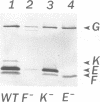
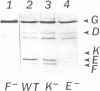
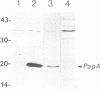
Abstract
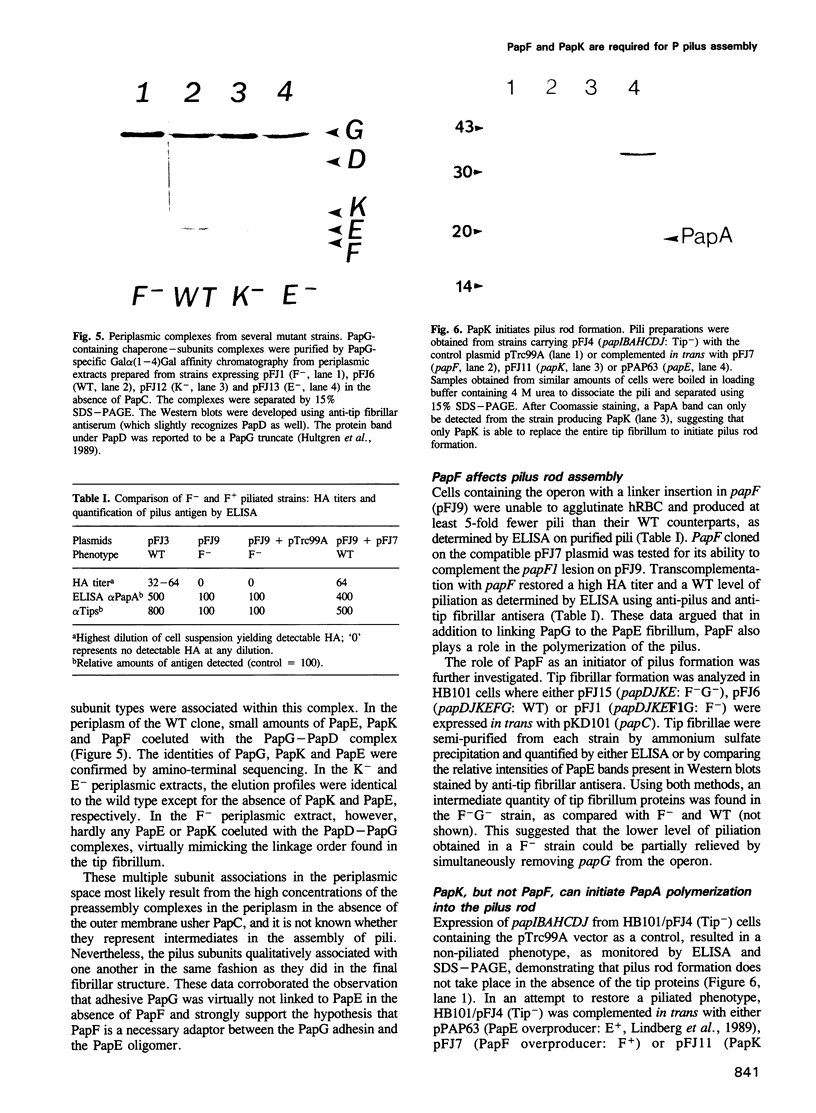
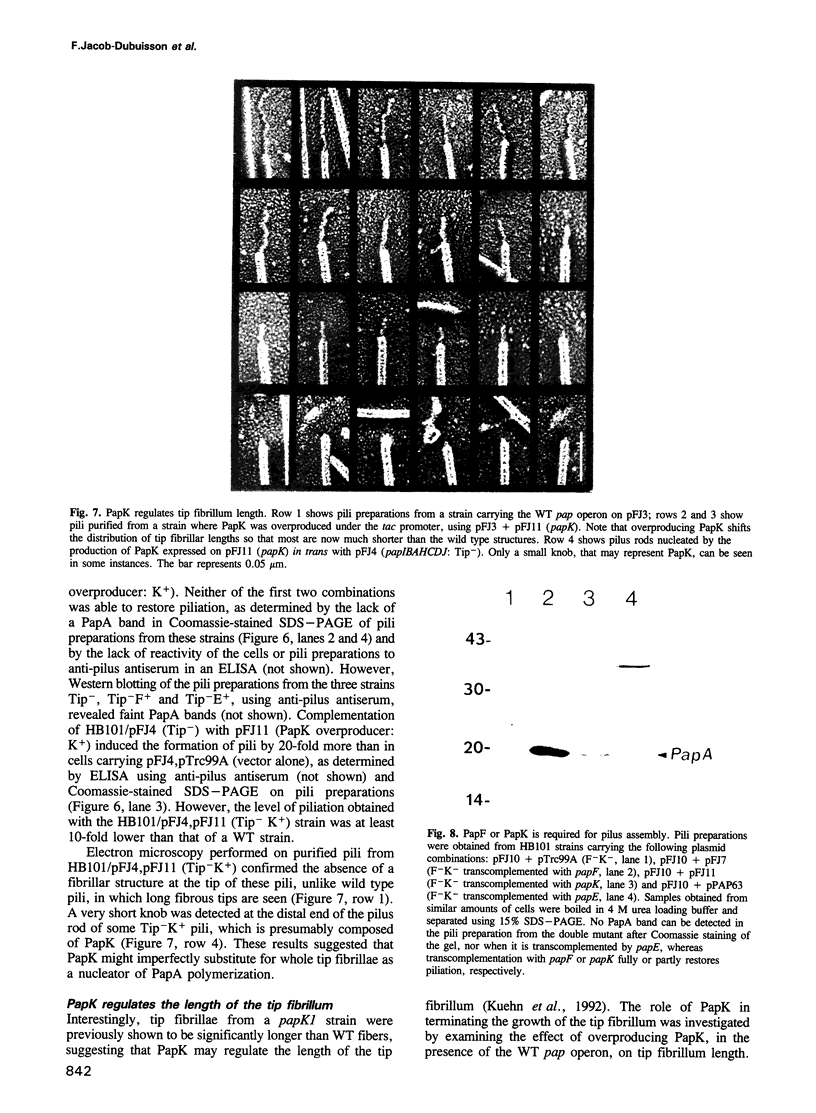
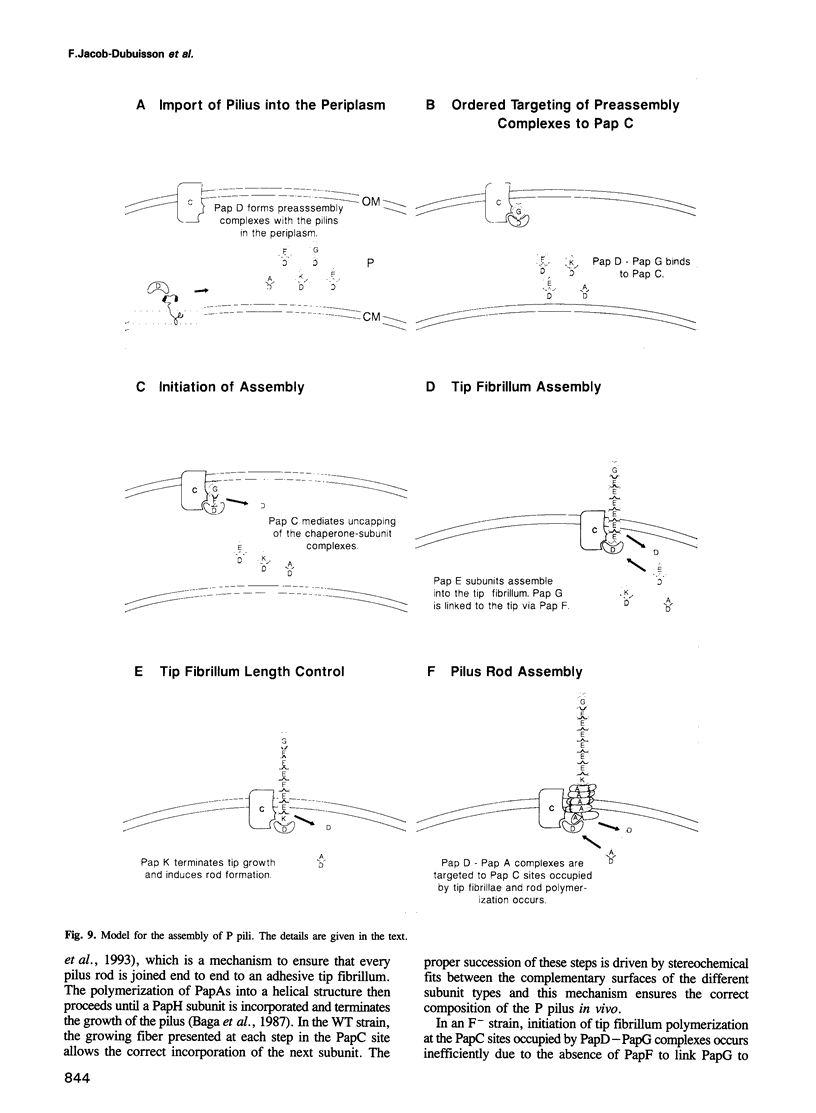
Uropathogenic Escherichia coli produce heteropolymeric surface fibers called P pili, which present an adhesin at their tip that specifically recognizes globoside receptors on the host uroepithelium. The initial attachment step is thought to be essential for pathogenesis. P pili are composite fibers consisting of a thin tip fibrillum joined end to end to a rigid helical rod. Here we show that the ordered assembly of these structures requires the activity of two proteins that are minor components of the tip fibrillum, PapF and PapK. PapF is required for the correct presentation of the adhesin at the distal end of the tip fibrillum. PapK regulates the length of the tip fibrillum and joins it to the pilus rod. We propose that these subunits function as adaptors, by providing complementary surfaces to different substructures of the pilus and promoting their proper associations. In addition, the conversion of chaperone-subunit complexes into pili depends on PapF and PapK since a papF- papK- double mutation abolishes piliation. We suggest that in addition to the adaptor functions of PapF and PapK, they are also required to initiate the formation of tip fibrillae and pilus rods.
Full text
PDF

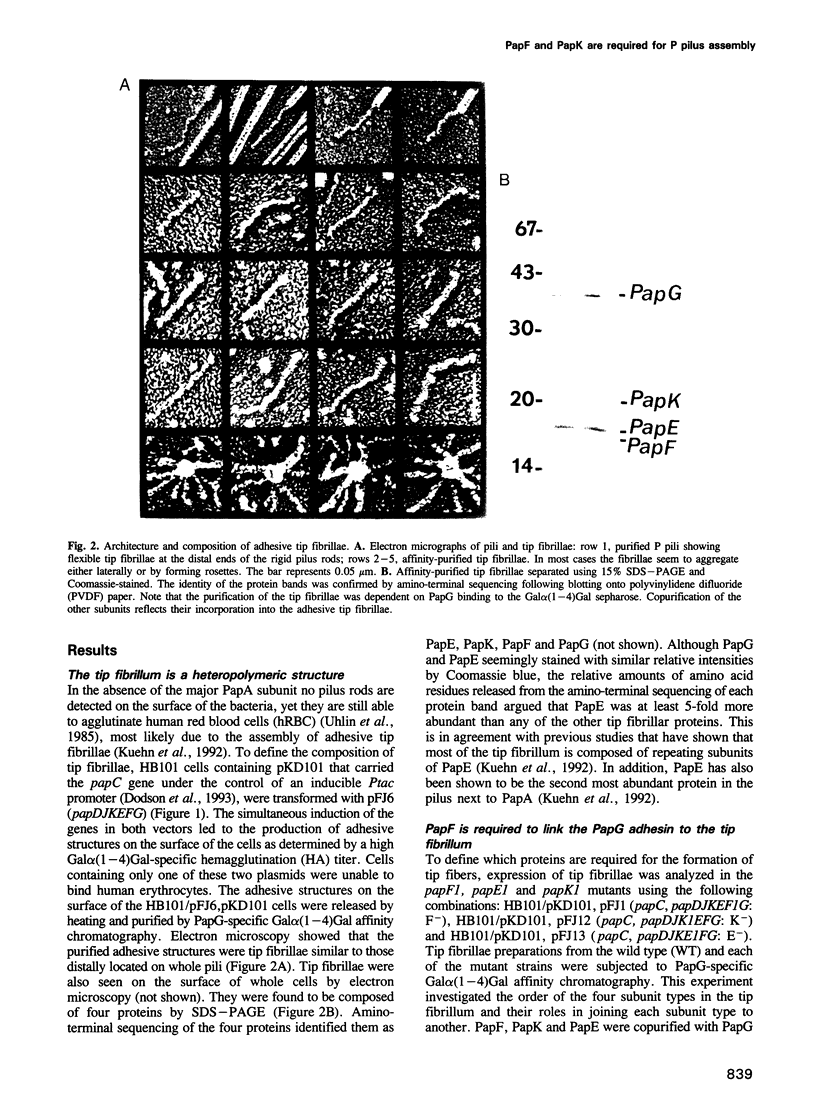





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Båga M., Norgren M., Normark S. Biogenesis of E. coli Pap pili: papH, a minor pilin subunit involved in cell anchoring and length modulation. Cell. 1987 Apr 24;49(2):241–251. doi: 10.1016/0092-8674(87)90565-4. