Abstract
Down syndrome (DS) is marked by intellectual disability (ID) and early-onset of Alzheimer’s disease (AD) neuropathology, including basal forebrain cholinergic neuron (BFCN) degeneration. The present study tested the hypothesis that maternal choline supplementation (MCS) lessens hippocampal dysfunction and protects against BFCN degeneration in the Ts65Dn mouse model of DS and AD. During pregnancy and lactation, dams were assigned to either a choline sufficient (1.1 g/kg choline chloride) or choline supplemented (5.0 g/kg choline chloride) diet. Between 13 and 17 months of age, offspring were tested in the radial arm water maze (RAWM) to examine spatial learning and memory followed by unbiased quantitative morphometry of BFCNs. Spatial mapping was significantly impaired in unsupplemented Ts65Dn mice relative to normal disomic (2N) littermates. Additionally, a significantly lower number and density of medial septum (MS) hippocampal projection BFCNs was also found in unsupplemented Ts65Dn mice. Notably, MCS significantly improved spatial mapping and increased number, density, and size of MS BFCNs in Ts65Dn offspring. Moreover, the density and number of MS BFCNs correlated significantly with spatial memory proficiency, providing powerful support for a functional relationship between these behavioral and morphometric effects of MCS for the trisomic offspring. Thus, increasing maternal choline intake during pregnancy may represent a safe and effective treatment approach for expectant mothers carrying a DS fetus, as well as a possible means of BFCN neuroprotection during aging for the population at large.
Keywords: Down syndrome, Alzheimer’s disease, medial septum, spatial memory, stereology, hippocampus, radial arm water maze, ventral diagonal band, nucleus basalis
INTRODUCTION
Down syndrome (DS), caused by triplication of human chromosome 21 (HSA21), is the most common genetic disorder resulting in intellectual disability (ID). By the third or fourth decade of life, DS individuals develop dementia and the histopathological characteristics of Alzheimer’s disease (AD) (Casanova et al., 1985; Fodale et al., 2006; Mann, 1988; Mann et al., 1986, 1984; Mann and Esiri, 1989; Wisniewski et al., 1985a, 1985b), including neurofibrillary tangles, neuritic plaques, and cholinergic basal forebrain pathology (Mufson et al., 2003; Isacson et al., 2002; Sendera et al., 2000; Whitehouse et al., 1982). Currently, there are no therapeutic interventions that prevent or reverse ID, age-related cognitive impairment, or brain pathology in DS.
Several mouse models have been developed to study the relationship between the triplication of specific genes in DS and distinct phenotypic features (see Das and Reeves, 2011; Rueda et al., 2011; Salehi et al., 2006 for reviews). The most well-characterized animal model is the Ts65Dn mouse, which is segmentally trisomic for the distal region of mouse chromosome 16 (MMU16) which contains more than 100 highly conserved genes that are orthologous to those on HSA21 (Sturgeon and Gardiner, 2011). This triplicated chromosomal segment also includes the “Down syndrome critical region,” (DSCR) which is considered necessary, although not solely sufficient, for the DS phenotype (Belichenko et al., 2009; Olson et al., 2007, 2004).
Similar to humans with DS, Ts65Dn mice are born with an intact basal forebrain cholinergic neuron (BFCN) system, which undergoes progressive atrophy starting between four and six months of age (Granholm et al., 2000; Holtzman et al., 1996; Hunter et al., 2003; Seo and Isacson, 2005). BFCN atrophy coincides with a decline in hippocampal-dependent memory function in Ts65Dn mice likely due to impairment of the cholinergic hippocampal projection system arising from the medial septum (MS) and vertical limb of the diagonal band (VDB) (Bimonte-Nelson et al., 2003; Crnic and Pennington, 2000; Fernandez and Garner, 2008; Granholm et al., 2000; Holtzman et al., 1996; Hunter et al., 2003; Hyde and Crnic, 2001; Hyde et al., 2001; Rye et al., 1984; Stasko and Costa, 2004). Cholinergic neurons within the nucleus basalis of Meynert (NBM) also atrophy, which correlates with cognitive decline in both DS and AD (Bierer et al., 1995; Davis et al., 1999; DeKosky et al., 2002; Mufson et al., 2003).
Based on these findings, interventions that maintain BFCN viability are likely to improve cognition in DS. A therapy which may hold promise in this regard is supplementation of the maternal diet with additional choline, a hypothesis based on two converging lines of evidence: (a) the pattern of cognitive impairment in DS (and the Ts65Dn mouse) is indicative of dysfunction of BFCNs and their projections to the hippocampus and frontal cortex (spatial memory and attention, respectively); and (b) in normal rodents, supplementing the maternal diet with additional choline (~ 4.5X the amount in normal chow) enhances these two cognitive domains in the offspring, and exerts structural and functional changes in the septo-hippocampal cholinergic system (reviewed in Meck and Williams, 2003; McCann et al., 2006; Zeisel and Niculescu, 2006). Prior findings from our lab have provided support for this hypothesis, demonstrating that maternal choline supplementation (MCS) produces lifelong improvements in performance of Ts65Dn offspring in behavioral tasks dependent on BFCN projections to the frontal cortex (Moon et al., 2010), and normalizes hippocampal neurogenesis in these animals (Velazquez et al., 2013).
The current study was designed to extend this prior work by testing the hypothesis that MCS improves performance of aged Ts65Dn offspring on tests of spatial learning and memory, and that the mechanism(s) underlying these cognitive benefits are related to protection of the BFCN hippocampal and cortical projection systems. To examine these hypotheses, we assessed the effects of supplementing the maternal diet with additional choline (versus a control diet with standard choline content) in Ts65Dn offspring and their normal disomic (2N) littermates, with respect to (1) radial arm water maze (RAWM) performance, and (2) morphometric indices of MS, VDB, and nucleus basalis of Meynert/substantia innominata (NBM/SI) cholinergic neurons.
METHODS
Subjects
Breeder pairs (Ts65Dn female and C57Bl/6J Eicher × C3H/HeSnJ F1 male mice) were purchased from Jackson Laboratories (Bar Harbor, ME), mated at Cornell University, (Ithaca, NY), and randomly assigned to either a choline-controlled, standard rodent chow diet (AIN-76A with 1.1 g/kg choline chloride; Dyets Inc., Bethlehem, PA) or a rodent chow diet with choline supplementation (AIN-76A with 5.0 g/kg choline chloride; Dyets Inc) as reported previously (Kelley et al., 2014a, 2014b; Velazquez et al., 2013). The two levels of maternal choline intake selected for these studies with the Ts65Dn model were based on numerous prior studies demonstrating lasting cognitive benefits of increased maternal choline intake in normal rodents (Meck and Williams, 1999; 2003; Meck et al., 2007). The choline content of the control diet is considered to provide adequate choline intake during pregnancy (Meck et al., 2007). The choline-supplemented diet provided approximately 4.5 times the concentration of choline in the normal diet, within the range of dietary variation observed in the human population (Detopoulou et al., 2008). The breeder pairs were provided ad libitum access to their assigned diet. Pups were weaned on postnatal day 21 (PND 21) and given ad libitum access to the control diet.
Breeder pairs yield litters with segmentally trisomic (Ts65Dn) mice and disomic (2N) littermates. At weaning, tail snips were sent to Jackson laboratories (Bar Harbor, ME) for genotyping by quantitative polymerase chain reaction (qPCR) for the detection of the extra chromosomal segment of MMU16, and determination of Pde6brd1 homozygosity. Pde6brd1 is a recessive mutation that leads to retinal degeneration (Keeler, 1966); mice homozygous for the Pde6brd1 mutation were excluded from the study.
