Abstract
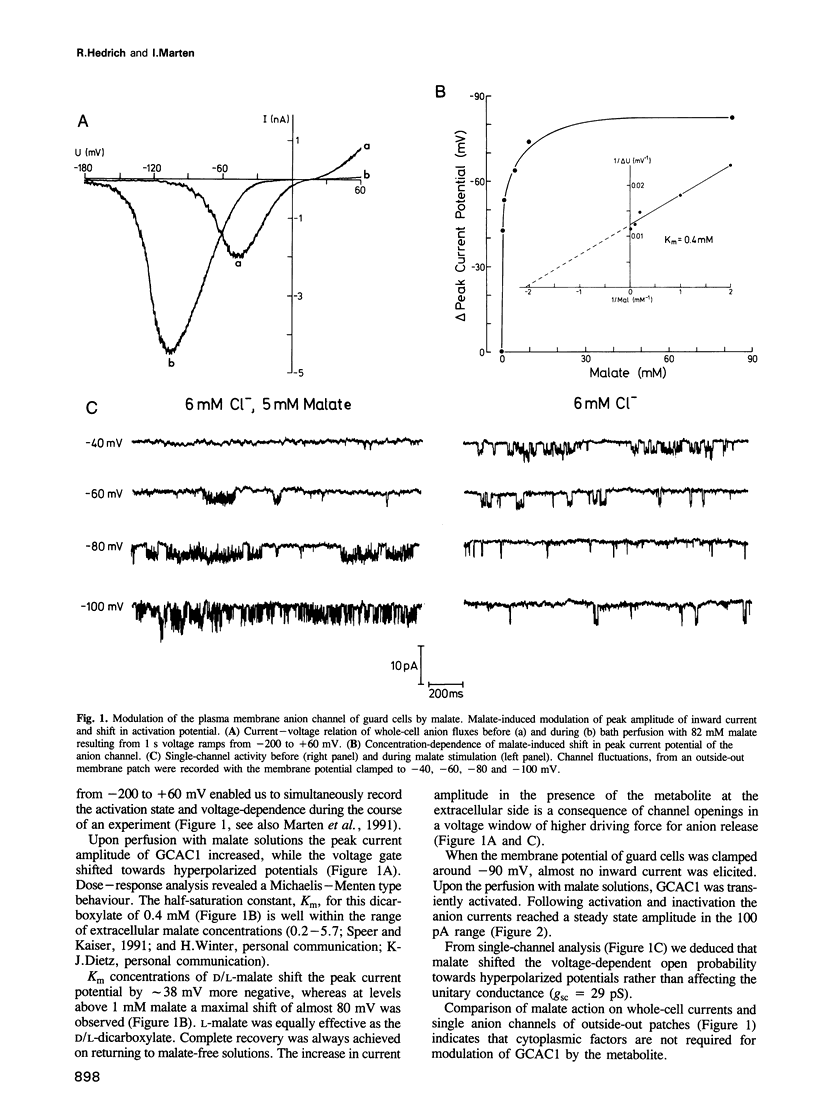
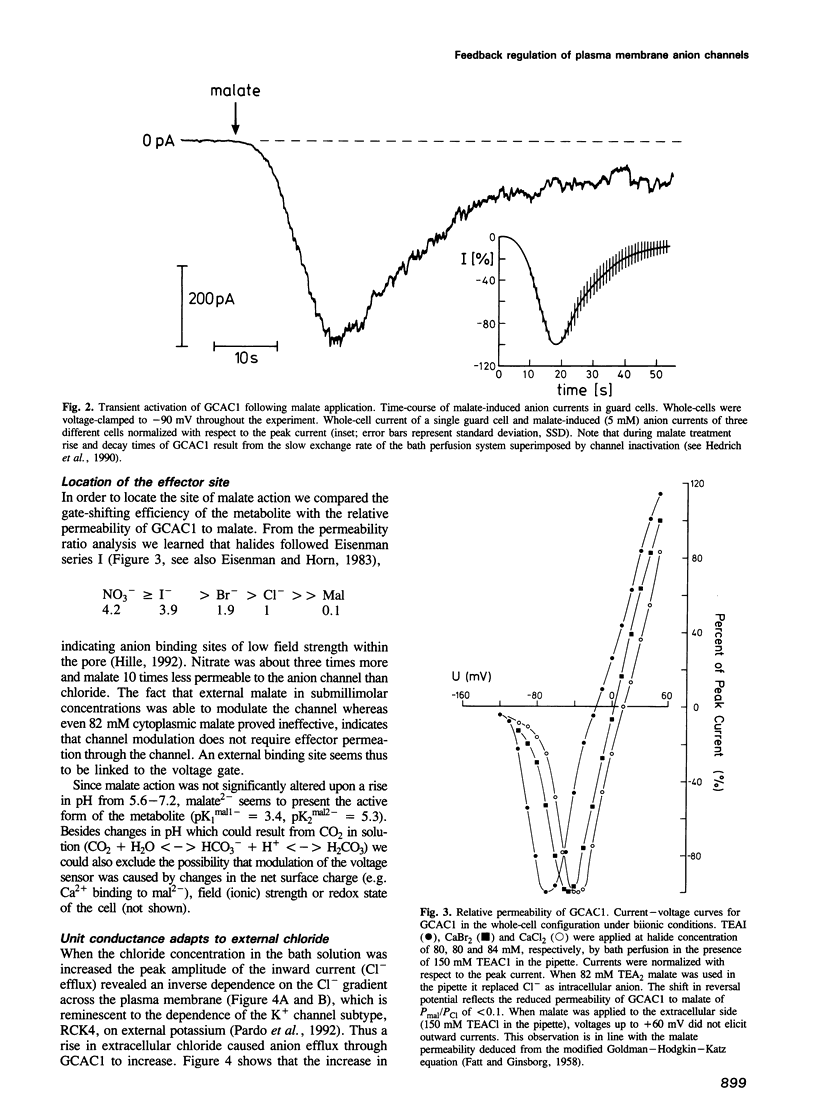
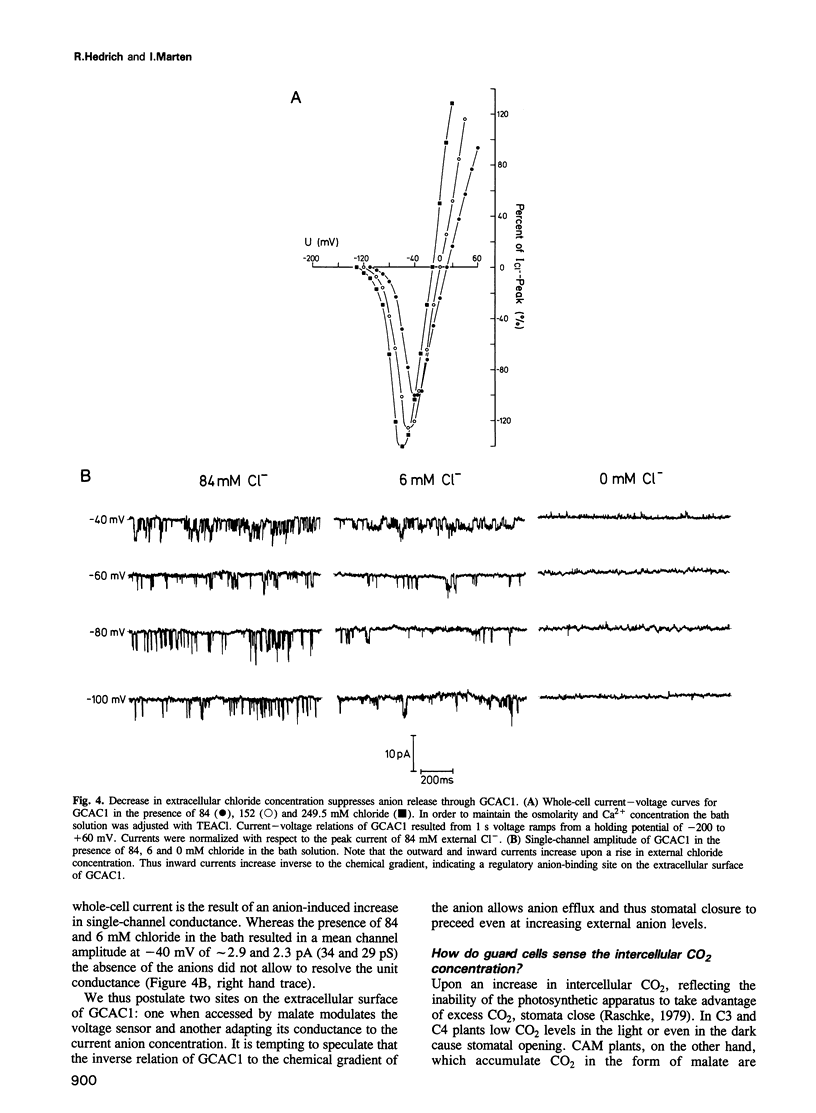
Plants have developed strategies to circumvent limitations in water supply through the adjustment of stomatal aperture in relation to the photosynthetic capacity (water-use efficiency). The CO2 sensor of guard cells, reporting on the metabolic status of the photosynthetic tissue, is, however, as yet unknown. We elucidated whether extracellular malate has the capability to serve as a signal metabolite in regulating the membrane properties of guard cells. Patch-clamp studies showed that slight variations in the external malate concentration induced major alterations in the voltage-dependent activity of the guard cell anion channel (GCAC1). Superfusion of guard cell protoplasts with malate solutions in the physiological range caused the voltage-gate to shift towards hyperpolarized potentials (Km(mal) = 0.4 mM elicits a 38 mV shift). The selectivity sequence of the anion channel NO3- (4.2) > or = I- (3.9) > Br- (1.9) > Cl- (1) >> mal (0.1) indicates that malate is able to permeate GCAC1. The binding site for shifting the gate is, however, located on the extracellular face of the channel since cytoplasmic malate proved ineffective. Single-channel analysis indicates that extracellular malate