Abstract
Inducible nitric-oxide synthase (iNOS) produces the reactive oxygen and nitrogen species (ROS/RNS) involved in bacteria killing and is crucial in the host defense mechanism. However, high level ROS/RNS can also be detrimental to normal cells and thus their production has to be tightly controlled. Availability or deficiency of tetrahydrobiopterin (BH4) cofactor and L-arginine substrate control coupling or uncoupling of NOS catalysis. Fully coupled reaction, with abundant BH4 and L-arginine, produces NO whereas the uncoupled NOS (in the absence of BH4 and/or L-arginine) generates ROS/RNS. In the current work we focus on direct rapid freeze EPR to characterize the structure and kinetics of oxygen-induced radical intermediates produced by ferrous inducible NOS oxygenase domain (iNOSox) in the presence or absence of BH4 and/or L-arginine. Fully reconstituted iNOSox (+BH4, +L-Arg) forms a dimer and yields a typical BH4 radical that indicates coupled reaction. iNOSox (–BH4) remains mainly monomeric and produces exclusively superoxide, that is only marginally affected by the presence of L-arginine. iNOSox (+BH4, −L-Arg) exists as a monomer/dimer mixture and yields both BH4 radical and superoxide. Present study is a natural extension of our previous work on the ferrous endothelial NOSox (eNOSox) [V. Berka, G. Wu, H.C. Yeh, G. Palmer, A.L. Tsai, J. Biol. Chem. 279 ( 2004) 32243–32251] and ferrous neuronal NOSox (nNOSox) [V. Berka, L.H. Wang, A.L. Tsai, Biochemistry 47 (2008) 405–420]. Overall, our data suggests different regulatory roles of L-arginine and BH4 in the production of oxygen-induced radical intermediates in NOS isoforms which nicely serve individual functional role.
1. INTRODUCTION
Nitric oxide (NO) is a gaseous signaling molecule and its production is catalyzed by three isoforms of nitric oxide synthase (NOS): neuronal nitric oxide synthase (nNOS/NOSI), inducible NOS (iNOS/NOSII) and endothelial NOS (eNOS/NOSIII). Both nNOS and eNOS are constitutively expressed Ca2+- calmodulin dependent enzymes that produce nM levels of NO [1–3]. In contrast, expression of Ca2+-independent iNOS, induced by inflammatory mediators, such as lipopolysaccharide (LPS) and cytokines, results in production of µM levels of NO [4, 5] to perform its bacterial killing function [6–11]. The difference in the NO output between eNOS/nNOS and iNOS points to possible differences in their reaction mechanism and deserves further investigation.
All three NOS isozymes catalyze NADPH-dependent conversion of substrate L-arginine and oxygen to NO and L-citrulline through 5-electron oxidation of N-atom of the guanidino group of L-arginine [12] [13]. The bioavailability of substrate L-arginine and cofactor BH4 is absolutely essential for production of NO from all three NOS isoforms. L-arginine and/or BH4 deficiency causes “uncoupling”, leading to production of superoxide [14–18] and/or hydrogen peroxide [19] instead of NO. In addition to NOS enzymes, L-arginine is also a substrate for arginase , another key enzyme involved in immunoregulation [20] leading to L-arginine deficiency for NOSs under certain pathological conditions [21–23]. Similarly, BH4 deficiency, or more precisely the ratio between cellular BH4 and its oxidized counterpart BH2, a competitive inhibitor of NOS, dictates the extent of NOS uncoupling, and is tightly associated with vascular dysfunction [24–26]. Thus, metabolic pathways that control the level of BH4 and L-arginine play crucial role in regulation of coupling of NOS catalysis and determine the NO/O2•− ratio. While uncoupling could be beneficial for iNOS function in cytoprotection to generate potent product including peroxynitrite formed from NO and O2•−, it is harmful for eNOS as it leads to vascular dysfunction and is detrimental to nNOS due to its high sensitivity to the ROS or RNS [27–30].
NOS isozymes are composed of two domains; an N-terminal heme-containing oxygenase domain and a C-terminal flavin-containing reductase domain, tethered by a Ca2+/calmodulin-binding loop. Both the oxygenase and reductase domains of NOS can fold and function independently of one another but fully functional wild type (WT) NOSs are active only as homodimers [31, 32]. The BH4, and partially L-arginine, are important for dimerization of NOS enzymes, especially for dimerization of iNOS, as BH4-deficient iNOS is fully monomeric [33]. The iNOS dimers are less stable than the other two NOS enzymes and thus control of monomer/dimer equilibrium may be, on top of substrate and/or BH4 availability, an additional factor that controls switching from NO to the ROS/RNS production in iNOS [12, 33].
In addition to the role in NOS dimerization, BH4 plays a critical role in NO biosynthesis. The proposed mechanism for NOS catalysis, especially the first reaction of L-arginine hydroxylation is relatively well characterized [13, 34, 35] and consists of the following steps: in the NADPH driven reaction, ferric heme is reduced to ferrous form by electron transfer from reductase flavins; oxygen then binds to the ferrous heme to form oxy-ferrous (Fe(II)O2) intermediate, which then receives an electron from BH4 to reduce FeIIO2 intermediate to form ferric peroxy intermediate and BH4 itself is converted to an enzyme-bound cationic BH4 radical. Several studies from different groups including us characterized spectroscopically the formation and decay kinetics of the Fe(II)O2 complex [36–43], as well as BH4 radical formation in all three NOS isoforms [39, 44–46]. Recently published papers provide even deeper insights into understanding of the structure/function aspects of BH4 radical [47–50]. Timely supply of this reducing equivalent is critical for coupling of the NO formation since NOS Fe(II)O2 intermediates are unstable and may produce superoxide or H2O2 if electrons from BH4 are not delivered to the heme at a sufficient rate or if both substrate and BH4 are missing [16, 18, 51–55].
With focus on the first step of NOS catalysis, we initiated the single-turnover studies of interaction of ferrous eNOSox and nNOSox [46] [56] with oxygen using direct rapid freeze quench EPR (RFQ EPR) rather than indirect spin-trapping method, which provide neither direct structural nor kinetic information for the various radical intermediates. Single-turnover RFQ EPR allowed us to identify, in addition to typical BH4 radical and superoxide, also novel radical species formed by eNOSox and nNOSox [40, 46, 56]. Moreover, the correlational spectral kinetic data enabled quantitative mechanistic interpretation. Our studies demonstrated that cofactor, substrate, and even reductant DTT affect the reactions and structures of radical intermediates of the two constitutive NOS enzymes differently [40, 46, 56].
In light of our previously identified differences between eNOS and nNOS together with the vastly different functional role and biological [NO] from eNOS/nNOS, we extended our study to iNOSox to reveal similarity and differences in reaction mechanisms of three NOSs. To address and to clarify the roles of BH4, L-arginine, and DTT in radical formation during reaction of iNOSox with oxygen, we measured the kinetics of heme transitions and radical formation in the first half of NO synthesis by single-turnover stopped-flow and RFQ EPR.
2. EXPERIMENTAL PROCEDURES
2.1. Materials and General Methods
All reagents and kits for DNA extraction and isolation were products of Qiagen (Valencia, CA). BH4 and BH2 were obtained from Schircks Laboratories (Jona, Switzerland). Restriction enzymes were purchased from New England Biolabs (Beverly, MA). The One ShotR BL21 Star™ (DE3) cells were obtained from Invitrogen (Carlsbad, Ca). Ni-NTA Agarose was obtained from Qiagen (Valencia, CA). The other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
2.2. Protein Expression and Purification of human iNOSox
Human iNOSox (amino acid 59–504) containing six-histidine (6His) at the C-terminus was expressed in Escherichia coli BL21 Star™ (DE3) using pCWori vector. Human iNOS cDNA in pBlueScript (SK(-))TA plasmid, kindly provided by Professor Timothy R. Billiar (University of Pittsburgh), was used as a template for PCR amplification of DNA fragment encoding calmodulin-free oxygenase domain, amino acids 59–504. The iNOSox domain forward primer 5'-AGGCATATGCTCGTGGAGACGGGAAAG-3' (translation start codon is underlined) also included the NdeI (in italics) site. The reverse primer 5'-CCGCAAGCTTTCAGTGATGGTGATGATGGTGCTCGTCCTGCCAGACATGGG-3' has added HindIII restriction site (in italics) after the stop codon (underlined) and 6His tag (in bold). The PCR product was first ligated into the TA vector (Invitrogen, Carlsbad, CA) and then introduced into XL10 Gold Escherichia coli cells for propagation according to the manufacturer’s recommendation. The sequence of iNOSox-6His DNA was confirmed by a primer extension sequencing (UT-HSC sequencing Core). The iNOSox- 6His DNA was excised from the plasmid by double-digestion with NdeI and HindIII restriction enzymes and subsequently ligated into the corresponding sites of the pCW vector. Purified pCW-iNOSox-6His plasmid was transformed into E. coli BL21 Star™ (DE3) cells containing the pT-groE plasmid [57] with a chloramphenicol-resistance gene for protein expression.
The expression and purification of human iNOSox in the absence of BH4 was performed similarly as we described previously for nNOSox [56] using 50 mM HEPES, 0.1 M NaCl, 10 % glycerol, pH 7.8 without DTT as our purification buffer. Our established bacterial expression system for human iNOSox domain yielded ~15 mg of the purified protein per liter of culture medium, with ~90 % purity, judged by the ratio of A280/A396, SDS-PAGE analysis and the heme content by pyridine hemochrome assay. For the experiments with BH4, BH2 and/or L-arginine, freshly isolated iNOSox was incubated overnight with 1 mM of biopterin at desired redox state, 1mM β-mercaptoethanol and/or 1mM L-arginine under anaerobic conditions and then stored in liquid nitrogen container. Unless otherwise indicated, excess of biopterin and ligands was removed before the experiments by passing through a 10-DG gel-filtration column.
2.3. Stopped-Flow Experiments
Kinetics of the reaction between Fe(II) iNOSox and oxygen were conducted at ambient temperature (22 °C) using an Applied Photophysics (Leatherhead, United Kingdom) Bio-Sequential SX-18MV stopped-flow instrument equipped with a rapid-scan diode array detector as previously described [46]. The sample handling and signal detection units were located in the anaerobic chamber filled with 5% H2 in N2 pre-filtered with a palladium-based O2 scrubber. During sample handling and kinetic measurements the gas analyzer (Model 10, Coy Laboratory Products, Inc.) tracked the anaerobic conditions to ensure that oxygen level was 0 ppm. Measurements were carried out using 8–10 µM ferrous enzymes and monitored by diode array detector or at different wavelengths as detailed in the Results section. Anaerobic ferrous samples were prepared in a tonometer with three 5-minute cycles of vacuum and argon replacement followed by stoichiometric titration using anaerobic sodium dithionite solution [58]. Extra care was taken to avoid excess dithionite addition by monitoring the absorbance at 315 nm (ε315 nm = 8 mM−1cm−1) [59]. The rates were calculated by fitting the obtained data to a single- or a double- exponential function, based on the profile of the kinetic data.
2.4. Quantitation of Superoxide
The superoxide released from the iNOSox sample during single-turnover reaction of ferrous iNOSox (10 µM before reaction) with oxygen (as air saturated buffer, ~240 µM of oxygen at 22 °C) was measured by the cytochrome c (at 50 µM ) trapping reaction, and was detected by stopped-flow for 5 sec. The total amount of superoxide was calculated using difference extinction coefficient of 21 mM−1 cm−1 at 550 nm [60] at the end of reaction. The optical change at 550 nm during oxidation of ferrous iNOSox with oxygen in the absence of cytochrome c was used as a baseline.
2.5. Hydrogen peroxide detection
The Amplex Red reagent (10-acetyl-3,7-dihydroxyphenoxazine, Molecular Probes), in combination with horseradish peroxidase (HRP), has been used to detect H2O2 released from different iNOSox samples. 3 ml solution of anaerobic ferrous iNOSox (10 µM) in 50 mM HEPES buffer, 0.1 mM NaCl, 10 % glycerol in tonometer was oxidized by adding 30 µl of aerobic buffer containing 5U of HRP and 20 µM Amplex Red. Resorufin, the red oxidation product of Amplex Red due to H2O2 formed by iNOSox was detected spectrophotometrically by 8453 Hewlett-Packard diode array spectrophotometer 15 sec after mixing. The total amount of hydrogen peroxide was calculated from difference extinction coefficient of 58 mM−1 cm−1 at 571 nm between ferric iNOSox and peak of resorufin.
2.6. Gel filtration Chromatography
Molecular weight of iNOSox proteins was estimated by size exclusion chromatography using a Bio-Rad Bio-Sil TSK-250 column. The column was pre-equilibrated with 50 mM HEPES buffer (pH 7.5), containing 10 % glycerol and 0.1 M NaCl at 22 °C. Equal amounts of iNOSox were injected through a 100 µl loading loop. The isocratic flow rate was 1 ml/min and the column eluate was monitored at 280 nm using Agilent 1100 series UV flow through detector. Molecular weights of protein peaks were estimated relative to protein molecular standards from Sigma Molecular Weight Marker Kit.