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C., Möller G. Induction of immunological tolerance requires that the B cells can respond to the polyclonal B-cell-activating properties of the thymus-independent antigens. J Exp Med. 1977 Jul 1;146(1):308–312. doi: 10.1084/jem.146.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., Makowski L. Helical structure of P pili from Escherichia coli. Evidence from X-ray fiber diffraction and scanning transmission electron microscopy. J Mol Biol. 1992 Dec 5;228(3):735–742. doi: 10.1016/0022-2836(92)90860-m. [DOI] [PubMed] [Google Scholar]
- Heuser J. Protocol for 3-D visualization of molecules on mica via the quick-freeze, deep-etch technique. J Electron Microsc Tech. 1989 Nov;13(3):244–263. doi: 10.1002/jemt.1060130310. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Kuehn M. J., Brändén C. I., Hultgren S. J. Conserved immunoglobulin-like features in a family of periplasmic pilus chaperones in bacteria. EMBO J. 1992 Apr;11(4):1617–1622. doi: 10.1002/j.1460-2075.1992.tb05207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Lindberg F., Magnusson G., Kihlberg J., Tennent J. M., Normark S. The PapG adhesin of uropathogenic Escherichia coli contains separate regions for receptor binding and for the incorporation into the pilus. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4357–4361. doi: 10.1073/pnas.86.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Normark S., Abraham S. N. Chaperone-assisted assembly and molecular architecture of adhesive pili. Annu Rev Microbiol. 1991;45:383–415. doi: 10.1146/annurev.mi.45.100191.002123. [DOI] [PubMed] [Google Scholar]
- Klemm P., Christiansen G. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1987 Jul;208(3):439–445. doi: 10.1007/BF00328136. [DOI] [PubMed] [Google Scholar]