affects the voltage-dependent mean open time rather than the unitary conductance of GCAC1. In contrast to malate the rise in the extracellular Cl- concentration increases the unit conductance of the anion efflux channel. We suggest that stomata sense changes in the intercellular CO2 concentration and thus the photosynthetic activity of the mesophyll via feedback regulation of anion efflux from guard cells through malate-sensitive GCAC1.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatt M. R. Ion channel gating in plants: physiological implications and integration for stomatal function. J Membr Biol. 1991 Nov;124(2):95–112. doi: 10.1007/BF01870455. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76(3):197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hedrich R., Busch H., Raschke K. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 1990 Dec;9(12):3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., Jeromin A. A new scheme of symbiosis: ligand- and voltage-gated anion channels in plants and animals. Philos Trans R Soc Lond B Biol Sci. 1992 Oct 29;338(1283):31–38. doi: 10.1098/rstb.1992.0126. [DOI] [PubMed] [Google Scholar]
- Marten I., Zeilinger C., Redhead C., Landry D. W., al-Awqati Q., Hedrich R. Identification and modulation of a voltage-dependent anion channel in the plasma membrane of guard cells by high-affinity ligands. EMBO J. 1992 Oct;11(10):3569–3575. doi: 10.1002/j.1460-2075.1992.tb05440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo L. A., Heinemann S. H., Terlau H., Ludewig U., Lorra C., Pongs O., Stühmer W. Extracellular K+ specifically modulates a rat brain K+ channel. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2466–2470. doi: 10.1073/pnas.89.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Hedrich R. Involvement of ion channels and active transport in osmoregulation and signaling of higher plant cells. Trends Biochem Sci. 1989 May;14(5):187–192. doi: 10.1016/0968-0004(89)90272-7. [DOI] [PubMed] [Google Scholar]
- Speer M., Kaiser W. M. Ion Relations of Symplastic and Apoplastic Space in Leaves from Spinacia oleracea L. and Pisum sativum L. under Salinity. Plant Physiol. 1991 Nov;97(3):990–997. doi: 10.1104/pp.97.3.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G., MacRobbie E. A., Blatt M. R. Membrane transport in stomatal guard cells: the importance of voltage control. J Membr Biol. 1992 Feb;126(1):1–18. doi: 10.1007/BF00233456. [DOI] [PubMed] [Google Scholar]
- Van Kirk C. A., Raschke K. Release of Malate from Epidermal Strips during Stomatal Closure. Plant Physiol. 1978 Mar;61(3):474–475. doi: 10.1104/pp.61.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]