After weaning, the pups were group-housed with same-sex littermates. Only male mice were used for these experiments. In pilot experiments, we observed that ad libitum access to food produces obesity in many of these mice over time, resulting in a tendency to float in the water rather than purposefully swim to search for the hidden escape platform. Therefore, to prevent obesity, a daily ration was calculated to yield body weights that were approximately 90% of their free-feeding weights. Two weeks prior to behavioral testing, mice were singly housed to prevent fighting between cage-mates, which often occurs when group-housed male mice of this strain are returned to the home-cage following daily behavioral testing. A combination of daily handling, testing and the provision of items in the home-cage (i.e., plastic igloos, tubes, and plastic-gel bones, Nestlets) countered the environmental impoverishment of single animal housing. All of these objects were always in the animals’ cages throughout the experiment. Once each week, the soiled objects were removed and replaced with clean objects. Mice were housed in a room with a 12:12-hour reversed light-dark cycle (lights off at 8:00 a.m.) and tested during the dark cycle. The behavioral testing room directly adjoined the housing room, preventing any light exposure during the transport of the animals between rooms.
All protocols were approved by the Institutional Animal Care and Use Committee of Cornell University and conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Behavioral Testing: Radial arm water maze (RAWM)
The RAWM was used to assess spatial learning and memory. Variations of this task have been used successfully in prior studies with Ts65Dn mice (Bimonte-Nelson et al., 2003; Howell and Gottschall, 2012; Hunter et al., 2003; Lockrow et al., 2011; Velazquez et al., 2013) and other AD mouse models (Arendash et al., 2004). Because this task requires mice to navigate the arms of the maze to find the escape platform, it prevents the thigmotactic behavior exhibited by Ts65Dn mice in the Morris water maze.
The RAWM was configured in a pool (100 cm diameter) containing six arms (25.5 cm high, 35 cm long, 20 cm wide) radiating from the center. This configuration created a central area of 40 cm diameter. Water temperature was maintained at 20–22 °C, cool enough to ensure adequate motivation to perform the task, but sufficiently warm to avoid hypothermia. The escape platform was a cylinder (surface 10 cm diameter, 7.5 cm tall) made of clear plastic, tinted black, situated 1 cm below the water surface. Because the inside of the pool was also black, the escape platform was not visible to the animals. Numerous distinctive extra-maze cues were available to allow the mice to navigate using spatial mapping. Two individuals conducted the behavioral testing, each testing an equal number of mice per treatment group. Animals were tested in two consecutive cohorts, in a stratified randomized balanced design across 4 groups, resulting in an age range of 12.8 to 16.7 months (Mean = 15.5, SD = 1.75; see Table 1) at the start of testing. Behavioral testing followed a 20-day protocol after which the animals were immediately sacrificed at ages 13.7 – 17.8 months. Whenever possible, one Ts65Dn and one 2N male pup were selected from each litter for behavioral testing. Experimenters conducting the behavioral testing were blind to the subject’s genotype and maternal diet.
Table 1.
Age and treatment groups
| Groupa | Age range & mean age at sacrifice | Final sample size (Mean age in months) | |||
|---|---|---|---|---|---|
| RAWM | ChAT b | p75NTR b | TrkA b | ||
| 2N | |||||
| Ch | 13.7–17.8 (15.6) | 19 (15.6) | 18 (15.6) | 15–20 (15.6) | 7–12 (15.5) |
| Ch+ | 13.7–17.6 (15.5) | 20 (15.5) | 19 (15.4) | 15–18 (15.5) | 9–13 (15.0) |
| Ts65Dn | |||||
| Ch | 13.7–17.4 (15.3) | 13 (15.3) | 15 (15.4) | 14–16 (15.3) | 4–8 (15.3) |
| Ch+ | 13.8–17.6 (15.7) | 21 (15.7) | 21–22 (15.8) | 20–23 (15.7) | 9–14 (15.7) |
2N: disomic wildtype mice whose dams were provided either a choline normal (Ch) or choline supplemented (Ch+) diet; Ts65Dn mice whose dams were provided either a choline normal (Ch) or choline supplemented (Ch+) diet. Tissue immunolabeled for antibodies: ChAT: choline acetyltransferase, p75NTR: pan-neurotrophin receptor protein 75 KDa, TrkA: tyrosine kinase receptor A.
RAWM testing comprised three phases: (1) training, (2) hidden platform (HP) task, and (3) visible platform (VP) task, as described below.
Training
For one session prior to maze testing, the mice were acclimated to the maze and the basic procedures of the task (e.g., swimming, finding the hidden platform). During this phase, all arms were blocked except for the start arm and the goal arm that contained the hidden platform, providing a direct escape route. This same procedure was used on the first trial of the first day of the hidden platform task (see below) but on all subsequent trials, all arms were accessible.
Hidden platform (HP) task
The HP task began on the day following training. In this task, mice were tested for five trials per session for a total of 15 sessions. The escape platform remained in the same location throughout testing for each animal, with the animals starting from a different arm on each of the five daily trials, pseudo-randomly determined. Each animal was assigned a different hidden platform location for the entirety of this task, with the goal location balanced across treatment groups. On each trial, the mice were given 60 seconds to locate the platform; if the platform was not located within that period, the mouse was guided to the platform. If at least half of the animal’s body entered a non-goal arm an error was tallied. After each trial, each animal was given a 15 second rest period on the platform and then returned to its home cage during the inter-trial interval (ITI) in order to prevent hypothermia, a particular concern for Ts65Dn mice (Iivonen et al., 2003; Stasko and Costa, 2004; Stasko et al., 2006). All mice were tested on trial 1 before any mouse was tested on trial 2, etc., with the result that each animal had a 10–20 minute rest period between consecutive trials. The animals were weighed every other day and fed immediately following the last trial of each day.
Visible platform (VP) task
On the day following completion of the HP task, the mice were tested on the VP task for five sessions, using the same water maze apparatus. Successful performance in the VP task does not require spatial mapping but depends on other abilities needed for success in the HP task, including the ability to swim, and climb onto the platform, motivation to escape from the water, arousal regulation, and visual acuity. In the VP task, a black curtain was placed around the water maze to obscure extramaze cues. The location of the platform was indicated by a prominent intramaze cue: a tall, white PVC pipe affixed to the platform (3.8 cm diameter × 30.5 cm high). The location of the platform was changed on every trial. All other testing characteristics were the same as in the HP task, including the amount of time allotted per trial to search for the platform, the resting period on the platform, and the ITI.
Tissue preparation
Upon completion of behavioral testing, mice were deeply anesthetized with ketamine (85 mg/kg)/xylazine (13 mg/kg) via intraperitoneal injection, perfused tran-scardially with 0.9% saline (50 ml), followed by 4% paraformaldehyde (50 ml) in phosphate buffer (PB; 0.1M; pH = 7.4). Ages at sacrifice are shown in Table 1. Brains were extracted from the calvaria, postfixed for 24 h in the same fixative, and placed in a 30% sucrose PB solution at 4 °C until sectioning. Each brain was cut in the coronal plane at 40 μm thickness, on a sliding freezing microtome into six adjacent series and stored at 4 °C in a cryoprotectant solution (30 % ethylene glycol, 30 % glycerol, in 0.1 M PB) prior to immunohistochemical staining (Kelley et al., 2014a, 2014b; Velazquez et al., 2013).