- Kuehn M. J., Heuser J., Normark S., Hultgren S. J. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature. 1992 Mar 19;356(6366):252–255. doi: 10.1038/356252a0. [DOI] [PubMed] [Google Scholar]
- Kuehn M. J., Normark S., Hultgren S. J. Immunoglobulin-like PapD chaperone caps and uncaps interactive surfaces of nascently translocated pilus subunits. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10586–10590. doi: 10.1073/pnas.88.23.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F. P., Lund B., Normark S. Genes of pyelonephritogenic E. coli required for digalactoside-specific agglutination of human cells. EMBO J. 1984 May;3(5):1167–1173. doi: 10.1002/j.1460-2075.1984.tb01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Tennent J. M., Hultgren S. J., Lund B., Normark S. PapD, a periplasmic transport protein in P-pilus biogenesis. J Bacteriol. 1989 Nov;171(11):6052–6058. doi: 10.1128/jb.171.11.6052-6058.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund B. I., Tennent J. M., Garcia E., Hamers A., Båga M., Lindberg F., Gaastra W., Normark S. Horizontal gene transfer of the Escherichia coli pap and prs pili operons as a mechanism for the development of tissue-specific adhesive properties. Mol Microbiol. 1992 Aug;6(16):2225–2242. doi: 10.1111/j.1365-2958.1992.tb01399.x. [DOI] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Structure of common pili from Escherichia coli. J Bacteriol. 1979 Jun;138(3):969–975. doi: 10.1128/jb.138.3.969-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., Wijfjes A., de Graaf F. K. Identification and characterization of precursors in the biosynthesis of the K88ab fimbria of Escherichia coli. J Bacteriol. 1983 Apr;154(1):41–49. doi: 10.1128/jb.154.1.41-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Båga M., Tennent J. M., Normark S. Nucleotide sequence, regulation and functional analysis of the papC gene required for cell surface localization of Pap pili of uropathogenic Escherichia coli. Mol Microbiol. 