2.7. RFQ EPR Kinetic Measurements
Ferrous iNOSox proteins were prepared as described previously [58]. Briefly, solutions containing 40–50 µM of iNOSox, biopterin free or BH4-reconstituted, in a HEPES buffer (50 mM HEPES, pH 7.5, 0.1 M NaCl, 10% glycerol) was reduced by anaerobic titration with a minimal amount of dithionite, in the presence or absence of 1 mM L-arginine, to shift the heme Soret peak to 413 nm, a marker for ferrous iNOSox formation. The ferrous iNOSox was then reacted with air-saturated buffer. Samples were collected for EPR analysis at different reaction time points by the RFQ apparatus (Update Instrument System 1000, Madison, WI) placed inside an anaerobic chamber (Coy Laboratory, MI). All liquid channels including sample syringes, tubings and mixers were kept inside the anaerobic chamber and only the exit end of the nozzle was exposed to air and was unplugged right before the shots. Single push program was used to obtain samples freeze-trapped with reaction time controlled by the length of tubing. At a ram velocity of 1.25 cm/s, the dead time is ~7 ms at room temperature determined by myoglobin azide reaction [61].
2.8. EPR Spectrometry
EPR spectra were recorded at liquid helium or liquid nitrogen temperature on aBruker EMX EPR spectrometer. For liquid helium system, a GFS600 transfer line and an ITC503 temperature controller were used to maintain the temperature. An Oxford ESR900 cryostat was used to accommodate the sample. For measurements at liquid nitrogen temperature, a silver-coated double-jacketed glass transfer line and a BVT3000 temperature controller were used. Data analysis was conducted using WinEPR program provided by Bruker. Radical spin concentration was routinely quantified by double integration against a copper standard [62].
Progressive power saturation of each radical intermediate was conducted as previously described and data obtained were fitted to:
| [1] |
where P½ is the power to achieve half-saturation of the signal, b is 1– 3 from pure inhomogeneous to pure homogeneous broadening and K is a proportional factor [63].
3. RESULTS
3.1. Stopped-Flow Detection of Transient Species during Single-Turnover Reaction of Ferrous iNOSox with Oxygen
It is well documented that fully reduced ferrous iNOSox (with typical Soret peak at 413 nm) binds oxygen rapidly and transiently forms the oxy-ferrous complex (peak at ~ 428 nm), which slowly decays to the original ferric form [36, 39]. This single turnover reaction of ferrous iNOSox with oxygen follows a simple scheme: Fe(II) + O2 ↔ Fe(II) − O2 → Fe(III) + O2−. We monitored this reaction by two complementary methods: diode array stopped-flow and RFQ EPR technique in the presence or absence of substrate L-arginine and BH4 cofactor (Fig. 1 – Fig. 4).
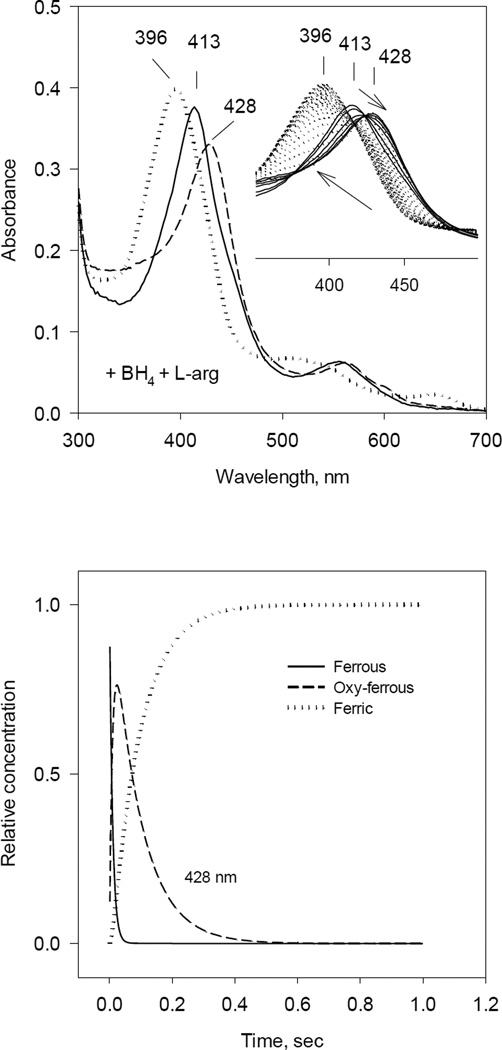
Fig. 1. Optical absorbance spectra and kinetics of the heme species observed during single turnover reaction of iNOSox(+BH4, +L-Arg) with oxygen.
Anaerobic ferrous iNOSox (8–10 µM) containing 500 µM of L-Arg and 10 µM of BH4 was rapidly mixed with an air-saturated buffer at 22 °C. The stopped-flow spectra were collected at 2.5 ms intervals using a rapid scan diode-array detector. Data are representative of three experiments.
A) UV-VIS spectra of starting ferrous Fe(II) (solid ine), oxy-ferrous intermediate Fe(II)O2 (dashed line) and final ferric Fe(III) (dotted line) species resolved by global analysis.
Inset: Original rapid-scan diode-array data. Arrows indicate directions of the spectral shift with time.
B) Kinetics of Fe(II) (solid line), Fe(II)O2 (dashed line) and Fe(III) (dotted line) resolved from global analysis. Data are expressed as percentage of the total heme concentration.
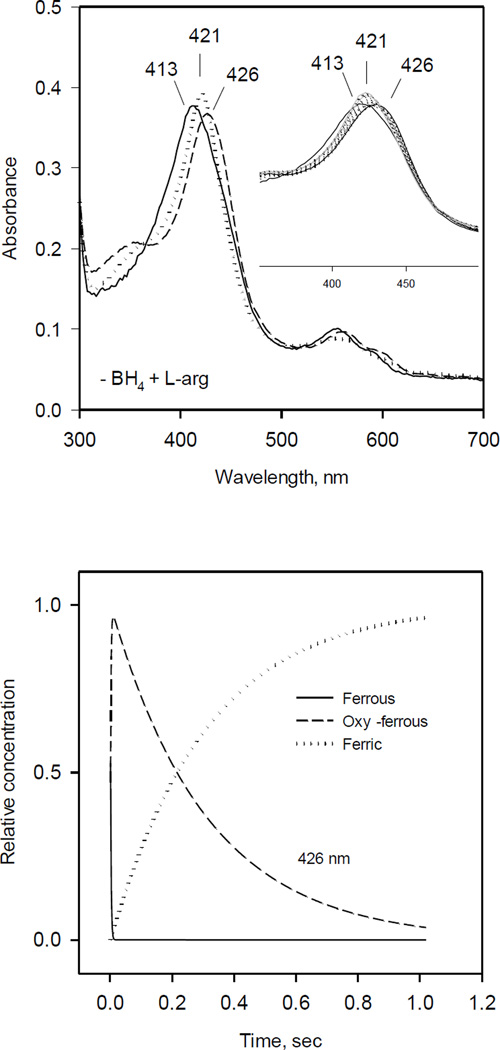
Fig. 4. Optical absorbance spectra and kinetics of the heme species observed during single turnover reaction of iNOSox(–BH4, +L-Arg) with oxygen.
The reaction was identical to that described in Fig. 1 except that no BH4 was added.
A) UV-VIS spectra of starting ferrous Fe(II) (solid line), oxy-ferrous intermediate Fe(II)O2− (dashed line) and final ferric Fe(III) (dotted line) species resolved by global analysis.
Inset: Original rapid scan traces.
B) Kinetics of Fe(II) (solid line), Fe(II) O2 (dashed line) and Fe(III) (dotted line) obtained from the global analysis. Data are expressed as percentage of the total heme concentration.
In the first experiment (+BH4, +L-Arg), 10 µM ferrous iNOSox in the presence of 0.5 mM L-arginine and freshly added 10 µM BH4 was rapidly mixed with an equal volume of air-saturated buffer. The presence of two sets of isosbestic points indicated two seqential chemical steps. An intermediate with 428 nm peak corresponds to oxy-ferrous complex (Fig 1. inset). iNOSox Fe(II)O2 intermediate was formed within 10 ms after mixing and further converted to the high-spin ferric form with Soret peak at 396 nm within 1s. Global analyses of these kinetic data indicated a best fit to an A↔B→C kinetic model, similar to that observed previously for eNOSox and nNOSox [46, 56]. The optical spectra of three species as resolved by global analysis are: A - original ferrous form with λmax at 413 nm, B - oxy-ferrous intermediate at λmax 428 nm, and C - final stage ferric form with λmax peak at 396 nm (Fig. 1, left). Kinetics of the Fe(II), Fe(II)O2 and Fe(III) species also resolved by global analysis (Fig. 1 right) gave estimated rates for oxy-ferrous formation and decay to ferric form at 106 and 18 s−1, respectively (Table 1).
Table 1.
Observed rate constants for different oxygen-induced radical intermediates of iNOSox obtained during single turnover reaction by Stopped–flow and RFQ EPR.
| iNOSox1 | +BH4, +L-arg | +BH4, −L-arg | −BH4, +L-arg | −BH4, −L-arg |
|---|---|---|---|---|
| Fe(II)-O2 formation (s−1) | 106 ± 7 | 108 ± 1 | ND3 | ND |
| Fe(II)-O2 decay (s−1) | 18.0 ± 3.7 | 54 ± 8 and 2.62 | 3.3 ± 2.5 | 7.3 ± 1.2 |
| Reduction of cyt c | 8 ± 4 % | 36 ± 12 % | 91± 10 % | 80 ± 17 % |
| Type of radical | BH4+· | BH4+· and O2−· | O2−· | O2−· |
| Radical formation (s−1) | 20.0 ± 2.5 | 66 ± 16 | ND | ND |
| Radical decay (s−1) | 0.8 ± 0.2 | 1.2 ± 0.3 | 2.5 ± 0.5 | 4.5±1 |
| Heme oxidation (s−1) | 15 ± 4 | 70 ± 19 | ND | ND |
Anaerobic ferrous iNOSox was rapidly mixed with air-saturated buffer at 22 °C. Observed changes were followed either by Stopped-flow or RFQ EPR. For more details see Material and Methods.
Biphasic decay, 2nd slow face ~15%.
ND - Not Detectable
Standard error, n = 3
Identical experiments were performed with ferrous iNOSox (+BH4, −L-Arg). Diode array spectra showed only a partially formed intermediate with optical absorption maximum at 420 nm (Fig. 2, inset) suggesting that the oxy-ferrous intermediate is too transient to fully develop during rapid scan kinetic measurement and very likely represent a mixture of oxy-ferrous and ferric form of protein. However, de-convolution of the collected data by global analysis using the A↔B→C→D kinetic model instead of previously used A↔B→C, with two stages from oxy-ferrous complex (B) to ferric iNOSox (C and D) yielded the four spectra (Fig. 2, left panel): A - starting ferrous enzyme with λmax at 413 nm, B - oxy-ferrous intermediate with λmax at 428 nm, C - high-spin ferric iNOSox with λmax at 398 nm and D - ferric iNOSox with λmax at 403 nm. Kinetics for A, B, C, D species were also resolved by global analysis (Fig. 2, right panel). Estimated rate for oxy-ferrous formation was 108 s−1 and rates for the two-stage decay to ferric form were 54 and 2.6 s−1 (Table 1). The slow shift, 2.6 s−1, from 398 nm peak to 403 nm indicates that lacking L-arginine, iNOSox heme pocket is relatively open, allowing water molecule to ligate the heme iron of a portion of the iNOSox molecules and producing a mixture of high- and low-spin ferric iNOSox.
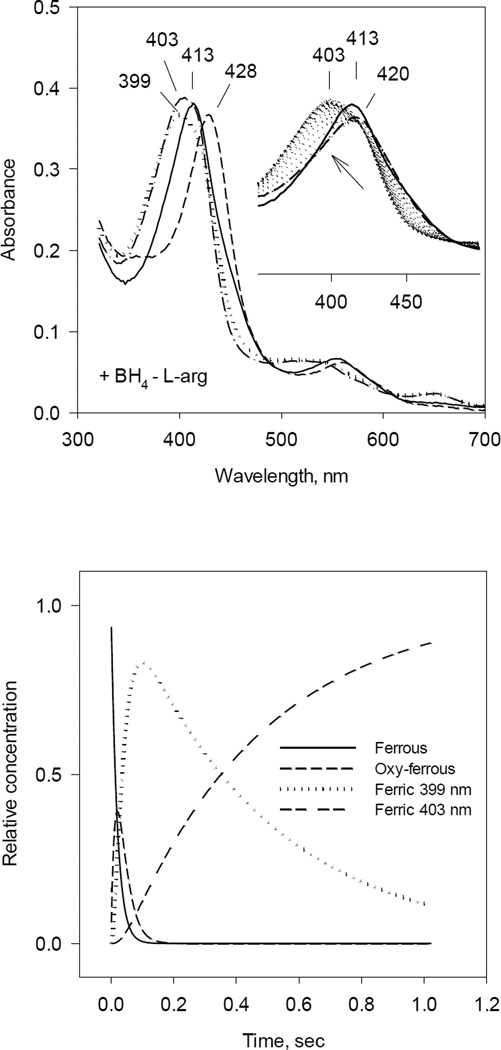
Fig. 2. Optical absorbance spectra and kinetics of the heme species observed during single turnover reaction of iNOSox (+BH4, −L-Arg) with oxygen.
The reaction was identical to that described in Fig. 1 except that L-Arg was not added.