Immunohistochemistry
Immunohistochemistry was performed as previously described (Kelley et al., 2014a, 2014b). Tissue was immunostained using the following primary antibodies: a goat polyclonal antibody against choline acetyltransferase (ChAT, 1:1000; Millipore, Billerica, MA), a rabbit polyclonal antibody against the pan-neurotrophin receptor p75NTR (1:2000; Millipore), and a rabbit polyclonal antibody against the cognate nerve growth factor (NGF) receptor tyrosine receptor kinase A (TrkA, 1:10,000, gift from Dr. L. Reichardt, UCLA). A 1/6 series of sections was singly immunolabeled for each marker, group sizes are as listed in Table 1. Briefly, sections were washed in 0.1 M phosphate buffer (PB; pH 7.4) to remove cryoprotectant, rinsed in Tris-buffered saline (TBS), and incubated in sodium (meta)periodate (2.139 g per 100 ml TBS) to inhibit endogenous peroxidase activity. To improve primary antibody penetrance throughout the full depth of the tissue, sections was washed in TBS with 0.25 % Triton X-100, followed by rinses in a blocking solution to prevent nonspecific binding consisting of 3 % serum (raised against host organism of secondary antibody: ChAT, horse serum; p75NTR and TrkA, goat serum) in TBS/Triton X-100. Tissue was then incubated overnight at room temperature with the primary antibody in TBS/Triton X-100 with 1 % serum and then washed in TBS and incubated for 1 h with a biotinylated IgG secondary antibody raised against the host of the primary antibody: ChAT, anti-goat; p75NTR, anti-rabbit; TrkA, anti-rabbit (Vector Laboratories, Burlingame, CA). Tissue was washed in TBS and incubated with an avidin-biotin complex (ABC) solution (Elite Kit, Vector Laboratories) for 1 h to amplify the immunochemical reaction. Immunolabeling was visualized using an acetate-imidazole buffer containing 0.05 % 3,3′-diaminobenzidine tetrahy-drochloride (DAB; Sigma-Aldrich, St. Louis, MO) and 0.0015 % freshly prepared H2O2. Sections were washed in acetate-imidazole buffer to terminate the immunochemical reaction, mounted onto chrome-alum-subbed slides, air dried for 24 h, dehydrated through a series of graded alcohols (70%, 95%, and 100 %), cleared in xylenes, and cover-slipped with distyrene/dibutylphthalate (plasticizer)/xylene (DPX) mounting medium (Kelley et al., 2014a, 2014b; Velazquez et al., 2013).
BFCN nomenclature
The BFCN subregions examined included the medial septum (MS), vertical limb of the diagonal band (VDB), and nucleus basalis of Meynert/substantia innominata (NBM/SI).
Stereology
ChAT-, p75NTR-, and TrkA-immunoreactive neuron counts were determined in the MS, VDB, and NBM/SI using the optical fractionator, a stereological model that pairs the optical disector probe (a three-dimensional counting space) with a two-dimensional grid that provides an unbiased random-start and systematic interval sampling of the region of interest. All analyses were conducted using Stereo Investigator software (version 9.14.5 32-bit, Micro-BrightField, Inc., Williston, VT, USA) coupled to a Nikon Optiphot-2 microscope. Values are presented as estimate per brain derived from a sampling of the region of interest bilaterally across a 1/6 series for each marker (X60, n.a. 1.40, 50 × 50 μm counting frame, 151 × 151 μm grid size, 10 μm disector height). Tissue thickness was measured at every site that contained cells and the reciprocal for (disector height)/(mean measured thickness) was used for reported numbers and statistical analyses. The large sampling fraction allowed for a CEm = 1 of ≤ 0.10 (Gundersen, 1999). Cell density is presented as cells per 1,000,000 μm3 (Kelley et al., 2014a). Calculation was performed for each animal, prior to group averages. Photomicrographs were taken on a Nikon Optiphot-2 microscope (Tokyo, Japan) connected to Stereo Investigator software (MicroBrightField, Inc.) Background correction was used at the time of image capture to establish evenness of illumination across the field, and scale bars were added within the Stereo Investigator software. Panels were compiled in PowerPoint (version 14.0.6129.5000, 32-bit, Microsoft, Redmond, WA, USA) and each micrograph was equally corrected for brightness and contrast. No retouching or further manipulations were performed.
Regional area analysis
Regional basal forebrain areas (μm2) were derived by planimetry. Previous comparison between Cavalieri estimator and planimetry values showed no difference between methods (Kelley et al., 2014a: slope m = 1.001, correlation r2 = 0.998, r = 0.999). Values represent summation from tracings outlining each basal forebrain subfield across a 1/6 series: an average of four MS sections, four VDB sections, and seven NBM/SI sections. An area estimate for each basal forebrain subregion was calculated by averaging measures for ChAT- and p75NTR-immunolabeled tissue prior to deriving group averages.
Neuron size
BFCN size was measured using a 5-ray nucleator probe for an average of 60 cells per stain, per region, per animal (X60 oil-immersion lens n.a. 1.40) using random sampling across rostrocaudal and dorsoventral axes derived with the optical fractionator. The nucleator involves taking five measurements from an approximate center of the cell to the perimeter of the cell in one plane (< 1.0 μm z-axis) of section (Gundersen, 1988). The probe derives an average radius for each cell and volume was calculated from this value using a weighted geometric formulae (shape assumption spheroid).
Antibody tissue penetration
A major criterion for the use of the optical disector is antibody penetration through the full depth of the stained section. The depth of ChAT, p75NTR, and TrkA antibody penetration through a tissue section in the z-axis was determined using the same optical disector system and software used to count labeled neurons in this study (see above). ChAT, p75NTR, and TrkA antibodies penetrated the full depth of the section allowing for the equal probability of counting all objects, a prerequisite for stereology (Mufson et al., 2000; Kelley et al., 2014a, 2014b; Velazquez et al., 2013).
Statistical Analyses
Statistical and correlation analyses were performed using the Statistical Analysis System (version 9.3; SAS Institute, Cary, NC). The statistical procedure used for each outcome measure is presented below.
HP and VP tasks
For both the HP and VP tasks, the dependent measure for each mouse was the mean number of errors committed per trial, averaged for each block of sessions. For the HP task, the 15 sessions were divided into five 3-day blocks for analysis; for the VP task, the 6 sessions were divided into three 2-day blocks. The data were analyzed using a generalized linear mixed model (PROC GLIMMIX), an established method for conducting repeated measures analyses with various probability distributions including Gaussian (Wolfinger and O’Connell, 1993). Fixed factors for the models included Genotype (Ts65Dn and 2N), Maternal Diet (diet containing normal levels of choline or choline-supplemented), and Cohort (cohorts 1 and 2), as well as appropriate interaction terms. The random factors in the model included Session-Block (defined above) and individual mouse performance. If the interaction between Genotype and Maternal Diet was significant, three post-hoc contrasts of interest were examined: (1) Comparison of unsupplemented Ts65Dn and 2N mice to determine the effect of genotype for mice born to dams maintained on a diet with normal choline levels; (2) Comparison of Ts65Dn mice born to unsupplemented dams versus supplemented dams to determine the effect of MCS for Ts65Dn mice; and (3) Comparison of supplemented 2N mice to unsupplemented 2N mice to determine the effects of MCS for the 2N mice. A Bonferroni correction was applied to these three comparisons, yielding an alpha level of 0.05/3 or 0.0167 as the threshold for significance. If the interaction was not significant, only the main effects of Maternal Diet and/or Genotype are reported.