1987 Sep;1(2):169–178. doi: 10.1111/j.1365-2958.1987.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Oudega B., de Graaf M., de Boer L., Bakker D., Vader C. E., Mooi F. R., de Graaf F. K. Detection and identification of FaeC as a minor component of K88 fibrillae of Escherichia coli. Mol Microbiol. 1989 May;3(5):645–652. doi: 10.1111/j.1365-2958.1989.tb00212.x. [DOI] [PubMed] [Google Scholar]
- Riegman N., Acton D., Sakkers R., van Die I., Hoekstra W., Bergmans H. Functional analysis of the fsoC gene product of the F7(1) (fso) fimbrial gene cluster. Mol Microbiol. 1990 Jan;4(1):101–106. doi: 10.1111/j.1365-2958.1990.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Riegman N., Hoschützky H., van Die I., Hoekstra W., Jann K., Bergmans H. Immunocytochemical analysis of P-fimbrial structure: localization of minor subunits and the influence of the minor subunit FsoE on the biogenesis of the adhesin. Mol Microbiol. 1990 Jul;4(7):1193–1198. doi: 10.1111/j.1365-2958.1990.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Riegman N., van Die I., Leunissen J., Hoekstra W., Bergmans H. Biogenesis of F7(1) and F7(2) fimbriae of uropathogenic Escherichia coli: influence of the FsoF and FstFG proteins and localization of the Fso/FstE protein. Mol Microbiol. 1988 Jan;2(1):73–80. [PubMed] [Google Scholar]
- Russell P. W., Orndorff P. E. Lesions in two Escherichia coli type 1 pilus genes alter pilus number and length without affecting receptor binding. J Bacteriol. 1992 Sep;174(18):5923–5935. doi: 10.1128/jb.174.18.5923-5935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferli D. M., Beachey E. H., Taylor R. K. Genetic analysis of 987P adhesion and fimbriation of Escherichia coli: the fas genes link both phenotypes. J Bacteriol. 