A) UV-VIS spectra of starting ferrous Fe(II) (solid line), oxy-ferrous intermediate Fe(II)O2 (dashed line) and final ferric Fe(III) (dotted line) species resolved by global analysis.
Inset: Original rapid-scan diode-array data.
B) Kinetics of Fe(II) (solid line), Fe(II)O2 (dashed line) and Fe(III) (dotted line) obtained from the global analysis. Data are expressed as percentage of the total heme concentration.
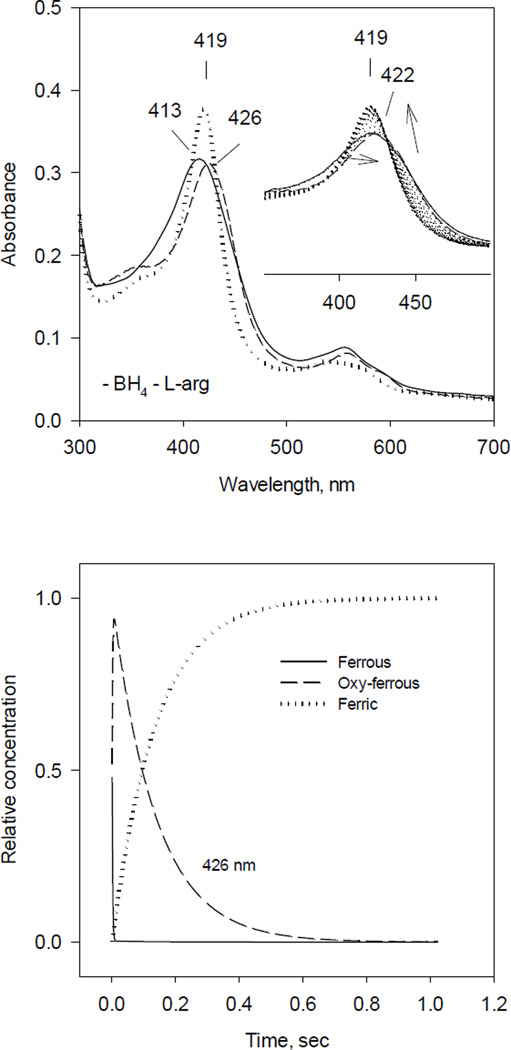
Ferrous iNOSox (–BH4, −L-Arg) deficient of both BH4 and L-arginine, when reacted with O2, again only partially converted to oxy-ferrous complex (422 nm) (Fig. 3). However, the rapid scan stopped-flow was not able to record any kinetics of oxy-ferrous complex formation, which was essentially completed in the dead time of our stopped-flow instrument. Nevertheless, diode array rapid scan was able to reliably capture the decay from oxy ferrous intermediate to ferric iNOSox manifested as a spectral shift from 422 nm to 419 nm (Fig. 3 inset). This spectral shift to 419 nm, instead of typical five coordinate high-spin ferric heme iNOSox (396 nm), indicates that the distal side of the heme iron in the BH4- and L-arginine-free enzyme was likely occupied by a water to form a six coordinate low-spin ferric heme iNOSox. Global analysis of obtained spectra, using the model A↔B→C, was able to predict oxy ferrous intermediate (B) at 426 nm and initial ferrous iNOSox (A) at 413 nm (Fig. 3, left panel). The kinetics of A, B and C were also resolved by global analysis (Fig. 3, right panel). During our experiments, estimated rate constant for formation of putative oxy-ferrous complex (B, observed at 422 nm) was ≥400 s−1 based on the ~1.5 ms dead time of our stopped-flow, and rate of decay to ferric form C (419 nm) was monophasic with estimated rate constant at 7.3 s−1 (Table 1).
Fig. 3. Optical absorbance spectra and kinetics of the heme species observed during single turnover reaction of iNOSox (–BH4, −L-Arg) with oxygen.
The reaction was identical to that described in Fig. 1 except that both BH4 and L-Arg were absent.
A) UV-VIS spectra of starting ferrous Fe(II) (solid line), oxy-ferrous intermediate Fe(II)O2 (dashed line) and final ferric Fe(III) (dotted line) species resolved by global analysis.
Inset: Original rapid-scan data. Arrows indicate directions of the spectral shift with time.
B) Kinetics of Fe(II) (solid line), Fe(II)O2 (dashed line) and Fe(III) (dotted line) obtained from the global analysis. Data are expressed as percentage of the total heme concentration.
The rapid scan stopped-flow data of iNOSox (–BH4, +L-Arg) showed that build-up of oxy-ferrous complex was very fast, again almost finished in the ~ 1.5 ms dead time of the stopped-flow. However, the presence of L-arginine stabilized oxy-ferrous intermediate exhibiting λmax at 428 nm, which decayed to final ferric form with λmax at 420 nm (Fig. 4 inset). The A↔B→C analysis, with fixed k1 = 400 s−1, revealed a beginning ferrous iNOSox with Soret peak at 413 nm, an oxy-ferrous intermediate at 428 nm and the final water-coordinated low-spin ferric form at 420 nm (Fig. 4, left panel). Thus, the presence of L-arginine in iNOSox(–BH4) caused a bathochromic shift in the Soret peak of oxy-ferrous species relative to those observed in the iNOSox (–BH4, −L-Arg) (422 nm vs 428 nm) and also slowed down the rate of oxy-ferrous decay to ferric iNOSox from 7.3 to 3.3 s−1 (Fig. 4, right panel and Table 1). The analysis of rate constants indicates that even in the presence of L-arginine, iNOSox reacts extremely fast with oxygen. However, monophasic decay rate of intermediate B (at 428 nm) to final ferric form C points to homogenous population of iNOSox (–BH4, +L-Arg). Comparison of iNOSox (+BH4, +L-Arg) vs. iNOSox (–BH4, +L-Arg) revealed not only differences in rates of oxy-ferrous heme formation (106 s−1 vs. >400 s−1) but also differences in decay rates (18 s−1 vs. 3.3 s−1). The ~5 times faster decay observed with BH4-reconstituted vs. BH4-deficient iNOSox is in agreement with the previous reports about the ability of BH4 to speed up the decay of oxy-ferrous complex in NOSs [36, 39].
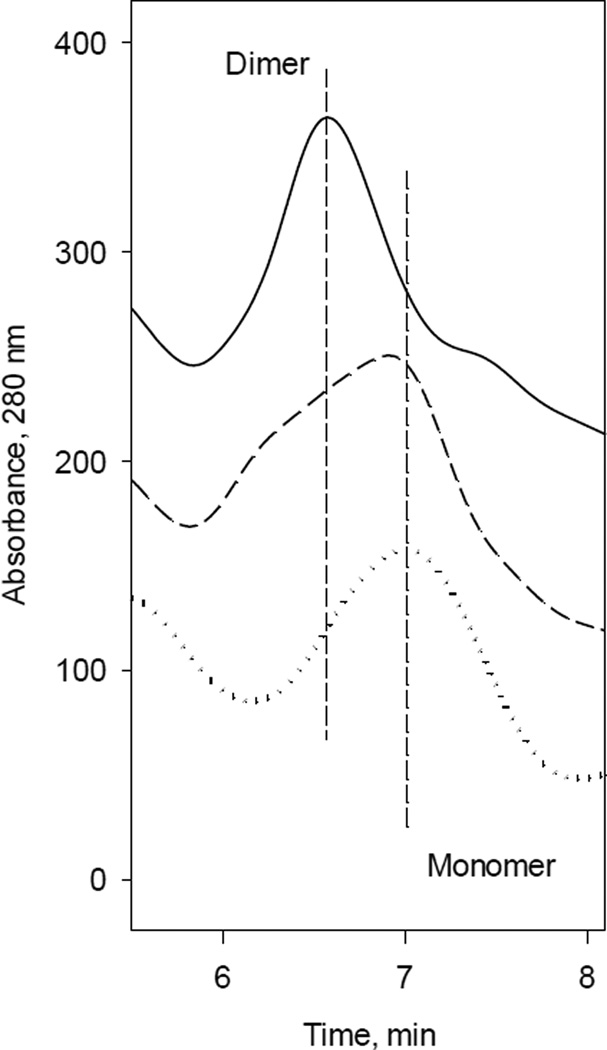
The cofactor BH4 not only provides one electron for L-arginine hydroxylation, but is also essential for iNOSox dimerization [33, 64–66], that is required for full coupling of iNOS catalysis. HPLC analyses of iNOSox samples confirmed that BH4 is required for dimerization of iNOSox (Fig. 5). However, while iNOSox fully dimerized in the presence of BH4 and L-arginine (Fig. 5, solid line), omission of L-arginine led to a monomer/dimer mixture (Fig. 5, dash line). In the absence of BH4, iNOSox was present exclusively in monomeric form, independent of L-arginine (Fig. 5, dotted line).
Fig. 5. Gel filtration analysis of Δ59 iNOSox.
iNOSox (100 µg) were loaded on a Bio-Sil TSK-250 column as described under “Material and Methods”. Dotted line, the elution profile which shows that iNOSox(–BH4, −L-arg), as isolated, is monomer. Dash line, the elution profile of protein following an anaerobic overnight incubation in 50 mM HEPES buffer (pH 7.5), containing 10 % glycerol, 0.1M NaCl and 100 µM of BH4 at 4 °C, iNOSox(+BH4, −L-arg), representing dimer-monomer mixture. Solid line, the elution profile of protein following an anaerobic overnight incubation with 1 mM L-arginine and 100 µM of BH4, iNOSox(+BH4, +L-arg), representing dimer. Two vertical lines are visual guides to indicate the retention time of the iNOSox dimer and monomer.
3.2. Superoxide Production during Single Turnover Reaction of iNOSox with Oxygen
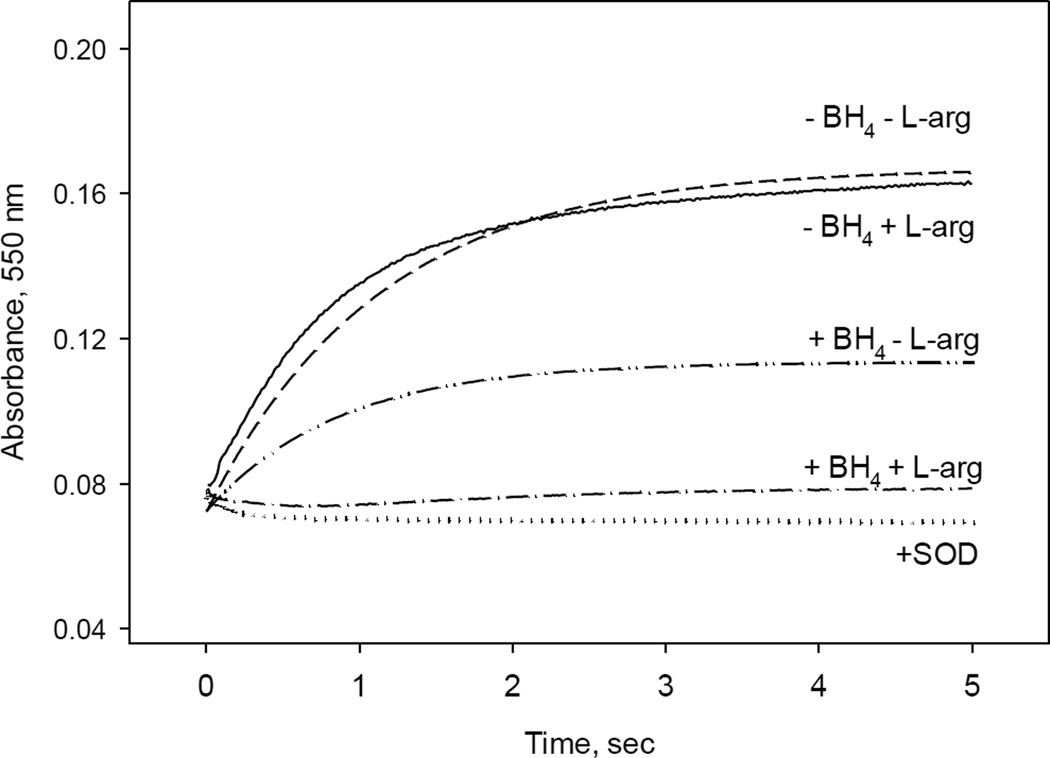
We next examined how the formation of superoxide, a primary product of uncoupling, is modulated by different conditions during a single turnover reaction. Superoxide can be oxidized by cytochrome c (cyt c), and thus production of superoxide by iNOSox was monitored at 550 nm as reduction of cyt c (Fig. 6). Experimental conditions were similar to those described in Fig. 1 – 4. The final concentration of iNOSox after reaction with air saturated buffer containing 50 µM cyt c was 5 µM. In the control experiments, we added also 5 U/mL of superoxide dismutase (SOD) that has a very high turnover rate for superoxide and prevents it from reacting with cyt c. A lack of absorbance increase at 550 nm (Fig. 6, dotted line) confirms that cyt c reduction observed in the experiments without SOD was indeed due to the superoxide release from iNOSox during reaction with oxygen. Small decrease in the absorbance at 550 nm in the experiment with SOD observed within the first 0.5 s (Fig. 6, dotted line) reflects optical changes corresponding to transition from ferrous to ferric iNOSox at 550 nm.
Fig. 6. Cyt c reduction by oxygen–induced superoxide radical during single turnover reaction of iNOSox in the presence/absence of BH4 and L-arg.