Morphometric analyses
Analyses of variance (ANOVA) assessed group differences in mean number, density, and size of neurons reactive for ChAT, p75NTR, and TrkA within each BFCN region (MS, VDB, NBM/SI) as described previously (Kelley et al., 2014a). Significant interactions between Maternal Diet and Genotype (p = 0.05) were followed up with the three comparisons of interest listed above using a Bonferroni correction.
Group differences in regional area of the various BFCN regions were assessed using the non-parametric Wilcoxon rank sum because normality assumptions of ANOVA were not met.
Correlations between behavioral and morphometric data
A non-parametric Spearman rank correlation was used to assess the relationship between each of the morphometric indices for the MS [count, density, and neuron size for each marker (ChAT, p75NTR, TrkA)], and the mean number of errors committed in block 3 of the HP task, the block of testing sessions in which the largest group differences were observed. The use of a non-parametric test mitigated the influence of a few outliers.
RESULTS
Sample size
The final sample size for all behavioral and morphometric measures is presented in Table 1. The four treatment groups were: 1) disomic mice born to dams maintained on a choline normal diet (2N Ch); (2) Ts65Dn mice born to dams maintained on a choline normal diet (Ts Ch); (3) disomic mice born to dams maintained on a choline supplemented diet (2N Ch+); and (4) Ts65Dn mice born to dams maintained on a choline supplemented diet (Ts Ch+). Note that the final sample size for the TrkA endpoints was smaller than for the other analyses due to tissue labeling variability.
Body Weight
Analysis of body weight at the start of testing revealed a main effect of Genotype (F (1,69) = 16.61, p < 0.0001) but no effect of Maternal Diet (F (1,69) = 0.35, p = 0.556), and no interaction of Genotype and Maternal Diet (F (1,69) = 0.09, p = 0.769). The average body weight of trisomic mice was 9% lower than the 2N mice (means ± S.E.M: 2N mice: 35.2 ± 0.50; Ts65Dn mice: 32.18 ± 0.55), consistent with prior reports (Bianchi et al., 2010; Fuchs et al., 2012; Roper et al., 2006).
BFCN Subregions
The nomenclature and topography of the mouse BFCN subfields are in accordance with earlier studies by our collaborative group (Kelley et al., 2014a; 2014b). BFCNs of the MS, VDB and NBM/SI appear as small, spherical and ovoid ChAT-immunoreactive neurons concentrated along the midline with extensions ventrolaterally between the subcallosal region, rostrally, and hemispheric crossing of the anterior commissure, caudally. More caudally, a diffuse group of large multipolar cholinergic neurons are located along the ventromedial aspect of the globus pallidus, which correspond to the NBM/SI (see Supplementary Fig. A.1 – A.3).
Effects of genotype and MCS on HP and VP task performance
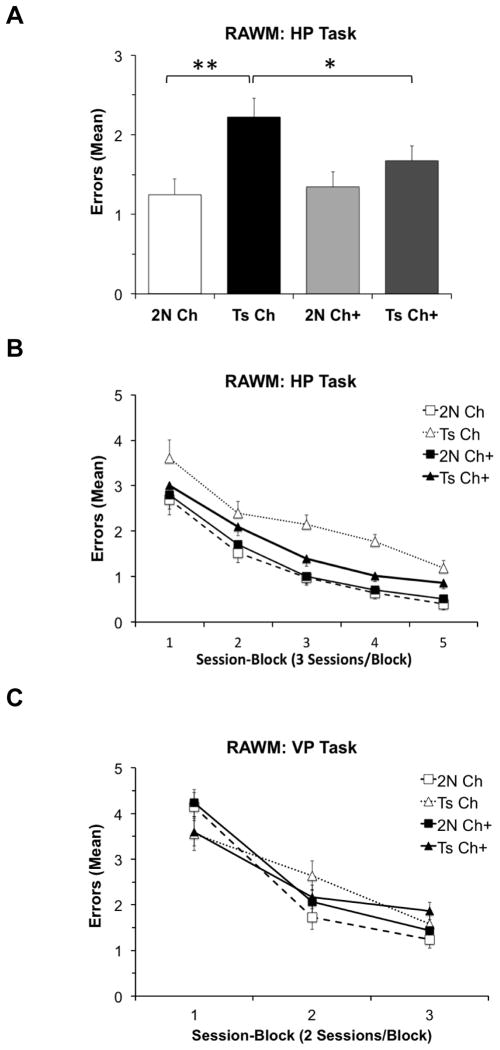
Analysis of mean errors in the HP task revealed a main effect of Genotype (F (1, 101.5) = 20.77, p < 0.0001). Although the main effect of Maternal Diet was not significant (F (1, 101.5) = 2.43, p = 0.122), there was a significant interaction of Genotype and Maternal Diet (F (1, 101.5) = 5.11, p = 0.026). Specifically, Ts65Dn mice born to dams on the unsupplemented diet committed a significantly higher number of errors than their 2N counterparts (p < 0.0001, Fig. 1A). Supplementing the maternal diet with additional choline significantly improved performance of the Ts65Dn offspring relative to Ts65Dn offspring born to dams on the unsupplemented diet (p = 0.012, Fig. 1A). The relationship between the groups did not vary by session-block (Fig. 1B). MCS did not significantly affect performance of the 2N mice on this task.
Fig. 1. RAWM performance.
(A) Average errors per trial (collapsed across sessions) in the HP Task was significantly higher for the unsupplemented Ts65Dn mice than their 2N counterparts. MCS significantly improved performance for Ts65Dn (p = 0.011) but not 2N mice; (B) Mean errors per trial across the 15 sessions of the HP task; (C) Mean errors in the VP task across the 6 sessions on this task; no significant groups differences were seen. Abbreviations: 2N Ch: disomic mice born to dams maintained on a choline normal diet (n = 19); Ts Ch: Ts65Dn mice born to dams maintained on a choline normal diet (n = 13); 2N Ch+: disomic mice born to dams maintained on a choline supplemented diet (n = 20); Ts Ch+: Ts65Dn mice born to dams maintained on a choline supplemented diet (n = 21); HP: Hidden platform task of the radial arm watermaze; VP: Visible platform task of the radial arm watermaze; MCS: maternal choline supplementation; * p ≤ 0.05, ** p ≤ 0.001.
In the VP task, neither the main effect of Genotype (p > 0.20), the main effect of Maternal Diet (p > 0.20) nor the interaction of Genotype and Maternal Diet (p > 0.20) was significant. Although a significant interaction was detected between Genotype and Session-block (F(2, 68.72) = 4.61; p = 0.013), the two genotypes did not differ significantly in any session-block (Fig 1C).
