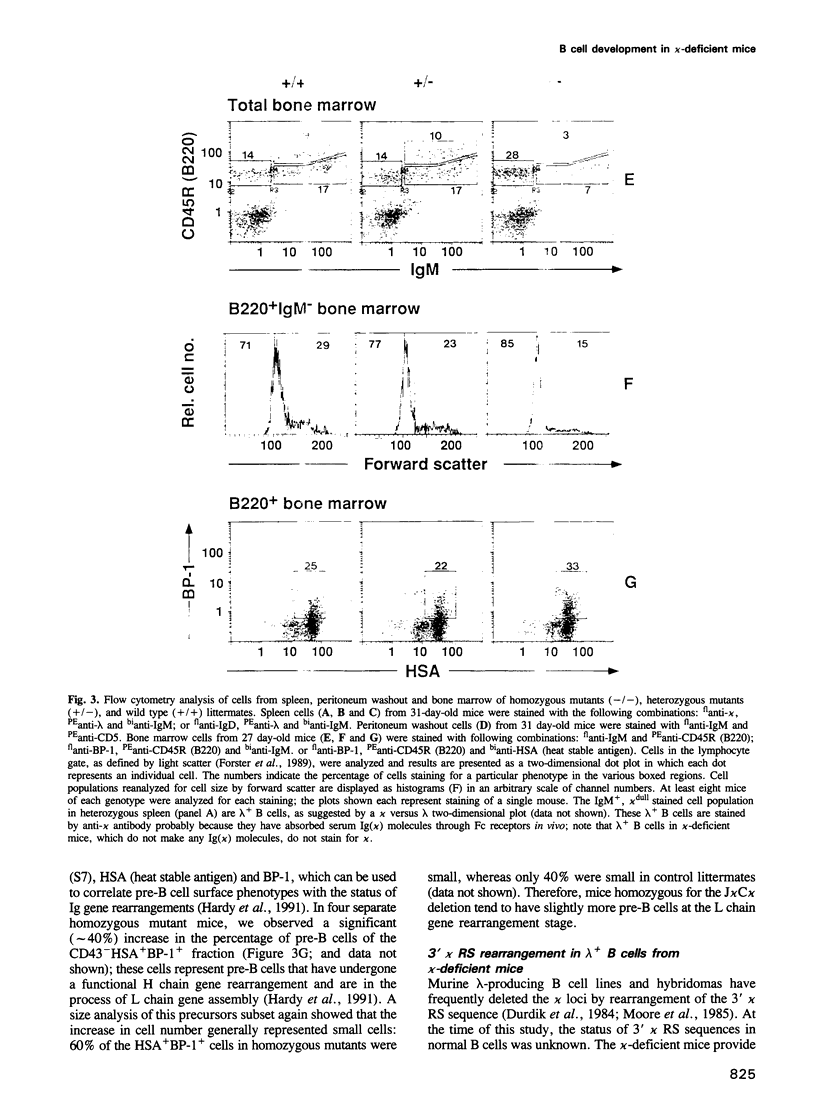
We have generated mice that lack the ability to produce immunoglobulin (Ig) kappa light chains by targeted deletion of J kappa and C kappa gene segments and the intervening sequences in mouse embryonic stem cells. In wild type mice, approximately 95% of B cells express kappa light chains and only approximately 5% express lambda light chains. Mice heterozygous for the J kappa C kappa deletion have approximately 2-fold more lambda+ B cells than wild-type littermates. Compared with normal mice, homozygous mutants for the J kappa C kappa deletion have about half the number of B cells in both the newly generated and the peripheral B cell compartments, and all of these B cells express lambda light chains in their Ig. Therefore, homozygous mutant mice appear to produce lambda-expressing cells at nearly 10 times the rate observed in normal mice. These findings demonstrate that kappa gene assembly and/or expression is not a prerequisite for lambda gene assembly and expression. Furthermore, there is no detectable rearrangement of 3' kappa RS sequences in lambda+ B cells of the homozygous mutant mice, thus rearrangements of these sequences, per se, is not required for lambda light chain gene assembly. We discuss these findings in the context of their implications for the control of Ig light chain gene rearrangement and potential applications of the mutant animals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Enea V., Bothwell A. L., Baltimore D. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 1980 Aug;21(1):1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- BERNIER G. M., CEBRA J. J. POLYPEPTIDE CHAINS OF HUMAN GAMMA-GLOBULIN: CELLULAR LOCALIZATION BY FLUORESCENT ANTIBODY. Science. 1964 Jun 26;144(3626):1590–1591. doi: 10.1126/science.144.3626.1590. [DOI] [PubMed] [Google Scholar]
- Berg J., McDowell M., Jäck H. M., Wabl M. Immunoglobulin lambda gene rearrangement can precede kappa gene rearrangement. Dev Immunol. 1990;1(1):53–57. doi: 10.1155/1990/56014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Alt F. W. Mechanism and developmental program of immunoglobulin gene rearrangement in mammals. Annu Rev Genet. 1989;23:605–636. doi: 10.1146/annurev.ge.23.120189.003133. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. Immunoglobulin gene rearrangement during pre-B cell differentiation. J Mol Cell Immunol. 1983;1(1):31–41. [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Crippen T. L., Jones I. M. Cell proliferation in the bone marrow, thymus and spleen of mice studied by continuous, in vivo bromodeoxycytidine labelling and flow cytometric analysis. Cell Tissue Kinet. 1989 May;22(3):203–212. doi: 10.1111/j.1365-2184.1989.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Durdik J., Moore M. W., Selsing E. Novel kappa light-chain gene rearrangements in mouse lambda light chain-producing B lymphocytes. Nature. 1984 Feb 23;307(5953):749–752. doi: 10.1038/307749a0. [DOI] [PubMed] [Google Scholar]