Single wavelength stopped-flow at 550 nm obtained during reaction of 10 µM ferrous iNOSox mixed with an air-saturated buffer containing 50 µM of cyt c at 22 °C. Data in the presence of 1U of SOD are shown as a negative control for that of iNOSox (–BH4, −L-Arg). Detected amounts of superoxide from these reactions expressed as percentage of the total heme concentration are summarized at Table 1. Data are similar from three separate experiments.
Percentages of superoxide production of iNOSox under different experimental conditions were calculated from the ratio of cyt c reduction to the amount of NOS heme and are listed in Table 1. The highest amounts of superoxide, ~ 80% and 91%, were detected in the single turnover reactions of iNOSox (–BH4, −L-Arg) and iNOSox (–BH4, +L-Arg), respectively. These data clearly indicate that in the absence of BH4, the main product of iNOSox is superoxide and substrate L-arginine has only a marginal effect. Small increase from 80% to 91% indicate that L-arginine-mediated stabilization of the oxy-ferrous intermediate (Fig. 3 vs. Fig. 4), which slows down the release of superoxide leading to more efficient superoxide interaction by cyt c before superoxide self-dismutation to H2O2 and O2. In the assumed coupled reaction, i.e., iNOSox (+BH4, +L-Arg), no superoxide production was expected and indeed, less than 8% of superoxide was detected in our system (Table 1).
3.3. Production of Hydrogen peroxide during Single Turnover Reaction of Fe(II) iNOSox with Oxygen
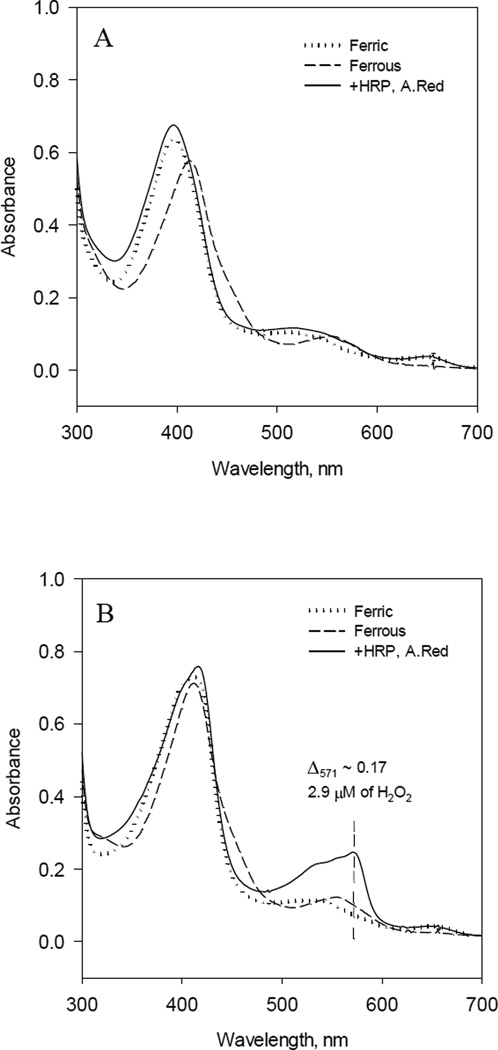
Several studies had suggested, that under the experimental conditions, +BH4, −L-Arg, BH4 provide electron to Fe(II)O2 complex yielding H2O2 as an alternative product of iNOSox instead of superoxide [51, 55, 67]. To test this uncoupling mechanism, we checked production of H2O2 by iNOSox (10 µM) using Amplex Red assay. Produced amount of H2O2 was detected spectrophotometrically by 8453 Hewlett-Packard diode array spectrophotometer. As expected, there was no detectable amount of H2O2 produced by the fully reconstituted iNOSox (+BH4, +L-Arg) (Fig. 7A) and approximately 33% of H2O2 were produced by iNOSox (+BH4, −L-Arg) (Fig. 7B, solid line). Surprisingly, a similar amount of H2O2 was produced by ferrous iNOSox (–BH4, ±L-Arg) (data not shown). This seemed at odds with the results of cyt c assay (Fig. 6) that showed mainly (80–91%) superoxide production. However, self-dismutation of superoxide is a rapid spontaneous process with the second order rate constant 3 ×105 M−1 sec−1 at pH 7.4 [68], thus we were not able to distinguish by a rather slow Amplex red assay between H2O2 originated from either iNOSox (+BH4, −L-Arg) uncoupling from Fe(II)O2− or via self-dismutation of superoxide.
Fig. 7. Detection of Hydrogen Peroxide after reaction of ferrous iNOSox with oxygen.
A) Optical spectra of 10 µM ferric iNOSox(+BH4, +L-arg) (dotted line) after anaerobic stoichiometric reduction with dithionite (dash line); oxidized by adding 30 µl of aerobic buffer containing 5U of HRP, 20 µM Amplex Red and opened to air (solid line). B) Optical spectra of 10 µM iNOSox(+BH4, −L-arg) in similar experiment. For details see “Materials and Methods.
3.4. EPR Characterization of Oxygen-Induced Radical Species of Ferrous iNOSox
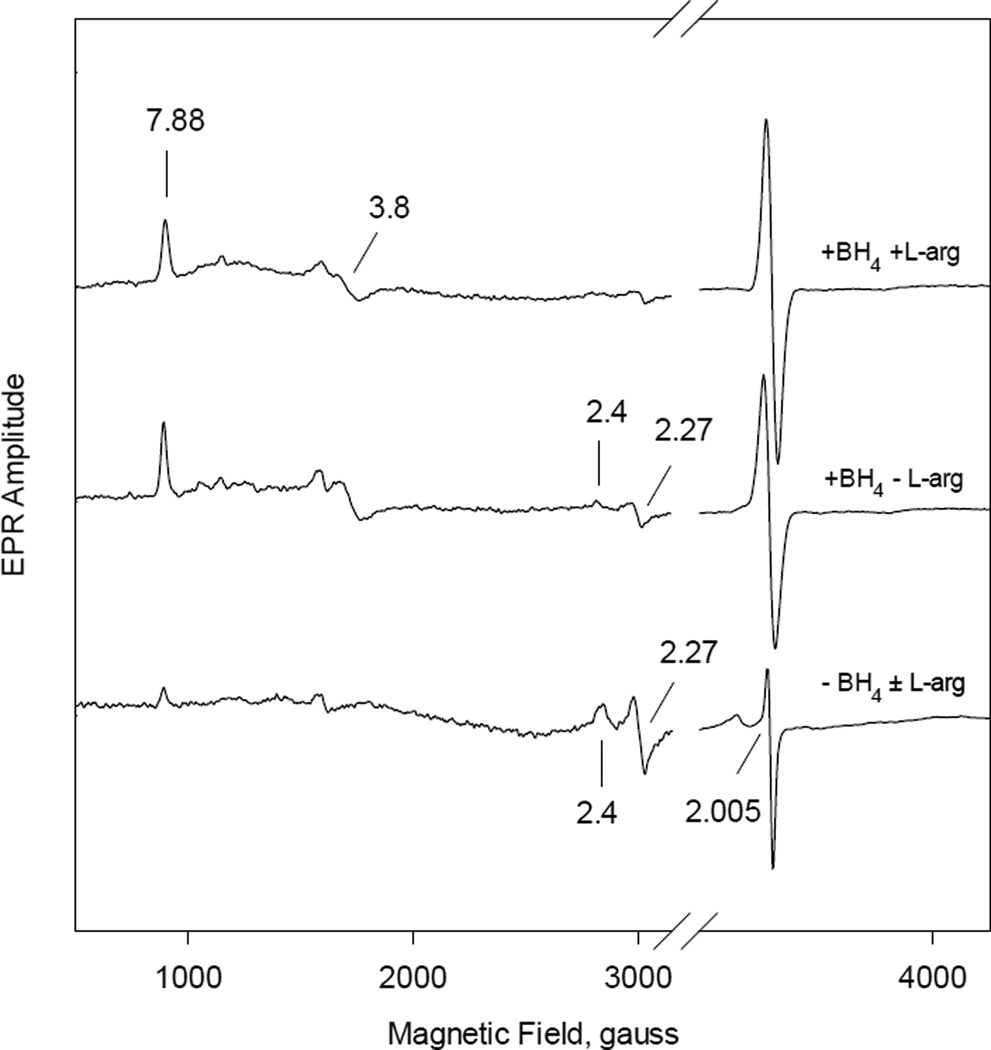
iNOSox catalysis involves either BH4 radical formation, heme oxidation, or superoxide production, detectable directly by RFQ EPR, depending on different coupling/uncoupling conditions (Fig. 8). The EPR spectra of samples freeze trapped at ~ 80 ms reaction time between 50 µM iNOSox and O2 in the presence or absence of L-arginine or BH4 recorded at 10 K showed clear differences in both the heme and radical signals. At fully-coupled condition (+BH4, +L-Arg) (top spectrum), the evolving rhombic high-spin heme signals at g = 7.88 and 3.80 and a symmetric radical signal g ~2 are the dominant EPR features. Little low-spin ferric heme was observed, matching the dominant 396 nm Soret peak observed for the ferric heme species (Fig. 1). EPR for the sample containing BH4 only (+BH4, −L-Arg) (middle spectrum), ferric heme present at both rhombic high- and low-spin (g = 7.88/3.80 and 2.40/2.27) and a symmetric radical signal were conspicuous, indicating a mixture of high- and low-spin heme formed together, corroborating with the 403 nm optical data of the evolving ferric heme in Fig. 2. For the reactions of iNOSox (−BH4, ±L-Arg) the EPR data showed dominant low-spin (g= 2.41 and 2.27) heme and a very big asymmetric radical signal (g ~2), typical for axial superoxide radical (bottom spectrum). The dominant low-spin heme matches the observed 419 nm peak for the ferric heme formation (Figs. 3 and 4).
Fig. 8. EPR spectra of iNOSox(±BH4, ±L-arg) formed at 80 ms in the reaction between ferrous iNOSox and oxygen.
Fully reduced ferrous 50 µM iNOSox containing equimolar BH4 and 500 µM L-Arg (top spectrum), with BH4 alone (middle spectrum), or in the absence of BH4 (bottom spectrum) was reacted with air saturated 50 mM HEPES buffer (pH 7.5), containing 10 % glycerol, 0.1M NaCl, pH 7.4 at a 1:1 ratio at 22 °C, and the reaction mixture was freeze-trapped at 80 ms reaction time in the prechilled isopentane. EPR conditions were: 4 mW microwave power, 10 G modulation and 11K. The g values of both the high-spin (7.88 and 3.80) and low-spin (2.41 and 2.27) heme species are labeled by solid lines.
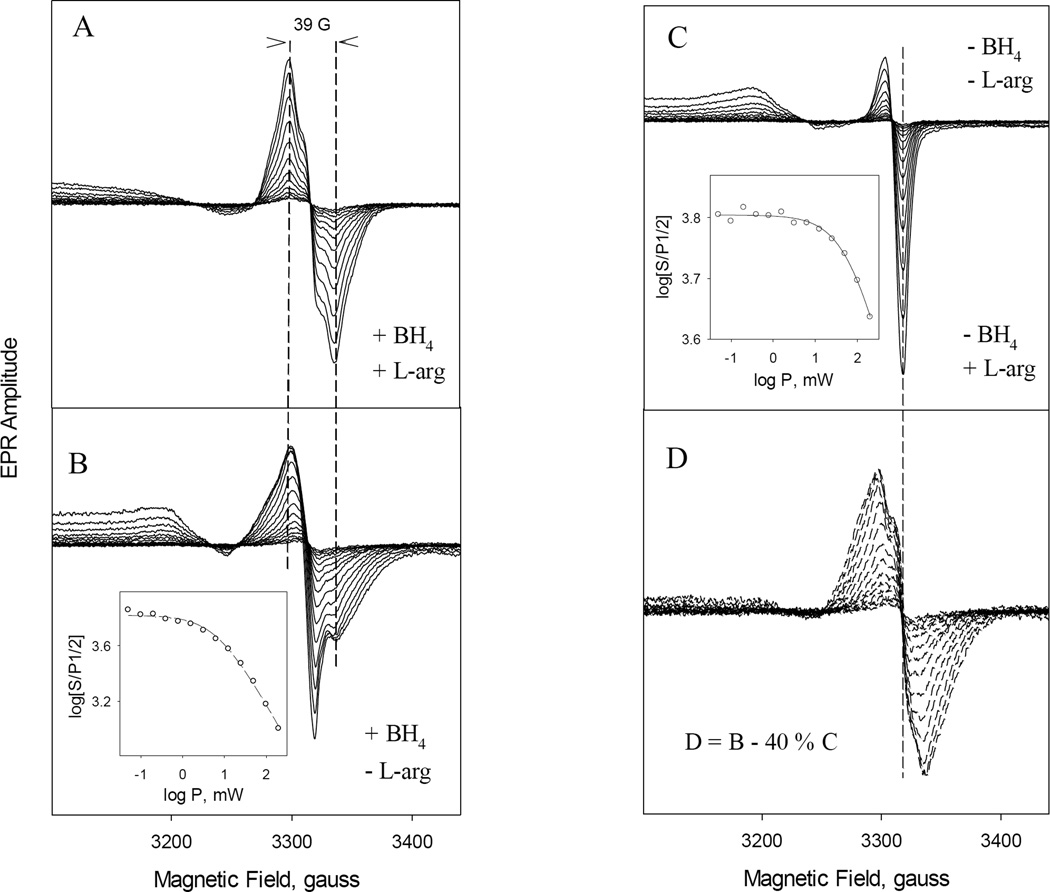
To characterize in detail the EPR data of the various radical species observed in Fig. 8 at g = 2 region, EPR spectra of rapid freeze samples were recorded at various microwave power and 400 G range at 115 K (Fig. 9). iNOSox (+BH4, +L-Arg) produced a typical BH4 radical [39, 40, 44–46, 48] with 39 G wide symmetric EPR centered at g =2.0023 (Fig. 9A) and a homogeneous microwave power dependence with a P1/2 ~26 mW (Table 2). Similar to eNOSox and nNOSox, iNOSox (–BH4, ±L-Arg) produced only superoxide radical (Fig. 9C) with a broad g‖ (or gz) = 2.080 and g┴ (or gx/gy) = 2.006 and the very high P1/2 ~155 mW (−L-Arg) and ~126 mW (+L-Arg) (Table 2), all typical features for an isolated superoxide anion radical (35). Thus, L-arginine had only a minimal effect on the chemical property of the radical intermediate. Ferrous iNOSox (+BH4, −L-Arg) generated a radical intermediate with a 39 G wide spectrum centered at gx/y=2.005 and an additional broad peak at gz = 2.076 and a broad trough at g = 1.99 (Fig. 9B). The microwave power dependence (P1/2), calculated from the center peak of the derivative signal, was 37 mW (Table 2). After normalizing against their spin concentrations, subtracting of 40 % of the superoxide radical formed in the absence of L-arginine and BH4 (spectra at Fig. 9C) from the EPR spectra obtained in the presence of BH4 (Fig. 9B) yielded a radical spectra showing a 39 G wide symmetric spectrum centered at 2.002 (Fig. 9D). The EPR line shape and power saturation of the serial difference spectra (P1/2 = 24 mW) showed strong similarity with typical BH4 radical of iNOSox (Fig. 9D vs. Fig. 9A). Thus the arithmetic treatment of the EPR spectra revealed that iNOSox (+BH4, −L-Arg) probably produced a mixture of superoxide and BH4 radical.