Effects of genotype and MCS on BFCN density
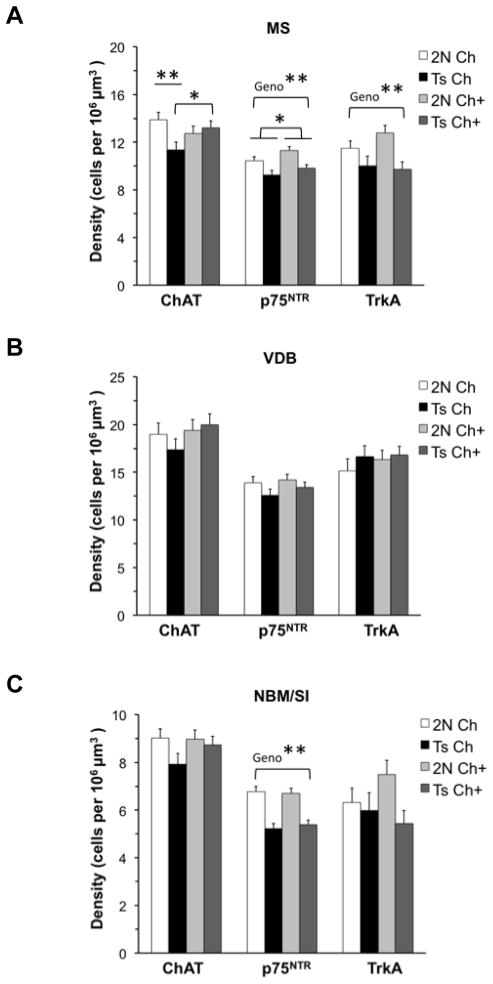
In the MS, there was a significant interaction between Genotype and Maternal Diet for ChAT-immunoreactive neuron density (F(1,70) = 2.70, p = 0.017). MS ChAT-immunoreactive neuron density was significantly reduced by 18% for unsupplemented Ts65Dn offspring relative to their 2N counterparts (p = 0.008, Fig. 2A). MCS significantly increased the density of MS ChAT-immunoreactive neurons by 17% for Ts65Dn mice (p = 0.036, Fig. 2A), achieving a level comparable to unsupplemented 2N mice. Maternal diet did not affect this measure for the 2N mice.
Fig. 2. Density of ChAT-, p75NTR-, and TrkA-immunoreactive cells in the basal forebrain.
(A) In the MS, Ts Ch mice showed a significantly lower ChAT-immunoreactive density relative to 2N Ch mice (p = 0.008), whereas both groups of Ts65Dn mice showed reduced density of p75NTR-immunoreactive and TrkA-immunoreactive neurons relative to 2N mice (p75NTR: p = 0.0001; TrkA: p = 0.002). MCS significantly increased the density of ChAT-immunoreactive neurons in Ts65Dn mice (p = 0.036); (B) No significant differences were seen in the VDB; (C) In the NBM/SI, both groups of Ts65Dn mice showed reduced p75NTR-immunoreactive neuron density relative to 2N mice (p < 0.0001). Abbreviations: 2N Ch: disomic mice born to dams maintained on a choline normal diet; Ts Ch: Ts65Dn mice born to dams maintained on a choline normal diet; 2N Ch+: disomic mice born to dams maintained on a choline supplemented diet; Ts Ch+: Ts65Dn mice born to dams maintained on a choline supplemented diet; Geno: Main effect of Genotype; MS: Medial Septum; VDB: Ventral Diagonal Band; NBM/SI: Nucleus Basalis of Meynert/Substantia Innominata; BFCNs: basal forebrain cholinergic neurons; ChAT: Choline Acetyltransferase; p75NTR: pan neurotrophin receptor; TrkA: tyrosine kinase A receptor; MCS: maternal choline supplementation;*p ≤ 0.05, **p ≤ 0.001.
The density of MS p75NTR- immunoreactive neurons was also significantly lower (13%) for Ts65Dn mice relative 2N counterparts (Main effect of Genotype: F(1,73) = 16.16, p = 0.0001, Fig. 2A). This same genotype effect was found for the density of MS TrkA-immunoreactive neurons (Main effect of Genotype: F(1,43) = 10.97, p = 0.002, Fig. 2A). MCS significantly increased the density of p75NTR-immunoreactive MS neurons by 7% for both genotypes (Main effect of Maternal Diet: F(1,73) = 4.31, p = 0.041, Fig. 2A), but did not affect TrkA-immunoreactive neuron density in this region, indicating a dissociation between NGF receptor phenotypes.
The NBM/SI of Ts65Dn mice showed a significant 22% reduction in the density of p75NTR-immunoreactive neurons relative to 2N mice (Main effect of Genotype: F(1,64) = 44.78, p < 0.0001, Fig. 2C) similar to that seen in the MS. MCS had no effect on this measure in the NBM/SI. In contrast to observations within the MS, neither ChAT- nor TrkA-immunoreactive neurons were significantly altered by Genotype, Maternal Diet or their interaction, in the NBM/SI.
For the VDB, there was no significant effect of Genotype, Maternal Diet or their interaction for BFCN density, regardless of the markers examined (Fig. 2B).
Effects of genotype and MCS on BFCN cell counts
Genotype did not significantly alter ChAT-immunoreactive neuron count in any of the three regions examined. MCS did alter the number of ChAT-immunoreactive BFCN neurons, but the effect varied by subregion and genotype. In the MS, an interaction between Genotype and Maternal Diet was seen (F(1, 70) = 4.63, p = 0.035). MCS produced a significant 30% increase in the number of MS ChAT-immunoreactive neurons in Ts65Dn mice (Ts65Dn Ch vs. Ts65Dn Ch+; p = 0.009, Fig. 3A), but not 2N mice (p > 0.20). In the VDB, MCS produced a significant 20% increase in the num- -ber of ChAT-immunoreactive neurons, with a comparable effect seen for the two genotypes (Main effect of Maternal Diet: F(1, 70) = 5.58, p = 0.021, Fig. 3B). A similar but non-significant 9% increase in ChAT-immunoreactive neurons was seen in the NBM/SI for both genotypes (Main effect of Maternal Diet: F(1, 68) = 2.30, p = 0.134, Fig. 3C).
Fig. 3. Number of ChAT-, p75NTR-, and TrkA-immunoreactive neurons in the basal forebrain.
(A) In the MS, MCS significantly increased the number of ChAT-immunoreactive neurons in Ts65Dn mice (p = 0.009); (B) In the VDB, both groups of Ts65Dn mice showed a significantly higher number of TrkA-immunoreactive neurons (p = 0.036). MCS increased the number of ChAT-immunoreactive BFCNs for both genotypes (p = 0.021); (C) In the NBM/SI, Ts65Dn mice exhibited a significantly higher number of p75NTR-immunoreactive neurons than the 2N mice (p < 0.0001). Abbreviations: 2N Ch: disomic mice born to dams maintained on a choline normal diet; Ts Ch: Ts65Dn mice born to dams maintained on a choline normal diet; 2N Ch+: disomic mice born to dams maintained on a choline supplemented diet; Ts Ch+: Ts65Dn mice born to dams maintained on a choline supplemented diet; Geno: Main effect of Genotype; MS: Medial Septum; VDB: Ventral Diagonal Band; NBM/SI: Nucleus Basalis of Meynert/Substantia Innominata; BFCNs: basal forebrain cholinergic neurons; ChAT: Choline Acetyltransferase; p75NTR: pan neurotrophin receptor; TrkA: tyrosine kinase A receptor; MCS: maternal choline supplementation; * p ≤ 0.05, ** p ≤ 0.001.
The number of p75NTR immunoreactive neurons was significantly reduced (23%) in trisomic mice relative to their 2N counterparts in the NBM/SI (Main effect of Genotype: F(1,64) = 17.61, p < 0.0001; Fig. 3C), with no Genotype effect for the MS (Fig. 3A) or VDB (Fig. 3B). MCS did not significantly affect the number of p75NTR- labeled neurons for either genotype in any BFCN subregion examined (Fig. 3A–C).
The number of TrkA-immunoreactive neurons was significantly higher in Ts65Dn mice than their 2N counterparts in the VDB (Main effect of Genotype: F(1, 37) = 4.72, p = 0.036; Fig 3B). In contrast, the genotypes did not differ significantly for TrkA-immunoreactive neuron count in the MS (p = 0.103, Fig. 3A) or NBM/SI (p = 0.150, Fig. 3C). MCS did not significantly alter TrkA-immunoreactive neuron counts for either genotype in any region examined.