1991 Feb;173(3):1230–1240. doi: 10.1128/jb.173.3.1230-1240.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll T., Hoschützky H., Morschhäuser J., Lottspeich F., Jann K., Hacker J. Analysis of genes coding for the sialic acid-binding adhesin and two other minor fimbrial subunits of the S-fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1989 Dec;3(12):1735–1744. doi: 10.1111/j.1365-2958.1989.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Simons B. L., Willemsen P. T., Bakker D., Roosendaal B., De Graaf F. K., Oudega B. Structure, localization and function of FanF, a minor component of K99 fibrillae of enterotoxigenic Escherichia coli. Mol Microbiol. 1990 Dec;4(12):2041–2050. doi: 10.1111/j.1365-2958.1990.tb00564.x. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Norgren M., Båga M., Normark S. Adhesion to human cells by Escherichia coli lacking the major subunit of a digalactoside-specific pilus-adhesin. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1800–1804. doi: 10.1073/pnas.82.6.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund B., van Die I., Kramer C., Kuusela P., Holthöfer H., Tarkkanen A. M., Virkola R., Riegman N., Bergmans H., Hoekstra W. Multifunctional nature of P fimbriae of uropathogenic Escherichia coli: mutations in fsoE and fsoF influence fimbrial binding to renal tubuli and immobilized fibronectin. Mol Microbiol. 1991 Dec;5(12):2965–2975. doi: 10.1111/j.1365-2958.1991.tb01856.x. [DOI] [PubMed] [Google Scholar]
- Zhang H., Scholl R., Browse J., Somerville C. Double stranded DNA sequencing as a choice for DNA sequencing. Nucleic Acids Res. 1988 Feb 11;16(3):1220–1220. doi: 10.1093/nar/16.3.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]