- Felsher D. W., Ando D. T., Braun J. Independent rearrangement of Ig lambda genes in tissue culture-derived murine B cell lines. Int Immunol. 1991 Jul;3(7):711–718. doi: 10.1093/intimm/3.7.711. [DOI] [PubMed] [Google Scholar]
- Förster I., Vieira P., Rajewsky K. Flow cytometric analysis of cell proliferation dynamics in the B cell compartment of the mouse. Int Immunol. 1989;1(4):321–331. doi: 10.1093/intimm/1.4.321. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Jorgensen H., Brink R. A., Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989 Nov 23;342(6248):385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- Hagman J., Rudin C. M., Haasch D., Chaplin D., Storb U. A novel enhancer in the immunoglobulin lambda locus is duplicated and functionally independent of NF kappa B. Genes Dev. 1990 Jun;4(6):978–992. doi: 10.1101/gad.4.6.978. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. R. Variable gene usage, physiology and development of Ly-1+ (CD5+) B cells. Curr Opin Immunol. 1992 Apr;4(2):181–185. doi: 10.1016/0952-7915(92)90010-c. [DOI] [PubMed] [Google Scholar]
- Hartley S. B., Crosbie J., Brink R., Kantor A. B., Basten A., Goodnow C. C. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991 Oct 24;353(6346):765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Huszar D., Sharpe A., Jaenisch R. Migration and proliferation of cultured neural crest cells in W mutant neural crest chimeras. Development. 1991 May;112(1):131–141. doi: 10.1242/dev.112.1.131. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992 May 29;69(5):823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Klobeck H. G., Zachau H. G. The human CK gene segment and the kappa deleting element are closely linked. Nucleic Acids Res. 1986 Jun 11;14(11):4591–4603. doi: 10.1093/nar/14.11.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Sharrow S. O., Goldman C. K., Leder P., Waldmann T. A. Normal human B cells display ordered light chain gene rearrangements and deletions. J Exp Med. 1982 Oct 1;156(4):975–985. doi: 10.1084/jem.156.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P. W., Zijderveld A., Linders K., Rudnicki M. A., Jaenisch R., Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991 Aug 11;19(15):4293–4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S., Rosenberg N., Alt F., Baltimore D. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982 Oct;30(3):807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- McGuire K. L., Vitetta E. S. kappa/lambda Shifts do not occur during maturation of murine B cells. J Immunol. 1981 Oct;127(4):1670–1673. [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 Mar 6;68(5):869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Moore M. W., Durdik J., Persiani D. M., Selsing E. Deletions of kappa chain constant region genes in mouse lambda chain-producing B cells involve intrachromosomal DNA recombinations similar to V-J joining. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6211–6215. doi: 10.1073/pnas.82.18.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B., Reth M. Ordered activation of the Ig lambda locus in Abelson B cell lines. J Exp Med. 1988 Dec 1;168(6):2131–2137. doi: 10.1084/jem.168.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B., Stappert H., Reth M. A physical map and analysis of the murine C kappa-RS region show the presence of a conserved element. Eur J Immunol. 1990 Jun;20(6):1409–1411. doi: 10.1002/eji.1830200631. [DOI] [PubMed] [Google Scholar]