Effects of genotype and MCS on BFCN size
ChAT-, p75NTR-, or TrkA-immunoreactive neuron size did not differ between the two genotypes in any region examined. MCS altered BFCN size only in the MS, producing a significant 15% increase in TrkA-immunoreactive neuron size in this region, with a comparable effect seen in both genotypes (Main effect of Maternal Diet: F(1, 42) = 3.89, p = 0.050, Fig. 4A). MCS did not alter the size of TrkA- immunoreactive neurons in the VDB (Fig. 4B) or NBM/SI (Fig. 4C), nor ChAT- or p75NTR-immunoreactive neurons in any subregions examined (Fig. 4A–C).
Fig. 4. Size of ChAT-, p75NTR-, and TrkA-immunoreactive cells in the basal forebrain.
(A) In the MS, MCS increased TrkA-immunoreactive BFCNs for both genotypes (p = 0.050); There was no effect of genotype, maternal diet, nor their interaction in (B) the VDB, or (C) the NBM/SI. Abbreviations: 2N Ch: dis-omic mice born to dams maintained on a choline normal diet; Ts Ch: Ts65Dn mice born to dams maintained on a choline normal diet; 2N Ch+: disomic mice born to dams maintained on a choline supplemented diet; Ts Ch+: Ts65Dn mice born to dams maintained on a choline supplemented diet; MS: Medial Septum; VDB: Ventral Diagonal Band; NBM/SI: Nucleus Basalis of Meynert/Substantia Innominata; BFCNs: basal forebrain cholinergic neurons; ChAT: Choline Acetyltransferase; p75NTR: pan neurotrophin receptor; TrkA: tyrosine kinase A receptor; MCS: maternal choline supplementation; * p ≤ 0.05, ** p ≤ 0.001.
Effects of genotype and MCS on region area
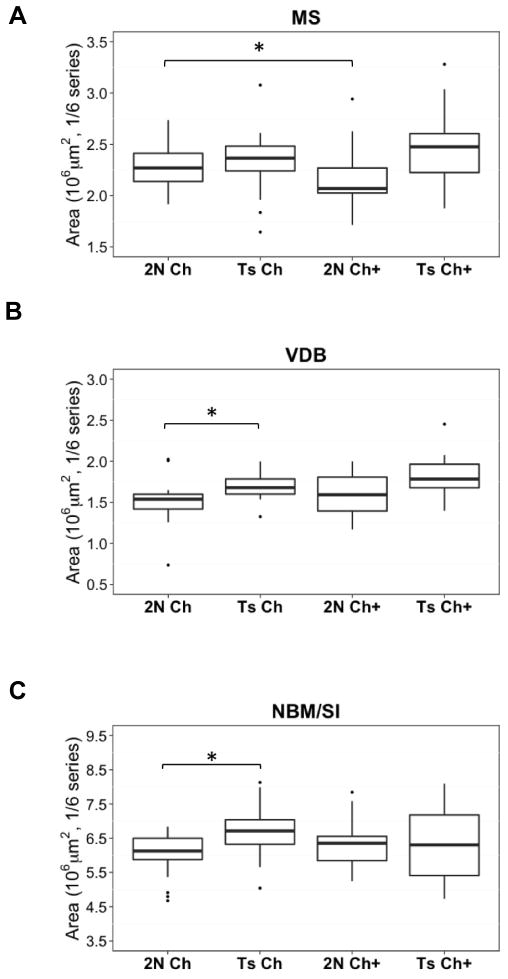
Region area of the VDB and NBM/SI was significantly larger in the unsupplemented Ts65Dn mice relative to their 2N counterparts (Wilcoxon Rank Sum Test, VDB: p = 0.016, Fig. 5B; NBM/SI: p = 0.033; Fig. 5C). In contrast, MS region area did not vary by genotype (Fig. 5A). The effect of MCS on region area varied by genotype and the specific subregion evaluated. MCS led to a significant 8% average decrease in MS area for 2N mice (Wilcoxon Rank Test, p = 0.046, Fig. 5A), with no effect for the VDB or NBM/SI. There was no effect of MCS on region area for Ts65Dn mice for any subfield examined (Fig. 5A–C).
Fig. 5. Regional basal forebrain areas.
(A) MCS decreased MS area for 2N mice (p = 0.046), but had no effect for Ts65Dn mice; (B) Unsupplemented Ts65Dn mice showed significantly larger VDB area than unsupplemented 2N mice (p = 0.016), a pattern also seen for the (C) NBM/SI (p = 0.033). MCS had no effect on VDB or NBM/SI for either genotype. Abbreviations: 2N Ch: disomic mice born to dams maintained on a choline normal diet; Ts Ch: Ts65Dn mice born to dams maintained on a choline normal diet; 2N Ch+: disomic mice born to dams maintained on a choline supplemented diet; Ts Ch+: Ts65Dn mice born to dams maintained on a choline supplemented diet; MS: Medial Septum; VDB: Ventral Diagonal Band; NBM/SI: Nucleus Basalis of Meynert/Substantia Innominata; BFCNs: basal forebrain cholinergic neurons; ChAT: Choline Acetyltransferase; p75NTR: pan neurotrophin receptor; TrkA: tyrosine kinase A receptor; MCS: maternal choline supplementation; * p ≤ 0.05, ** p ≤ 0.001.
Correlations between maze performance and BFCN indices
Correlational analyses revealed a significant negative association between the mean number of errors in block 3 of the HP task and density of ChAT-immunoreactive MS neurons (rs= −0.31, p = 0.014; Fig. 6A). A similar trend was seen for the density of TrkA-immunoreactive MS neurons (rs =−0.27, p = 0.095; Fig. 6B). There were no significant relationships between maze performance and size of ChAT- or TrkA-immunoreactive neurons.
Fig. 6. Correlations between HP performance and BFCN indices in the MS.
Mean errors in block 3 of the HP task was negatively correlated with (A) Density of ChAT-immunoreactive BFCNs (p = 0.014), (B) Density of TrkA-immunoreactive BFCNs (p = 0.095); (C) Cell size of p75NTR-immunoreactive BFCNs (p = 0.036), and (D) Number of p75NTR- immunoreactive BFCNs (p = 0.062). Abbreviations: 2N Ch: disomic mice born to dams maintained on a choline normal diet; Ts Ch: Ts65Dn mice born to dams maintained on a choline normal diet; 2N Ch+: disomic mice born to dams maintained on a choline supplemented diet; Ts Ch+: Ts65Dn mice born to dams maintained on a choline supplemented diet; HP: Hidden platform task of the radial arm watermaze; BFCNs: basal forebrain cholinergic neurons; ChAT: Choline Acetyltransferase; p75NTR: pan neurotrophin receptor; TrkA: tyrosine kinase A receptor.
A significant negative correlation was also observed between the mean number of block 3 errors and size of p75NTR-immunoreactive neurons in the MS (rs =−0.26; p = 0.036; Fig. 6C), with a similar trend seen for MS p75NTR-immunoreactive neuron numbers (rs =−0.23, p = 0.062; Fig. 6D).
DISCUSSION
The present study demonstrated that supplementing the maternal diet with additional choline during pregnancy and lactation significantly improved spatial cognition of Ts656Dn offspring and provided BFCN neuroprotection in a phenotypic- and subregion-specific manner. Moreover, several of these BFCN indices, notably those in the MS, correlated significantly with maze performance, providing powerful support for a functional relationship between these behavioral and morphometric effects of MCS. These data have significant translational potential for minimizing BFCN dysfunction in DS, and add to the growing evidence that maternal choline intake should be increased during pregnancy.