Fig. 9. Progressive power-dependent EPR spectra of the radical intermediates formed in the reaction between ferrous iNOSox and oxygen.
The reaction was identical to that described in Fig. 8. EPR spectra were recorded at microwave powers ranging from 0.025–100 mW with 3 dB increment for 40–50 µM iNOSox with equimolar concentration of BH4 and 500 µM L-Arg present (A) or with BH4 alone (B) or with L-Arg alone (C) at 115 K. Almost identical EPR spectra shown in (C) were obtained for iNOSox in the absence of both BH4 and L-Arg (data not shown). Difference spectra obtained by subtracting of 40% spectra (C) from spectra (B) to maximally remove the g= 2.005 sharp dip signal are shown at panel (D). Spectra D are very similar to typical EPR spectra of BH4 radical of iNOSox (A). Vertical dash lines are visual guides. Insets in (B) and (C) are plots to obtain the half-saturation power via non-linear regression to Equation 1. Symbols are the data and lines are the fit.
Table 2.
EPR parameters for different oxygen-induced radical intermediates in eNOSox [46], nNOSox [56] and iNOSox.
| +BH4, +L-arg | −BH4, −L-arg | −BH4, +L-arg | +BH4, −L-arg | ||
|---|---|---|---|---|---|
| eNOSox* | radical species | BH4+· | O2−· | O2−· | new radical |
| g value | 2.002 | 2.078a, 2.006b | 2.078a, 2.006b | 2.005b, 1.99c | |
| P1/2 (mW) | 13.8 ± 1.0 | 54 ± 3 | 51 ± 2 | 4.0 ± 1.5 | |
|
nNOSox -DTT |
radical species | BH4+· | O2−· | O2−· and new 38G | BH4+· and O2−· |
| g value | 2.002 | 2.077a, 2.005b | 2.077a, 1.99 | 2.076a and 2.005 | |
| P1/2 (mW) | 27.0 ± 2.7 | 100.0 ± 4.7 | 100 and 10 ± 1 | 26 ± 7 and 12 ± 2 | |
|
nNOSox +DTT |
radical species | BH4+· | O2−· | O2−· and new 38G | wide 75 G and 20% O2−· |
| g value | 2.002 | 2.077a, 2.005b | 2.077a, 1.99 | 2.003b, 1.97c | |
| P1/2(mW) | 27.0 ± 2.7 | 100.0 ± 4.7 | 100 and 10 ±1 | 37 ± 5 and 14.7 | |
| iNOSox* | radical species | BH4+· | O2−· | O2−· | BH4+ and O2−· |
| g value | 2.002 | 2.080a, 2.006b | 2.080a, 2.006b | 2.076a and 2.005 | |
| P1/2 (mW) | 26 ± 1 | 155 ± 7 | 126 ± 14 | 37 ± 4 | |
Broad peak.
Crossover.
Satellite signal.
No Difference between samples with or without DTT. Standard error, n = 3
Because we had shown previously that radical formation by nNOSox (+BH4, −L-Arg) is affected by DTT [56], we repeated the experiment with iNOSox in the presence of 1 mM DTT. The oxygen-induced radical intermediate species produced by iNOSox (+BH4, −L-Arg) were not affected by the presence of DTT and the obtained spectra were identical to those shown in Fig. 9B. The EPR parameters, the P1/2 and g values, for all iNOSox radical intermediates are summarized in Table 2 together with our previous data for eNOSox and nNOSox for comparison.
3.5. Correlation of Kinetics of Heme Redox Changes and Oxygen-Induced BH4/superoxide Radical Intermediates in iNOSox
As mentioned earlier, a single turnover catalytic reaction of ferrous iNOSox with oxygen follows a simple reaction scheme: Fe(II) + O2 ↔ Fe(II) − O2 → Fe(III) + O2•−. The kinetics of heme oxidation measured by stopped-flow were compared with kinetics of 1) heme oxidation state detected by RFQ EPR as high spin g = 7.88 or low spin g = 2.27 signal at 10 K; and 2) BH4 and/or superoxide radicals obtained by rapid freeze experiments and detected by EPR as g = 2 signal at 115 K. Table 1 summarizes the rate constants of iNOSox obtained in the presence or absence of BH4 and/or L-arginine.
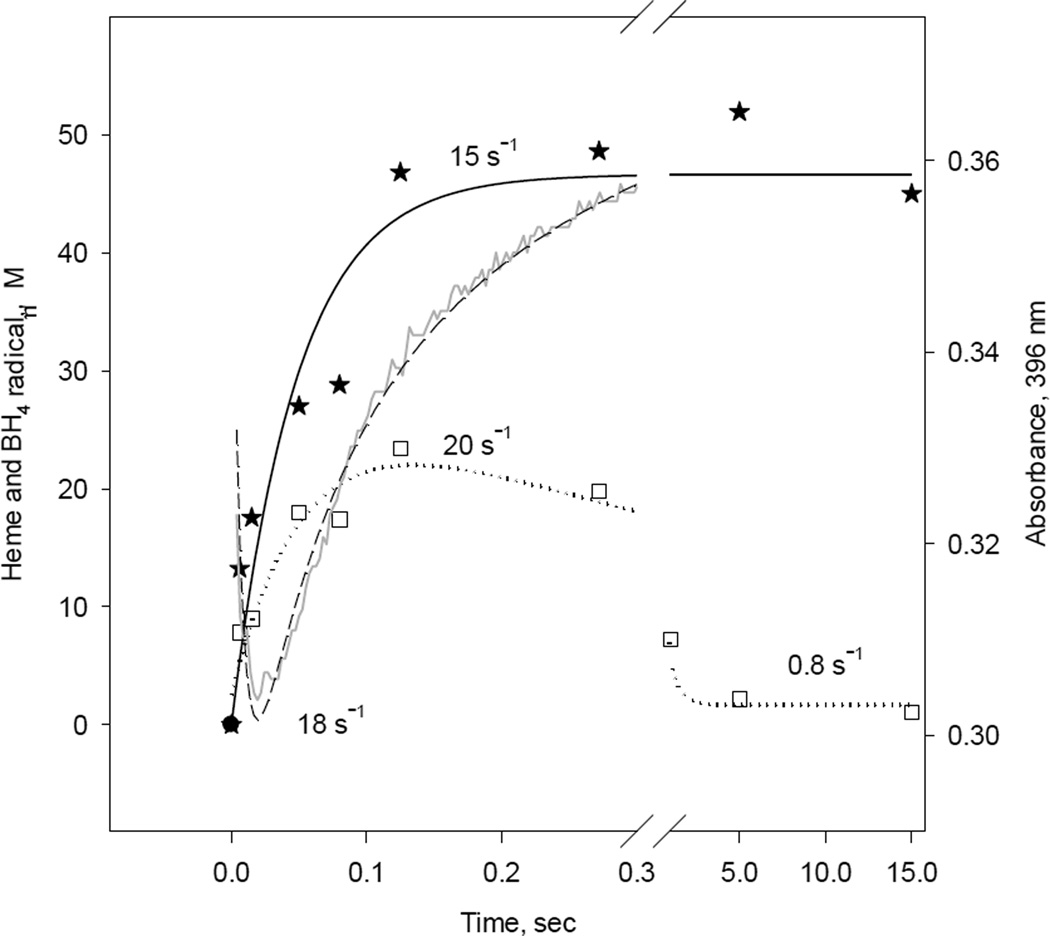
A representative single wavelength stopped-flow and EPR kinetics of ferrous iNOSox (+BH4, +L-Arg) during reaction with oxygen are shown in Figure 10. The rate constant of oxy-ferrous decay to the ferric form of iNOSox (396 nm) obtained by stopped-flow was 18 s−1 (Fig. 10; dashed line). The BH4 radical formation, as EPR signal change at g = 2, had a rate constant of 20 s−1 and was followed by a slow decay of 0.8 s−1 (Fig. 10, dotted line and Table 1). A rate constant of the ferric high-spin heme formation at g = 7.88 was 15 s−1 (Fig. 10, solid line). These data confirms that decay of oxy-ferrous heme complex correlates nicely with the BH4 radical formation and the formation of ferric heme in iNOSox (+BH4, +L-Arg).
Fig. 10. Kinetic correlation between heme redox changes and BH4 radical during reaction of reduced iNOSox(+BH4, +L-arg) with oxygen.
Single wavelength stopped-flow at 396 nm obtained during reaction of 10 µM iNOSox(+BH4, +L-arg) with an air-saturated buffer (dash line) at 22 °C and parallel RFQ EPR kinetics of 50 µM protein to obtain amplitudes of BH4 radical, g= 2 signal (square), and high spin ferric heme, g= 7.8 signal (star) recorded at 10K. The lines and corresponding rate constants were obtained by a one- exponential fit for the g= 7.8 signal and an irreversible A→B→C sequential mechanism for g= 2 signal and A↔B→C for A396 stopped-flow data.
