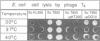Abstract
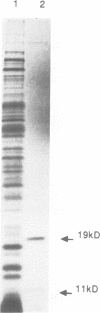
When present on a multicopy plasmid, a newly discovered gene (sugE) mapping to 94 min on the Escherichia coli chromosome, suppresses a groEL mutation and mimics the effects of groE overexpression. A groEL mutant of E.coli, transformed with the Klebsiella pneumoniae nif gene cluster, failed to accumulate nitrogenase components [Govezensky et al. (1991) J. Bacteriol., 173, 6339-6346]. Transformation with sugE reversed the mutant phenotype. In wild type K.pneumoniae, transformation with sugE accelerated the rate of nitrogenase biogenesis after nif derepression. In E.coli, transformation with sugE enabled bacteriophage T4 growth in a groEL mutant. A continuous 178 codon open reading frame (ORF) in sugE encloses another, in-frame, 105 codon ORF similar to a predicted ORF in Proteus vulgaris. In vivo products of both sugE ORFs were observed in transformants expressing the gene from a T7 promoter. In non-transformed cells, a typical sigma 70-dependent promoter found upstream of the larger ORF directs sugE transcription during growth at 30 degrees C. At elevated temperatures or in stationary phase cells, another promoter, found within the coding sequence upstream of the smaller ORF, is activated independently of sigma 32. The results suggest that sugE encodes a chaperonin-related system whose composition might vary with temperature and growth phase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W., Rump A., Klipp W., Priefer U. B., Pühler A. Nucleotide sequence of a 24,206-base-pair DNA fragment carrying the entire nitrogen fixation gene cluster of Klebsiella pneumoniae. J Mol Biol. 1988 Oct 5;203(3):715–738. doi: 10.1016/0022-2836(88)90205-7. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J., Gershoni J. M., Zamir A. Expression of nitrogen fixation genes in foreign hosts. Assembly of nitrogenase Fe protein in Escherichia coli and in yeast. J Biol Chem. 1985 May 10;260(9):5240–5243. [PubMed] [Google Scholar]
- Berman J., Zilberstein A., Salomon D., Zamir A. Expression of a nitrogen-fixation gene encoding a nitrogenase subunit in yeast. Gene. 1985;35(1-2):1–9. doi: 10.1016/0378-1119(85)90151-9. [DOI] [PubMed] [Google Scholar]
- Bloom M., Skelly S., VanBogelen R., Neidhardt F., Brot N., Weissbach H. In vitro effect of the Escherichia coli heat shock regulatory protein on expression of heat shock genes. J Bacteriol. 1986 May;166(2):380–384. doi: 10.1128/jb.166.2.380-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. T. Nucleotide sequence and comparative analysis of the frd operon encoding the fumarate reductase of Proteus vulgaris. Extensive sequence divergence of the membrane anchors and absence of an frd-linked ampC cephalosporinase gene. Eur J Biochem. 1987 Sep 15;167(3):481–488. doi: 10.1111/j.1432-1033.1987.tb13362.x. [DOI] [PubMed] [Google Scholar]
- Cowing D. W., Bardwell J. C., Craig E. A., Woolford C., Hendrix R. W., Gross C. A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci U S A. 1985 May;82(9):2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Erickson J. W., Gross C. A. Identification of the sigma E subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes Dev. 1989 Sep;3(9):1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- Fayet O., Louarn J. M., Georgopoulos C. Suppression of the Escherichia coli dnaA46 mutation by amplification of the groES and groEL genes. Mol Gen Genet. 1986 Mar;202(3):435–445. doi: 10.1007/BF00333274. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C., Ang D. The Escherichia coli groE chaperonins. Semin Cell Biol. 1990 Feb;1(1):19–25. [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
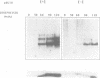