MCS improves spatial cognition of Ts65Dn offspring
Unsupplemented Ts65Dn mice performed significantly less well in the HP task than their 2N littermates. In contrast, these groups did not differ in the VP task, indicating that the impaired performance of the unsupplemented trisomic mice in the HP task cannot be attributed to impairments in visuomotor skills, swimming ability, or motivation to escape from the water. Rather, these data collectively indicate that the poor performance of Ts65Dn mice in the HP task is due to impaired spatial mapping, subserved by the hippocampus, consistent with prior findings with spatial mazes (Belichenko et al., 2007; Bimonte-Nelson et al., 2003; Chang and Gold, 2008; Escorihuela et al., 1995; Holtzman et al., 1996; Hunter et al., 2003; Reeves et al., 1995; Sago et al., 1998, Velazquez et al., 2013), as well as other hippocampal-dependent tasks (Bianchi et al., 2010; Hyde and Crnic, 2001; Hyde et al., 2001; Lockrow et al., 2011).
Importantly, supplementing the maternal diet with additional choline significantly improved performance of the trisomic offspring in the HP task. These results extend our prior findings that MCS improves attention and emotion regulation in Ts65Dn offspring (Moon et al., 2010), functions subserved by BFCN projections to the cortex. A prior study from our lab also demonstrated that MCS normalized hippocampal neurogenesis in Ts65Dn offspring, which correlated with their improved spatial mapping ability (Velazquez et al., 2013). Together, these findings suggest that increased maternal choline intake during pregnancy and lactation results in lasting improvement in cognitive and affective functions in Ts65Dn offspring, perhaps due to the normalization of BFCN projection systems.
MCS did not significantly affect performance of the 2N mice on the spatial learning task. This lack of effect in the 2N mice is likely due to the task not being sufficiently demanding. This inference is based on prior water maze studies, which have shown benefits of MCS for normal rats when the location of the hidden escape platform was changed daily, but not when it remained in the same location across sessions (Tees, 1999; Tees and Mohammadi, 1999), as was the case in the version of the HP task used in the present study. The less demanding reference memory version of the HP task was selected for the present study only after extensive pilot testing demonstrated that the Ts65Dn mice could not solve the more demanding explicit memory version of this task.
MCS protects MS BFCNs in Ts65Dn offspring
We observed differences in BFCN number and density between unsupplemented trisomic and unsupplemented 2N mice, which varied by BFCN subregion and phenotypic marker. In the MS, the densities of ChAT-, p75NTR-, and TrkA-immunoreactive neurons were significantly reduced in the unsupplemented trisomic mice relative to their 2N counterparts. A similar pattern, albeit non-significant, was seen for total number of BFCNs in this region, for all three markers, consistent with previous reports using Ts65Dn mice in the age-range examined here (Contestabile et al., 2006; Cooper et al., 2001; Hunter et al., 2001; Salehi et al., 2006; Seo and Isacson, 2005). In the VDB, there were no genotype differences in the number of ChAT- or p75NTR-immuno-reactive neurons, consistent with earlier studies using 18–19 month old mice (Contestabile et al., 2006; Hunter et al., 2004). In contrast, the number of VDB TrkA-immunoreactive neurons was significantly higher for Ts65Dn mice than 2N mice. In the NBM/SI, the trisomic mice exhibited significantly reduced number and density of p75NTR-immunoreactive neurons relative to 2N mice, but no differences for ChAT- or TrkA-immunoreactive neurons.
MCS normalized select BFCN phenotypes, with the greatest effect on MS neurons. Ts65Dn mice born to supplemented dams had a significantly higher number and density of ChAT-immunoreactive neurons within the MS than their unsupplemented counterparts. Moreover, MCS increased the density of p75NTR-containing neurons in the MS, and the number of ChAT-immunoreactive neurons in the VDB for both genotypes. Although, neither count nor density of these neurons was significantly affected by MCS in the NBM/SI, the patterns across groups were similar. Taken together, these data collectively suggest that MCS has beneficial effects on cholinotrophic BFCN subtypes. The regional variability of MCS effects may be related to differences in BFCN target-derived support related to its survival neurotrophin, NGF, and/or the differences in the specific projection sites of each BFCN subregion. In this regard, the MS and VDB project to the hippocampus, whereas the NBM/SI projects to the neocortex (Rye et al., 1984, Mesulam et al., 1983).
In contrast to the lower number and density of ChAT- and p75NTR-immunoreactive BFCNs for unsupplemented Ts65Dn mice, the size of these neurons did not vary by genotype within any basal forebrain subregion examined. Previous findings indicate atrophy for both ChAT- and p75NTR-immunolabeled BFCNs in the MS and VDB for Ts65Dn mice at younger (6–10 mos) and older (18–22 mos) ages (Lockrow et al., 2011; Salehi et al., 2006; Cooper et al., 2001; Granholm et al., 2000; Holtzman et al., 1996). However, similar to the current findings, no differences between genotypes were reported for BFCN cell size in mice 11 to 14 months of age (Granholm et al., 2002; Seo and Isacson, 2005). These findings suggest atrophy of BFCNs in unsupplemented trisomic mice which is detectable in young and old animals but not during middle-age. These results imply either an increase in cell-size for trisomic mice from young adulthood to middle age, possibly as a neuroplastic compensation for a frank loss in number and density of cholinergic neurons, or alternatively, degenerative neuronal atrophy in 2N mice from young adulthood to middle age.
Supplementing the maternal diet with additional choline significantly increased the size of TrkA-immunoreactive MS neurons in both Ts65Dn and 2N offspring. Similar patterns, albeit non-significant, were seen for the size of ChAT- and p75NTR-immunoreactive neurons in this region. A prior study reported that MCS significantly increased p75NTR-immunoreactive cell size in the MS/VDB at 6 to 7 months of age in normal rats, relative to rats born to dams on a standard choline diet (Williams et al., 1998), but no studies to date have examined the effects of this maternal choline dietary intervention on the size of TrkA-immunoreactive neurons. The increase in TrkA-immunoreactive cell size in the MS suggests upregulation of the NGF cognate cell survival receptor (Bothwell, 1995; Hempstead, 2006). These findings lend further support to the concept that MCS has a complex, heterogeneous effect on intracellular mechanisms and signaling pathways associated with cholinotrophic neuronal activity, which our morphometric data suggests may be region- and age-dependent.
Possible Mechanisms
The current investigation demonstrated that supplementing the maternal diet with additional choline improved spatial cognition in the Ts65Dn offspring and offered some protection to BFCNs, with the greatest effect on those within the MS, which provide the primary cholinergic innervation to the hippocampus (Mesulam et al., 1983). Importantly, errors in the maze task were significantly (inversely) correlated with the density of ChAT- and TrkA-immunoreactive neurons in the MS as well as the number and size of p75NTR- immunoreactive neurons in the MS. The data reported herein suggest that the normalization of MS cholinergic hippocampal projection neurons in Ts65Dn mice due to MCS contributes to their improved spatial cognition. Our group has also shown that MCS partially normalized adult hippocampal neurogenesis in trisomic offspring relative to their unsupplemented counterparts, and that the degree of neurogenesis was a significant predictor of water maze performance (Velazquez et al., 2013). These findings suggest that both of these effects of MCS (increased hippocampal neurogenesis and BFCN neuroprotection) contribute to improved spatial cognition in supplemented Ts65Dn mice.