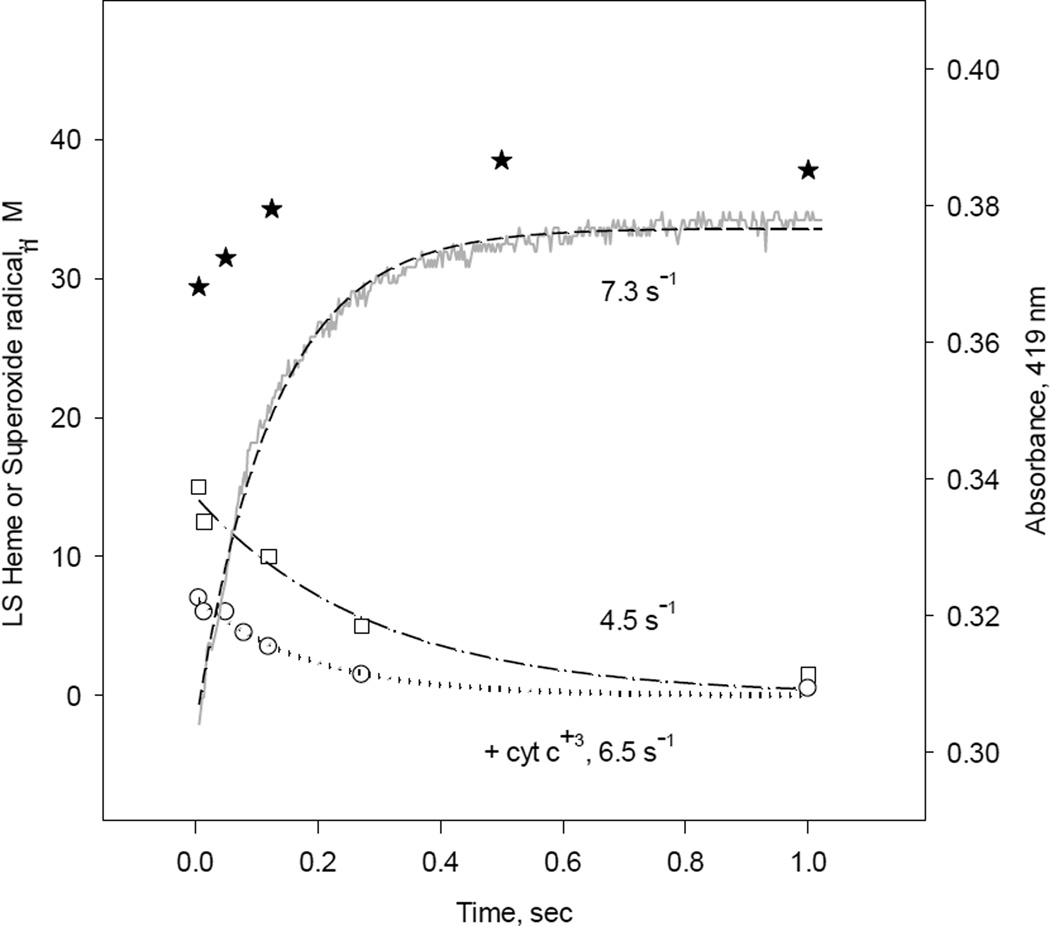
Stopped-flow data showed only putative formation of oxy-ferrous complex produced by ferrous iNOSox (−BH4, −L-Arg) and iNOSox (−BH4, +L-Arg) during reaction with oxygen (Figs. 3 and 4). Increases of absorbance at 419 nm represent decay from these complexes to low-spin ferric iNOSox (−BH4, −L-Arg) (Fig. 11, dash line); data for iNOSox (−BH4, +L-Arg) are very similar (not shown). The oxy-ferrous complex decay rates obtained by stopped-flow were 7.3 s−1 and 3.3 s−1, for these two iNOSox samples, respectively (Table 1). Kinetics of superoxide radical production by iNOSox (−BH4, −L-Arg) and iNOSox (−BH4, +L-Arg) obtained by RFQ EPR are also very similar for both samples; majority of the formation kinetics of superoxide radical was lost in the dead time of our rapid freeze apparatus, ~ 7 ms, and rates for radical decays were of 4.5 and 2.5 s−1, respectively (Table 1 and Fig. 11 square symbols for representative kinetics). The total amount of detected superoxide at 8 ms, was less than ~30% compared to the amount of iNOSox heme. Significant amount of superoxide signal missing in the dead time of RFQ EPR probably due to self dismutation of superoxide to hydrogen peroxide and oxygen. The superoxide scavenger cyt c further decreased detectable superoxide by RFQ EPR to ~15% which decayed at 6.5 s−1 (Fig. 11, circles). Loss of kinetic data of oxy-ferrous complex formation, heme oxidation (Fig. 11, stars symbols) as well as superoxide radical formation in the dead time of the instruments point to a very fast Fe(II)O2 bond cleavage in oxy-ferrous iNOSox (−BH4, ±L-Arg) complexes. These data indicate that in monomeric, BH4-deficient iNOSoxthe heme pocket is wide open for immediate water ligation to the iron. The result of this process is a transient formation of high-spin (396 nm) ferric iNOSox undetectable by stopped-flow. Appearance of Soret peak at 419–420 nm represents final stage of conversion of high-spin to the terminal water-ligated low-spin ferric iNOSox (−BH4, ±L-Arg) (Figs. 3, 4 and 8).
Fig. 11. Kinetic correlation between the heme redox changes and superoxide radical production during reaction of reduced iNOSox(–BH4, −L-arg) with oxygen.
Single wavelength stopped-flow at 419 nm obtained during reaction of 10 µM iNOSox(–BH4, −L-arg) with an air-saturated buffer (dash line) at 22 °C and parallel RFQ EPR kinetics of 50 µM protein to obtain amplitudes of superoxide radical g= 2 signal (square) recorded at 115K and low-spin ferric heme g= 2.27 signal (star) recorded at 10 K. The lines and corresponding rate constants were obtained by a one-exponential fit to the g= 2 signal and an A↔B→C sequential mechanism for A419 stopped-flow data.
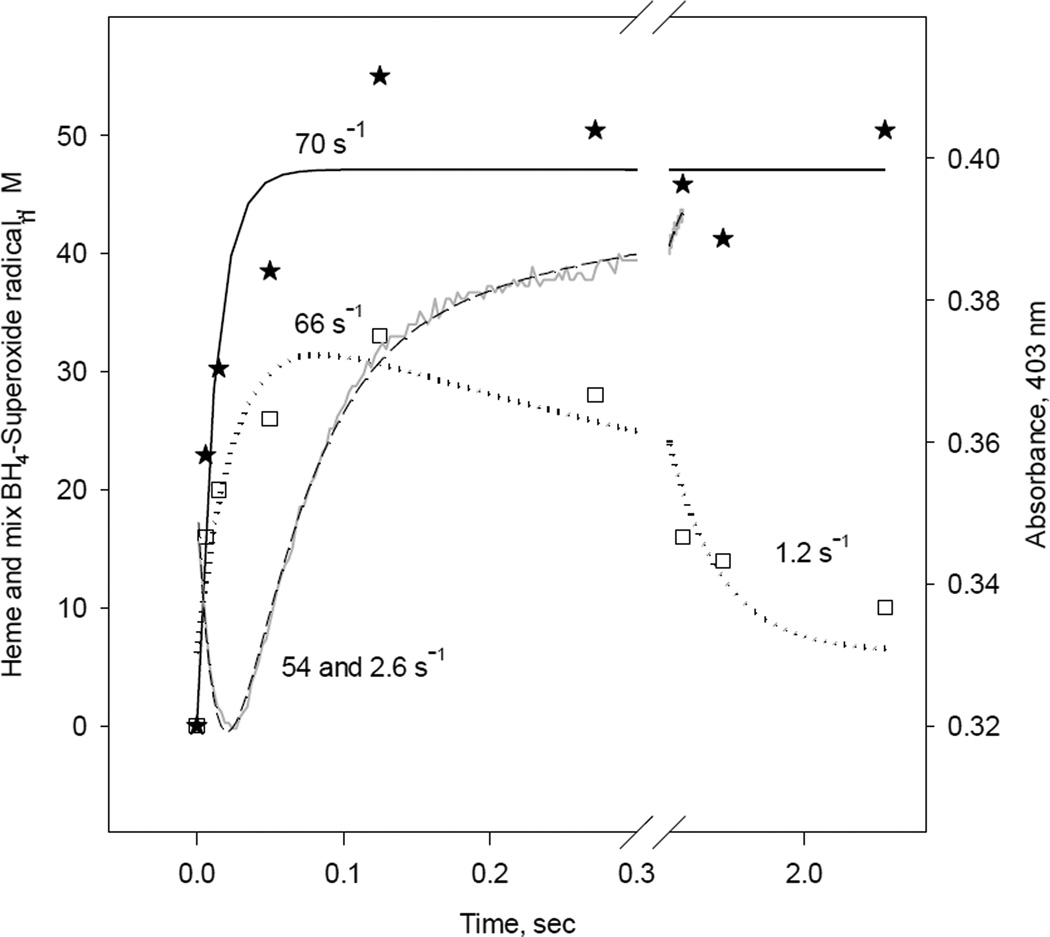
In iNOSox (+BH4, −L-Arg), that produced unusual EPR spectra representing a mixture of BH4 and superoxide radical (Fig. 9B), the RFQ kinetic measurements revealed a radical formation rate of 66 s−1 (Fig. 12, squares) and a heme oxidation rate of 70 s−1 (Fig. 12, stars). The 66 s−1 rate of formation of BH4/superoxide radical mixture is faster than the 20 s−1 rate of BH4 radical formation by iNOSox (+BH4, +L-Arg) but slower than that of iNOSox (−BH4, −L-Arg), which is not detectable by RFQ-EPR. The oxy-ferrous decay rate obtained by stopped-flow was biphasic with rates 54 s−1 (~ 85 %) and 2.6 s−1 (~15%) (Fig. 12, dash line and Table 1). Thus again, stopped-flow and RFQ data showed comparable rates of oxy-ferrous complex decay (54 s−1) and radical formation (66 s−1). As we described earlier (Figs. 2 and 8), the second slow face of oxy-ferrous decay (2.6 s−1) represents conversion from five coordinate high-spin to partially water ligated low-spin ferric iNOSox (+BH4, −L-Arg).
Fig. 12. Kinetic correlation between heme redox changes and BH4/Superoxide radical during reaction between reduced iNOSox(+BH4, −L-arg) and oxygen.
Single wavelength stopped-flow at 403 nm obtained during reaction of 10 µM iNOSox(+BH4, −L-arg) with an air-saturated buffer (dash line) at 22 °C and parallel RFQ EPR kinetics of 50 µM protein to obtain amplitudes of radical (mixture of two radicals, BH4 and superoxide), g= 2 signal (square), and ferric heme, g= 7.8 signal (star), recorded at 10 K. The lines and corresponding rate constants were obtained by an A↔B→C→D sequential mechanism for stopped-flow data, one-exponential fit for the g= 7 signal, and an irreversible A→B→C sequential mechanism for g= 2 signal.
4. DISCUSSION
4.1. New information derived from our single turnover stopped-flow and RFQ EPR kinetic measurements of iNOSox
In our previous studies, we simulated physiological as well as pathophysiological conditions by supplying or depleting of BH4 and L-arginine during the reactions of reduced eNOSox or nNOSox with oxygen [46, 56]. It was clear that the substrate and/or cofactor control the formation and decay of oxy-ferrous intermediate and various EPR detectable radical species from eNOS and nNOS oxygenase domains (Tables 2 and 3) [46, 56]. Thiol reductant also showed discrete effect on the oxygen-induced radical intermediates in the nNOSox.
Table. 3.
Comparison of rate constants from single-turnover reactions catalyzed by three NOSox isoforms obtained by stopped-flow and RFQ EPR.
In the current study, we focused on the study of iNOSox. To obtain direct structural and correlated kinetic information of the heme center, BH4 cofactor and the various radical intermediates for individual steps of the catalysis in the presence or absence of BH4 and/or L-arginine, single turnover rather than the steady-state conditions were used. Our stopped-flow data from iNOSox catalysis measured at 22 °C indicate that formation of fully developed oxy-ferrous intermediate, judged by absorbance change at 428 nm, is detectable only in the presence of L-arginine and BH4 (Fig. 1). In the absence of BH4, the rate of formation of Fe(II)O2 was very fast (≥ 400 s−1), and resulted in a Soret maximum between 422–428 nm (Figs. 3 and 4). This outcome expands our conclusion derived from the previous study of nNOSox and eNOSox further to iNOSox, that heme, L-arginine and BH4 “triad” reinforces each other in rigidifying the heme pocket scaffold via H-bonding network and thus the Fe-O bond strength in the Fe(II)O2 intermediate. The significance of BH4 in NOS is further underscored by the faster decay rate of oxy-ferrous to ferric form in the presence of BH4. The oxy-ferrrous intermediate of all NOS isoforms is kinetically unstable and spontaneously decays to superoxide and ferric enzyme if it is not reduced in a timely manner [13]. Thus, faster reduction of the iNOSox oxy-ferrous intermediate in the presence of BH4 (18 s−1) compared to BH4-deficient iNOSox (3.3 s−1) prevents the uncoupling of catalysis and emphasizes the role of BH4 as an effective electron source to provide the second reducing equivalent in the monooxygenation reaction in NOS proteins.
EPR study of radical formation revealed that ferrous iNOSox is also capable of forming multiple radical intermediates as the other two NOS isoforms [46, 56] upon reaction with oxygen depending on the availability of L-arginine and BH4. In addition to typical BH4 radical formed by iNOSox (+BH4, +L-Arg) or superoxide radical formed by iNOSox (–BH4, ±L-Arg), iNOSox (+BH4, −L-Arg) formed a mixture of BH4 and superoxide radical. This is clearly a specific property of iNOSox, because eNOSox and nNOSox produced new radical intermediates under similar conditions (Table 2). The new wide 75 G radical formed by the nNOSox (+BH4, −L-Arg) in the presence of thiol, detected by RFQ-EPR [56], was interpreted by pulsed EPR/ENDOR studies as the BH4 radical signal broadened by an adjacent low-spin ferric heme via dipolar or/and exchange coupling (Krzyaniak, M.D., Bowman, M.K., et al, manuscript in preparation). This is in contrast to the typical 39 G BH4 radical observed for fully coupled NOS (46, 56), which is magnetically coupled to a fast relaxing high-spin ferric heme (Krzyaniak, M.D., Bowman, M.K., et al, manuscript in preparation). Furthermore, in iNOSox(+BH4, −L-Arg) w/o thiol there is no such magnetic interaction with a nearby low-spin ferric heme, since RFQ-EPR of iNOSox(+BH4, −L-Arg ) clearly showed a mixture of BH4 and superoxide radical rather than the new wide 75 G radical formed by the nNOSox (+BH4, −L-Arg) in the presence of thiol. This characteristic might be a consequence of the critical role of BH4 in stabilization of NOS dimers, which is more prominent in iNOSox than in the constitutive NOS isozymes [33]. Interaction of BH4 with the heme propionate and the helix α7a initiates dimer formation and the dimer is further reinforced through the interaction of dihydroxyprolyl side chain interaction with the enzyme's N-terminal hook to create a tight active iNOSox dimer [69]. The role of BH4 in iNOSox dimer reinforcement is significantly stronger than that of L-arginine [33]. However, the relative strength of all three NOS homodimers follows eNOS > nNOS > iNOS [33, 70] indicating that iNOS forms the least stable dimers and even the smallest decrease of BH4 and/or L-arginine levels can lead to dissociation of the dimeric iNOSox and consequently to an uncoupling reaction (Table 1 and Table 2). This weaker interaction between two monomers in iNOS than nNOS also corroborates the EPR/ENDOR observation for nNOSox(+BH4, −L-Arg) plus thiol, implying that the BH4 radical formed interacting magnetically with the nearby low-spin heme center of the other subunit, whereas the distance is too long between BH4 radical and the heme iron in iNOSox(+BH4, −L-Arg) w/o thiol due to much looser dimer formation.