- Govezensky D., Greener T., Segal G., Zamir A. Involvement of GroEL in nif gene regulation and nitrogenase assembly. J Bacteriol. 1991 Oct;173(20):6339–6346. doi: 10.1128/jb.173.20.6339-6346.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govezensky D., Zamir A. Structure-function relationships in the alpha subunit of Klebsiella pneumoniae nitrogenase MoFe protein from analysis of nifD mutants. J Bacteriol. 1989 Oct;171(10):5729–5735. doi: 10.1128/jb.171.10.5729-5735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R., Roberts R. E., Wilde R. J. Cloning of the aspartase gene (aspA) of Escherichia coli. J Gen Microbiol. 1984 May;130(5):1271–1278. doi: 10.1099/00221287-130-5-1271. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Holland D., Zilberstein A., Govezensky D., Salomon D., Zamir A. Nitrogenase MoFe protein subunits from Klebsiella pneumoniae expressed in foreign hosts. Characteristics and interactions. J Biol Chem. 1987 Jun 25;262(18):8814–8820. [PubMed] [Google Scholar]
- Jaurin B., Grundström T. ampC cephalosporinase of Escherichia coli K-12 has a different evolutionary origin from that of beta-lactamases of the penicillinase type. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4897–4901. doi: 10.1073/pnas.78.8.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A. J., March J. B., Oliver I. R., Masters M. A DNA fragment containing the groE genes can suppress mutations in the Escherichia coli dnaA gene. Mol Gen Genet. 1986 Mar;202(3):446–454. doi: 10.1007/BF00333275. [DOI] [PubMed] [Google Scholar]
- Jenkins D. E., Auger E. A., Matin A. Role of RpoH, a heat shock regulator protein, in Escherichia coli carbon starvation protein synthesis and survival. J Bacteriol. 1991 Mar;173(6):1992–1996. doi: 10.1128/jb.173.6.1992-1996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone D. B., Farr S. B. AppppA binds to several proteins in Escherichia coli, including the heat shock and oxidative stress proteins DnaK, GroEL, E89, C45 and C40. EMBO J. 1991 Dec;10(12):3897–3904. doi: 10.1002/j.1460-2075.1991.tb04959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusukawa N., Yura T. Heat shock protein GroE of Escherichia coli: key protective roles against thermal stress. Genes Dev. 1988 Jul;2(7):874–882. doi: 10.1101/gad.2.7.874. [DOI] [PubMed] [Google Scholar]
- Lohmeier E., Hagen D. S., Dickie P., Weiner J. H. Cloning and expression of fumarate reductase gene of Escherichia coli. Can J Biochem. 1981 Mar;59(3):158–164. doi: 10.1139/o81-023. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Vaughn V., Phillips T. A., Bloch P. L. Gene-protein index of Escherichia coli K-12. Microbiol Rev. 1983 Jun;47(2):231–284. doi: 10.1128/mr.47.2.231-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel H. R., Stitt B. L., Lielausis I., Wood W. B. Role of the host cell in bacteriophage T4 development. I. Characterization of host mutants that block T4 head assembly. J Virol. 1980 Jan;33(1):366–376. doi: 10.1128/jvi.33.1.366-376.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MYu, Goldberg A. L. Heat shock in Escherichia coli alters the protein-binding properties of the chaperonin groEL by inducing its phosphorylation. Nature. 1992 May 14;357(6374):167–169. doi: 10.1038/357167a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Takano T., Kakefuda T. Involvement of a bacterial factor in morphogenesis of bacteriophage capsid. Nat New Biol. 1972 Sep 13;239(89):34–37. doi: 10.1038/newbio239034a0. [DOI] [PubMed] [Google Scholar]
- Tilly K., Murialdo H., Georgopoulos C. Identification of a second Escherichia coli groE gene whose product is necessary for bacteriophage morphogenesis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1629–1633. doi: 10.1073/pnas.78.3.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. P., Kaguni J. M. A novel sigma factor is involved in expression of the rpoH gene of Escherichia coli. J Bacteriol. 1989 Aug;171(8):4248–4253. doi: 10.1128/jb.171.8.4248-4253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilstra-Ryalls J., Fayet O., Georgopoulos C. The universally conserved GroE (Hsp60) chaperonins. Annu Rev Microbiol. 1991;45:301–325. doi: 10.1146/annurev.mi.45.100191.001505. [DOI] [PubMed] [Google Scholar]
- Zhou Y. N., Kusukawa N., Erickson J. W., Gross C. A., Yura T. Isolation and characterization of Escherichia coli mutants that lack the heat shock sigma factor sigma 32. J Bacteriol. 1988 Aug;170(8):3640–3649. doi: 10.1128/jb.170.8.3640-3649.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]