- Nadel B., Cazenave P. A., Sanchez P. Murine lambda gene rearrangements: the stochastic model prevails over the ordered model. EMBO J. 1990 Feb;9(2):435–440. doi: 10.1002/j.1460-2075.1990.tb08128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltz E. M., Yancopoulos G. D., Morrow M. A., Rolink A., Lee G., Wong F., Kaplan K., Gillis S., Melchers F., Alt F. W. A novel regulatory myosin light chain gene distinguishes pre-B cell subsets and is IL-7 inducible. EMBO J. 1992 Jul;11(7):2759–2767. doi: 10.1002/j.1460-2075.1992.tb05341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond D. G. Proliferation kinetics and the lifespan of B cells in central and peripheral lymphoid organs. Curr Opin Immunol. 1991 Apr;3(2):179–185. doi: 10.1016/0952-7915(91)90047-5. [DOI] [PubMed] [Google Scholar]
- Park Y. H., Osmond D. G. Dynamics of early B lymphocyte precursor cells in mouse bone marrow: proliferation of cells containing terminal deoxynucleotidyl transferase. Eur J Immunol. 1989 Nov;19(11):2139–2144. doi: 10.1002/eji.1830191125. [DOI] [PubMed] [Google Scholar]
- Persiani D. M., Durdik J., Selsing E. Active lambda and kappa antibody gene rearrangement in Abelson murine leukemia virus-transformed pre-B cell lines. J Exp Med. 1987 Jun 1;165(6):1655–1674. doi: 10.1084/jem.165.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden D. A., Wu G. E. Mouse kappa light-chain recombination signal sequences mediate recombination more frequently than do those of lambda light chain. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10721–10725. doi: 10.1073/pnas.88.23.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B., Penit C., Baron C., Vasseur F., Dautigny N., Freitas A. A. Accumulation of bromodeoxyuridine-labeled cells in central and peripheral lymphoid organs: minimal estimates of production and turnover rates of mature lymphocytes. Eur J Immunol. 1990 Aug;20(8):1697–1708. doi: 10.1002/eji.1830200812. [DOI] [PubMed] [Google Scholar]
- Rolink A., Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell. 1991 Sep 20;66(6):1081–1094. doi: 10.1016/0092-8674(91)90032-t. [DOI] [PubMed] [Google Scholar]
- Russell D. M., Dembić Z., Morahan G., Miller J. F., Bürki K., Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991 Nov 28;354(6351):308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Waldschmidt T. J., Conrad D. H., Lynch R. G. The expression of B cell surface receptors. I. The ontogeny and distribution of the murine B cell IgE Fc receptor. J Immunol. 1988 Apr 1;140(7):2148–2154. [PubMed] [Google Scholar]
- Weiss S., Lehmann K., Raschke W. C., Cohn M. Mice completely suppressed for the expression of immunoglobulin kappa light chain. Proc Natl Acad Sci U S A. 1984 Jan;81(1):211–215. doi: 10.1073/pnas.81.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]