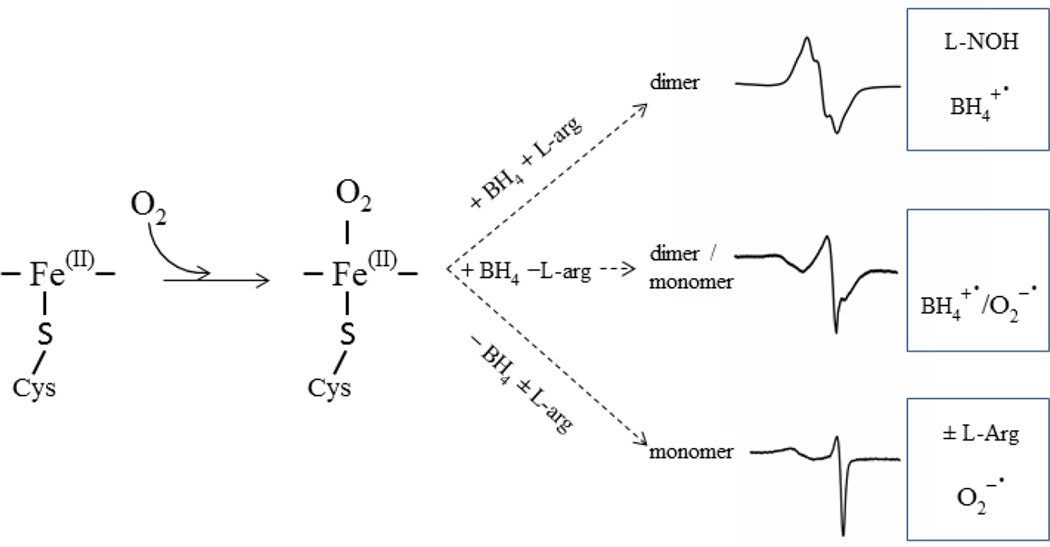
Data from all our experiments show that a fully reconstituted iNOSox (+BH4, +L-Arg) forms dimer and only BH4 radical in Fe(II) iNOSox is induced by oxygen. In the absence of BH4 ± L-arginine, iNOSox remains monomeric and produces superoxide up to 80% the amount of heme. In the presence of BH4 only, iNOSox (+BH4, −L-Arg) exists as a monomer/dimer mixture and thus, not unexpectedly, produces a mixture of BH4 and superoxide radical (Fig. 7 and Table 2). Our data clearly show that in addition to the reductase domain of iNOS [15], iNOS oxygenase domain is also able to produce a large amount of superoxide when uncoupled by a lack of BH4 or L-arginine. Earlier reports demonstrate that exogenous addition of superoxide to iNOS greatly decreases NO generation through BH4 oxidation associated with shift of the enzyme from the active dimer to the inactive monomer [71]. Thus, in correlation with our data, superoxide internally produced from iNOSox in the absence of BH4 or L-arginine may damage protein itself and consequently increases the production of superoxide by iNOS even further. On the other hand, it is well known that the reaction of NO with superoxide generates the potent oxidizing peroxide, peroxynitrite (ONOO−) [11, 72]. Interestingly, ONOO− induces the irreversible inactivation of iNOS and decreases both NO and O2− production [71].
We have shown here that Fe(II)iNOSox forms only BH4 or superoxide radical or a mixture of these two radicals upon reaction with oxygen (Scheme 1). The heme-BH4-L-arginine triad interplay seems to dictate the monomer/dimer ratio as well as the identity and amount of the oxygen-induced radical species, an interesting and intricate structure/function relationship of NOS.
Scheme 1.
Radical formation mechanism of iNOSox in the presence or absence of BH4 and/or L-arginine.
It is worth mentioning that WT NOS is active only as a homodimer and the electron transfer is conceivable between NOS reductase and oxygenase domain only in a “inter-molecular” fashion where electron transfer occurs between flavin and heme groups located on adjacent subunits in the dimer [73]. Nevertheless, engineered NIH 3T3 cells incapable of de novo BH4 biosynthesis are able to express human iNOS, in a predominantly monomeric form inactive in NO synthesis, or an active dimers form in cytosols when supplemented with BH4 alone [66]. In the absence of L-arginine and BH4 the heme iron in an iNOS is solvent-exposed [64] and has a low midpoint potential (–347 mV), difficult to accept electrons from the reductase domain flavins FADH/FMNH2 (–290 mV) [74]. On the other hand, binding of BH4 or L-arginine alone increases the heme midpoint potential to more favorable –295 mV or –235 mV, respectively. In the presence of both BH4 and L-arginine, these values in iNOSox domain and in WT iNOS are –263 mV and –270 mV, respectively [74, 75]. Thus, thermodynamically heme reduction should be achieved only in the presence of L-arginine and BH4. However, a midpoint potential of –340 mV for NADPH can provide sufficient push, and is able to reduce NOS heme even in L-arginine and/or BH4 deficient iNOS. Indeed, NADPH oxidation was observed for macrophage NOS in the absence of L-arginine, even though only ~16% relative to L-arginine bound NOS [76].
Based on iNOSox data presented here, it is possible to assume that part of heme containing monomeric WT iNOS can be reduce by NADPH and in the absence of L-arginine, iNOS produces superoxide instead of NO, thus justifies the biological significance in using isolated iNOSox to study the dynamics of oxygen-induced radical species. Moreover, inactive iNOS is not more preferentially degraded than active iNOS. Protein turnover rates of iNOS monomers and dimers are similar, with cellular half-life of 1.6 ± 0.3 h [77].
4.2. Comparison between eNOSox, nNOSox, and iNOSox
Although stopped-flow conducted at decreased temperature or protein concentration helped to slow down the reaction to acquire more kinetic data for oxy-ferrous heme formation [38, 42, 43, 78, 79], we decided to use identical temperature, 22 °C, and similar final protein concentration after 2 × dilution in stopped-flow or ~ 4 × dilutions in rapid freeze EPR experiments to enable direct comparison of oxy-ferrous decay with heme oxidation and radical formation, as well as comparison with data from other two NOSox isoforms. These comparative studies led to important conclusions at two different aspects.
4.2.1. Kinetics of oxygen-induced heme and radical changes
Our results, summarized in Table 3, indicate that the decay rates of oxy-ferrous intermediates are different among the three NOSox (+BH4, +L-Arg) enzymes and display a rank of nNOSox (34.9 s−1) > iNOSox (18.0 s−1) > eNOSox (15.0 s−1). The rate of BH4 radical formation follow the identical trend, nNOSox (67.0 ± 10.5 s−1) > iNOSox (20.0 ± 2.5 s−1) > eNOSox (13.1 ± 2.7 s−1). Our data obtained at 22 °C are in agreement with the previous results acquired at 10 °C [43]. Regardless of the intrinsic differences between NOS isozymes and huge differences in NO production, these data support the existence of a common kinetic mechanism for L-arginine hydroxylation in the three NOSs: the BH4 radical forms only after O2 binds to the ferrous heme and the rate of BH4 radical formation matches the rate of oxy-ferrous disappearance in all three NOSs.
4.2.2. Structures of radical intermediates
The radical species from all three NOS isoforms obtained by rapid-freeze EPR are summarized in Table 2. Only nNOSox, not iNOSox or eNOSox, showed thiol-dependent radical EPR structural changes [56]. Under fully coupled conditions, i.e. +BH4, +L-Arg, eNOSox, nNOSox, and iNOSox produce solely BH4 radical with a 39 G wide symmetric spectrum and crossover gx/gy of 2.002 (Table 2, first column). There are only subtle differences among NOSox isozymes in the reaction between oxygen and ferrous NOSox (–BH4, ±L-Arg). All three isozymes produce superoxide EPR with gz of 2.077–2.08 and crossover gx/gy of 2.005–2.006. The EPR line shape and the very high P1/2 in the range of 54–155 mW are typical features of a superoxide radical (Table 2, second column). Only exception is the data from nNOSox(–BH4, +L-Arg) where in addition to a typical superoxide radical our analysis of rapid-freeze EPR spectra revealed formation of a novel (38 gauss) radical with 3-line hyperfine structure, probably involving the guanidine nitrogen nucleus of L-arginine (Table 2, second column and [56]). Thus, L-arginine decreases the amount of superoxide produced by nNOSox by converting it to a new radical. The most interesting data were obtained in the presence of BH4 and absence of L-arginine, NOSox(+BH4, −L-Arg) (Table 2, last column and [46, 56]). Reconstitution with BH4 only (−L-Arg) yields a novel BH4-based radical in eNOSox and also a new 75G radical in DTT-containing nNOSox, as an ensemble of magnetically coupled BH4 radical and a low-spin ferric heme. On the other hand, iNOSox and also nNOSox in the absence of DTT produce a mixture of superoxide and typical BH4 radical that couples to a high-spin ferric heme. This gives the implication that DTT also enhances a tighter dimer formation in nNOSox.
4.2.3. Conclusions
In summary, our spectroscopic analyses showed that during normal catalytic cycle, iNOSox produces a typical BH4 radical and consequently NO, whereas under uncoupling conditions it produces either superoxide radical alone or a mixture with BH4 radical. No additional new radical was observed under any of the tested conditions. iNOS is unique for its potential in forming large (µM) quantities of both superoxide and NO and further the potent oxidizing peroxynitrite, ONOO−, efficient for killing pathogens. However, production of large quantities of these radicals also induces disease conditions such as sepsis, oxidative stress, tissue damage, or chronic inflammatory diseases. One way to avoid these unwanted side-effects is to prevent the expression or to enhance degradation of iNOS itself due to its inductive nature and fast protein turnover, facilitated by its less rigid protein scaffold than nNOS and eNOS.
In contrast, nM quantities of NO produced by constitutively expressed eNOS and nNOS enzymes are sufficient to fulfill their physiological functions. Thus the need for a limited, tightly controlled production of NO from these two NOS isoforms is crucial and is particularly profound in the case of nNOS since even small changes in quantities of NO or superoxide is toxic to neuronal cells with decreased production of glutathione [80]. Thiols are also important to avoid NOS uncoupling and preserving NOS activity in three ways; 1) to prevent BH4 oxidation [40] and 2) to enhance the monomer interaction in the dimer and 3) to prevent irreversible oxidation of surface cysteines [56]. The latter two findings are specific only for nNOS and further underscore the neuroprotective role of glutathione. The differences between iNOS and the constitutive NOS isoforms can be expanded also to explain how these enzymes handle unavoidable uncoupling due to L-arginine deficiency. A self-controlling mechanism evolved in eNOS and nNOS to reduce superoxide production by chemical conversion to a novel surrogate radical species involving BH4 radical via magnetic interaction with an adjacent low-spin ferric heme, and such mechanisms are not employed by iNOS. The differences among all three isoforms are even more apparent under BH4 deficiency, i.e., –BH4, +L-Arg. While L-arginine has marginal effect on the rate or the amount of superoxide production by iNOSox, it decreases only the rate and not the total amount of superoxide radical produced in eNOSox [46], whereas L-arginine decreased superoxide production by forming a new radical in nNOSox [56].
Highlights.
We studied the effect of BH4 and L-arg on radical formation during the reaction of O2 with iNOSox.
Parallel single turnover stopped-flow and rapid freeze trap EPR measurements were performed.
We report differences between O2 intermediates formed by 3 NOSs isoforms.
Acknowledgment
This work was supported by National Institutes of Health grant RO1 HL 095820 to A.-L.T.
Abbreviations
- NOS
nitric oxide synthase
- iNOSox, eNOSox, nNOSox
the oxygenase domain of inducible, endothelial and neuronal NOSs, respectively
- L-Arg
L-arginine
- BH4
(6R)-5,6,7,8-tetrahydro-L-biopterin
- BH2
7,8-dihydro-L-biopterin
- iNOSox (+BH4,+L-Arg)
BH4 reconstituted and L-Arg containing iNOSox
- iNOSox (+BH4, −L-Arg)
BH4 reconstituted and L-Arg deficient iNOSox
- iNOSox (−BH4, −L-Arg)
BH4 and L-Arg deficient iNOSox
- iNOSox (−BH4,+L-Arg)
BH4 deficient and L-Arg containing iNOSox
- cyt c
cytochrome c
- SOD
superoxide dismutase
- HRP
horseradish peroxidase
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- RFQ EPR
rapid freeze quench EPR
- DTT
dithiothreitol
- BME
β–mercaptoethanol
- HEPES
4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid
- WT
wild type
- Fe(II)O2
oxy-ferrous complex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Forstermann U, Gath I, Schwarz P, Closs EI, Kleinert H. Biochem. Pharmacol. 1995;50:1321–1332. doi: 10.1016/0006-2952(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 2.Bredt DS, Snyder SH. Proc. Natl. Acad. Sci. U. S. A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Proc. Natl. Acad. Sci. U. S. A. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moncada S, Palmer RM, Higgs EA. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 5.Nathan C, Xie QW. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 6.MacMicking J, Xie QW, Nathan C. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 7.Pautz A, Art J, Hahn S, Nowag S, Voss C, Kleinert H. Nitric Oxide. 2010;23:75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Schulz R, Rassaf T, Massion PB, Kelm M, Balligand JL. Pharmacol. Ther. 2005;108:225–256. doi: 10.1016/j.pharmthera.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Robinson MA, Baumgardner JE, Otto CM. Free Radic. Biol. Med. 2011;51:1952–1965. doi: 10.1016/j.freeradbiomed.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. J. Leukoc. Biol. 2011;89:873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacher P, Beckman JS, Liaudet L. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alderton WK, Cooper CE, Knowles RG. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daff S. Nitric Oxide. 2010;23:1–11. doi: 10.1016/j.niox.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. J. Biol. Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 15.Xia Y, Roman LJ, Masters BS, Zweier JL. J. Biol. Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- 16.Xia Y, Tsai AL, Berka V, Zweier JL. J. Biol. Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 17.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Proc. Natl. Acad. Sci. U. S. A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinzel B, John M, Klatt P, Bohme E, Mayer B. Biochem. J. 1992;281((Pt 3)):627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris SM., Jr Sci Signal. 2010;3:27. doi: 10.1126/scisignal.3135pe27. [DOI] [PubMed] [Google Scholar]
- 21.Bronte V, Zanovello P. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 22.Gobert AP, McGee DJ, Akhtar M, Mendz GL, Newton JC, Cheng Y, Mobley HL, Wilson KT. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13844–13849. doi: 10.1073/pnas.241443798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaturvedi R, Asim M, Lewis ND, Algood HM, Cover TL, Kim PY, Wilson KT. Infect. Immun. 2007;75:4305–4315. doi: 10.1128/IAI.00578-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNeill E, Channon KM. Thromb. Haemost. 2012;108:832–839. doi: 10.1160/TH12-06-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. J. Clin. Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ionova IA, Vasquez-Vivar J, Whitsett J, Herrnreiter A, Medhora M, Cooley BC, Pieper GM. Am J Physiol Heart Circ Physiol. 2008;295:2178–2187. doi: 10.1152/ajpheart.00748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manoury B, Montiel V, Balligand JL. Cardiovasc. Res. 2012;94:304–315. doi: 10.1093/cvr/cvr360. [DOI] [PubMed] [Google Scholar]
- 28.Bian K, Murad F. Front. Biosci. 2003;8:264–278. doi: 10.2741/997. [DOI] [PubMed] [Google Scholar]
- 29.Thippeswamy T, McKay JS, Quinn JP, Morris R. Histol. Histopathol. 2006;21:445–458. doi: 10.14670/HH-21.445. [DOI] [PubMed] [Google Scholar]
- 30.Olson N, van der Vliet A. Nitric Oxide. 2011;25:125–137. doi: 10.1016/j.niox.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baek KJ, Thiel BA, Lucas S, Stuehr DJ. J. Biol. Chem. 1993;268:21120–21129. [PubMed] [Google Scholar]
- 32.Ghosh DK, Stuehr DJ. Biochemistry. 1995;34:801–807. doi: 10.1021/bi00003a013. [DOI] [PubMed] [Google Scholar]
- 33.Panda K, Rosenfeld RJ, Ghosh S, Meade AL, Getzoff ED, Stuehr DJ. J. Biol. Chem. 2002;277:31020–31030. doi: 10.1074/jbc.M203749200. [DOI] [PubMed] [Google Scholar]
- 34.Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. J. Biol. Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 35.Feng C, Chen L, Li W, Elmore BO, Fan W, Sun X. J. Inorg. Biochem. 2014;130:130–140. doi: 10.1016/j.jinorgbio.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abu-Soud HM, Gachhui R, Raushel FM, Stuehr DJ. J. Biol. Chem. 1997;272:17349–17353. doi: 10.1074/jbc.272.28.17349. [DOI] [PubMed] [Google Scholar]
- 37.Sato H, Sagami I, Daff S, Shimizu T. Biochem. Biophys. Res. Commun. 1998;253:845–849. doi: 10.1006/bbrc.1998.9851. [DOI] [PubMed] [Google Scholar]
- 38.Ledbetter AP, McMillan K, Roman LJ, Masters BS, Dawson JH, Sono M. Biochemistry. 1999;38:8014–8021. doi: 10.1021/bi990619h. [DOI] [PubMed] [Google Scholar]
- 39.Wei CC, Wang ZQ, Wang Q, Meade AL, Hemann C, Hille R, Stuehr DJ. J. Biol. Chem. 2001;276:315–319. doi: 10.1074/jbc.M008441200. [DOI] [PubMed] [Google Scholar]
- 40.Berka V, Yeh HC, Gao D, Kiran F, Tsai AL. Biochemistry. 2004;43:13137–13148. doi: 10.1021/bi049026j. [DOI] [PubMed] [Google Scholar]
- 41.Moreau M, Boucher JL, Mattioli TA, Stuehr DJ, Mansuy D, Santolini J. Biochemistry. 2006;45:3988–3999. doi: 10.1021/bi051488p. [DOI] [PubMed] [Google Scholar]
- 42.Bec N, Gorren AC, Voelker C, Mayer B, Lange R. J. Biol. Chem. 1998;273:13502–13508. doi: 10.1074/jbc.273.22.13502. [DOI] [PubMed] [Google Scholar]
- 43.Wei CC, Wang ZQ, Durra D, Hemann C, Hille R, Garcin ED, Getzoff ED, Stuehr DJ. J. Biol. Chem. 2005;280:8929–8935. doi: 10.1074/jbc.M409737200. [DOI] [PubMed] [Google Scholar]
- 44.Hurshman AR, Krebs C, Edmondson DE, Huynh BH, Marletta MA. Biochemistry. 1999;38:15689–15696. doi: 10.1021/bi992026c. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt PP, Lange R, Gorren AC, Werner ER, Mayer B, Andersson KK. J Biol Inorg Chem. 2001;6:151–158. doi: 10.1007/s007750000185. [DOI] [PubMed] [Google Scholar]
- 46.Berka V, Wu G, Yeh HC, Palmer G, Tsai AL. J. Biol. Chem. 2004;279:32243–32251. doi: 10.1074/jbc.M404044200. [DOI] [PubMed] [Google Scholar]
- 47.Wei CC, Wang ZQ, Tejero J, Yang YP, Hemann C, Hille R, Stuehr DJ. J. Biol. Chem. 2008;283:11734–11742. doi: 10.1074/jbc.M709250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoll S, NejatyJahromy Y, Woodward JJ, Ozarowski A, Marletta MA, Britt RD. J. Am. Chem. Soc. 2010;132:11812–11823. doi: 10.1021/ja105372s. [DOI] [PubMed] [Google Scholar]
- 49.Brunel A, Santolini J, Dorlet P. Biophys. J. 2012;103:109–117. doi: 10.1016/j.bpj.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tejero J, Stuehr D. IUBMB Life. 2013;65:358–365. doi: 10.1002/iub.1136. [DOI] [PubMed] [Google Scholar]
- 51.Stuehr D, Pou S, Rosen GM. J. Biol. Chem. 2001;276:14533–14536. doi: 10.1074/jbc.R100011200. [DOI] [PubMed] [Google Scholar]
- 52.Vasquez-Vivar J, Hogg N, Martasek P, Karoui H, Pritchard KA, Jr, Kalyanaraman B. J. Biol. Chem. 1999;274:26736–26742. doi: 10.1074/jbc.274.38.26736. [DOI] [PubMed] [Google Scholar]
- 53.Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. Biochem. J. 2002;362:733–739. doi: 10.1042/0264-6021:3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosen GM, Tsai P, Weaver J, Porasuphatana S, Roman LJ, Starkov AA, Fiskum G, Pou S. J. Biol. Chem. 2002;277:40275–40280. doi: 10.1074/jbc.M200853200. [DOI] [PubMed] [Google Scholar]
- 55.Weaver J, Porasuphatana S, Tsai P, Pou S, Roman LJ, Rosen GM. Biochim. Biophys. Acta. 2005;1726:302–308. doi: 10.1016/j.bbagen.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Berka V, Wang LH, Tsai AL. Biochemistry. 2008;47:405–420. doi: 10.1021/bi701677r. [DOI] [PubMed] [Google Scholar]
- 57.Yasukawa T, Kanei-Ishii C, Maekawa T, Fujimoto J, Yamamoto T, Ishii S. J Biol Chem. 1995;270:25328–25331. doi: 10.1074/jbc.270.43.25328. [DOI] [PubMed] [Google Scholar]
- 58.Du M, Yeh HC, Berka V, Wang LH, Tsai AL. J. Biol. Chem. 2003;278:6002–6011. doi: 10.1074/jbc.M209606200. [DOI] [PubMed] [Google Scholar]
- 59.Di Iorio EE. Methods Enzymol. 1981;76:57–72. doi: 10.1016/0076-6879(81)76114-7. [DOI] [PubMed] [Google Scholar]
- 60.Kuthan H, Ullrich V, Estabrook RW. Biochem J. 1982;203:551–558. doi: 10.1042/bj2030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai AL, Berka V, Kulmacz RJ, Wu G, Palmer G. Anal. Biochem. 1998;264:165–171. doi: 10.1006/abio.1998.2774. [DOI] [PubMed] [Google Scholar]
- 62.Kulmacz RJ, Tsai AL, Palmer G. J Biol Chem. 1987;262:10524–10531. [PubMed] [Google Scholar]
- 63.Tsai AL, Berka V, Chen PF, Palmer G. J. Biol. Chem. 1996;271:32563–32571. doi: 10.1074/jbc.271.51.32563. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh DK, Wu C, Pitters E, Moloney M, Werner ER, Mayer B, Stuehr DJ. Biochemistry. 1997;36:10609–10619. doi: 10.1021/bi9702290. [DOI] [PubMed] [Google Scholar]
- 65.Presta A, Siddhanta U, Wu C, Sennequier N, Huang L, Abu-Soud HM, Erzurum S, Stuehr DJ. Biochemistry. 1998;37:298–310. doi: 10.1021/bi971944c. [DOI] [PubMed] [Google Scholar]
- 66.Tzeng E, Billiar TR, Robbins PD, Loftus M, Stuehr DJ. Proc. Natl. Acad. Sci. U. S. A. 1995;92:11771–11775. doi: 10.1073/pnas.92.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pignitter M, Gorren AC, Nedeianu S, Schmidt K, Mayer B. Free Radic. Biol. Med. 2006;41:455–463. doi: 10.1016/j.freeradbiomed.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Bielski BHJ, Allen AO. The Journal of Physical Chemistry. 1977;81:1048–1050. [Google Scholar]
- 69.Crane BR, Arvai AS, Ghosh DK, Wu C, Getzoff ED, Stuehr DJ, Tainer JA. Science. 1998;279:2121–2126. doi: 10.1126/science.279.5359.2121. [DOI] [PubMed] [Google Scholar]
- 70.Venema RC, Ju H, Zou R, Ryan JW, Venema VJ. J. Biol. Chem. 1997;272:1276–1282. doi: 10.1074/jbc.272.2.1276. [DOI] [PubMed] [Google Scholar]
- 71.Sun J, Druhan LJ, Zweier JL. Arch. Biochem. Biophys. 2010;494:130–137. doi: 10.1016/j.abb.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beckman JS. Ann. N. Y. Acad. Sci. 1994;738:69–75. doi: 10.1111/j.1749-6632.1994.tb21791.x. [DOI] [PubMed] [Google Scholar]
- 73.Siddhanta U, Presta A, Fan B, Wolan D, Rousseau DL, Stuehr DJ. J. Biol. Chem. 1998;273:18950–18958. doi: 10.1074/jbc.273.30.18950. [DOI] [PubMed] [Google Scholar]
- 74.Presta A, Weber-Main AM, Stankovich MT, Stuehr DJ. J. Am. Chem. Soc. 1998;120:9460–9465. [Google Scholar]
- 75.Gao YT, Smith SM, Weinberg JB, Montgomery HJ, Newman E, Guillemette JG, Ghosh DK, Roman LJ, Martasek P, Salerno JC. J. Biol. Chem. 2004;279:18759–18766. doi: 10.1074/jbc.M308936200. [DOI] [PubMed] [Google Scholar]
- 76.Abu-Soud HM, Stuehr DJ. Proc. Natl. Acad. Sci. U. S. A. 1993;90:10769–10772. doi: 10.1073/pnas.90.22.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kolodziejski PJ, Koo JS, Eissa NT. Proc. Natl. Acad. Sci. U. S. A. 2004;101:18141–18146. doi: 10.1073/pnas.0406711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marchal S, Gorren AC, Sorlie M, Andersson KK, Mayer B, Lange R. J. Biol. Chem. 2004;279:19824–19831. doi: 10.1074/jbc.M313587200. [DOI] [PubMed] [Google Scholar]
- 79.Sorlie M, Gorren AC, Marchal S, Shimizu T, Lange R, Andersson KK, Mayer B. J. Biol. Chem. 2003;278:48602–48610. doi: 10.1074/jbc.M305682200. [DOI] [PubMed] [Google Scholar]
- 80.Aoyama K, Watabe M, Nakaki T. J Pharmacol Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]