The protection of BFCNs in Ts65Dn mice may reflect the effects of increased maternal choline intake on neurotrophic factors. For example, normal adult rat offspring of choline-supplemented dams exhibit increased brain levels of NGF and brain-derived neurotrophic factor (BDNF) relative to those born to unsupplemented dams (Glenn et al., 2007; Sandstrom et al., 2002). Based on these data, it is reasonable to posit that MCS enhances target-derived neuroprotection of Ts65Dn BFCNs, which typically begin to atrophy at six months of age due to impaired retrograde transport of NGF (Cooper et al., 2001; Granholm et al., 2000; Holtzman et al., 1996, 1992; Salehi et al., 2006). The resulting increased functionality of these neurons would then contribute to improvement in various cognitive tasks, especially related to BFCN projection systems, notably the hippocampus and frontal cortex.
Although much remains to be learned regarding the specific mechanism(s) by which supplementing the maternal diet with additional choline exerts life-long effects on offspring cognitive functioning, BFCN projection neurons, hippocampal neurogenesis and neurotrophins, all of these effects likely reflect one or both of two broad categories of effects: First, these effects may be due to organizational brain changes, secondary to choline’s role as the precursor to phosphatidyl choline, a major constituent of cellular membranes, and its role as the precursor of acetylcholine, an important ontogenetic signal (Cermak et al., 1999; Meck et al., 1989; Zeisel and Niculescu, 2006). Second, these effects may be related to epigenetic modifications with lasting effects on gene expression, secondary to choline’s role as a methyl donor (Niculescu et al., 2004, 2006; Waterland and Jirtle, 2003; Zeisel, 2009). Choline has a primary role as a methyl donor, through the betaine-methionine pathway, and alterations in dietary levels of choline during early development can produce life-long effects on gene expression through DNA methylation and histone modifications (Blusztajn and Mellot, 2012; Davison et al., 2009).
Increased need for choline during early development
The lifelong beneficial effects of MCS seen in the Ts65Dn offspring in the current study should be interpreted within the context of current choline intake recommendations. Dietary recommendations for choline were first established in 1998 by the Institute of Medicine, based on the level of choline needed to prevent liver dysfunction. However, several converging lines of evidence suggest that these recommendations are insufficient during pregnancy to optimize brain development, cognitive functioning, and lifelong health of the offspring. First, data from both animal models and humans indicate that pregnancy causes a pronounced depletion of maternal choline pools indicating that choline requirements during pregnancy are increased and that the need for this nutrient by the fetus may commonly exceed the amount consumed by the mother (Gwee and Sim, 1979; 1978; Yan et al., 2012; Zeisel et al., 1995). Indeed, a doubling of choline intake by pregnant women does not increase the urinary excretion of choline, a water-soluble biomolecule, indicating that the higher intake level does not exceed metabolic requirements (Yan et al., 2012). Second, and perhaps most importantly, numerous rodent studies indicate that supplementing the maternal diet with additional choline produces lifelong beneficial cognitive effects for the offspring and reduces age-related cognitive decline (e.g., McCann et al., 2006; Meck and Williams, 2003; Meck et al., 2008). Finally, consistent with the present findings, MCS not only improves cognitive functioning in normal offspring but also offers protection against a variety of neural insults including those associated with fetal alcohol syndrome (Thomas et al., 2009), Rett syndrome (Nag and Berger-Sweeney, 2007), epilepsy (Holmes et al., 2002; Wong-Goodrich et al., 2008), and schizophrenia (Stevens et al., 2008), providing additional support for increasing maternal choline intake during pregnancy.
Conclusions
The present behavioral and morphometric findings indicate that increasing maternal choline intake normalizes BFCNs in the Ts65Dn mouse model of DS in a complex, phenotype- and subregion-specific manner. Support for the functional effects of these changes is provided by powerful correlative analyses, which link select BFCN changes to improved performance in a task subserved by the septo-hippocampal system. Although extrapolations to humans with DS must be tempered by the fact that the Ts65Dn mouse does not recapitulate all of the genetic or phenotypic features of DS, the present findings raise the exciting possibility that increasing maternal choline intake during pregnancy may represent a safe and beneficial intervention for expectant mothers carrying a DS fetus, as well as possibly provide BFCN neuroprotection during aging for the population at large.
Supplementary Material
Highlights.
Evaluated maternal choline supplementation (MCS) in a mouse model of Down Syndrome (84)
MCS improved spatial mapping in the Ts65Dn offspring (model of Down syndrome) (79)
MCS normalized number and density of cholinergic neurons in the medial septum (MS) (84)
Spatial cognition correlated with several features of cholinergic neurons in the MS (85)
MCS may improve cognition & prevent neurodegeneration in humans with Down Syndrome (84)
Acknowledgments
Role of the Funding Source
Grant sponsor: National Institute of Child Health and Human Development, grant number HD057564 (to BJS); National Institute on Aging, grant number AG014449 (to EJM); AG043375 (EJM & SDG), and the Alzheimer’s Association, grant number IIRG-12-237253 (to SDG); National institute of Health, grant number HD45224, HD57564 (to BJS, EJM, SDG).
We would like to thank Melissa J. Alldred, Ph.D. for assistance with tissue accrual and brain dis-sections, and Zoe Luscher for her help in running the behavioral task.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arendash GW, et al. Multi-metric behavioral comparison of APPsw and P301L models for Alzheimer’s disease: linkage of poorer cognitive performance to tau pathology in forebrain. Brain Res. 2004;1012:29–41. doi: 10.1016/j.brainres.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Belichenko NP, et al. The “Down syndrome critical region” is sufficient in the mouse model to confer behavioral, neurophysiological, and synaptic phenotypes characteristic of Down syndrome. J Neurosci. 2009;29:5938–48. doi: 10.1523/JNEUROSCI.1547-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belichenko PV, et al. Synaptic and cognitive abnormalities in mouse models of Down syndrome: exploring genotype-phenotype relationships. J Comp Neurol. 2007;504:329–45. doi: 10.1002/cne.21433. [DOI] [PubMed] [Google Scholar]
- Bianchi P, et al. Early pharmacotherapy restores neurogenesis and cognitive performance in the Ts65Dn mouse model for Down syndrome. J Neurosci. 2010;30:8769–79. doi: 10.1523/JNEUROSCI.0534-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer LM, et al. Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem. 1995;64:749–60. doi: 10.1046/j.1471-4159.1995.64020749.x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res. 2003;139:47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Mellott TJ. Choline nutrition programs brain development via DNA and histone methylation. Cent Nerv Syst Agents Med Chem. 2012;12:82–94. doi: 10.2174/187152412800792706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, WL, Whitehouse PJ, Price DL. Abnormalities of the nucleus basalis in Down’s syndrome. Ann Neurol. 1985;18:310–313. doi: 10.1002/ana.410180306. [DOI] [PubMed] [Google Scholar]
- Cermak JM, et al. Prenatal availability of choline alters the development of acetylcholinesterase in the rat hippocampus. Dev Neurosci. 1999;21:94–104. doi: 10.1159/000017371. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Age-related changes in memory and in acetylcholine functions in the hippocampus in the Ts65Dn mouse, a model of Down syndrome. Neurobiol Learn Mem. 2008;89:167–77. doi: 10.1016/j.nlm.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A, et al. Choline acetyltransferase activity at different ages in brain of Ts65Dn mice, an animal model for Down’s syndrome and related neurodegenerative diseases. J Neurochem. 2006;97:515–26. doi: 10.1111/j.1471-4159.2006.03769.x. [DOI] [PubMed] [Google Scholar]
- Cooper JD, et al. Failed retrograde transport of NGF in a mouse model of Down’s syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc Natl Acad Sci U S A. 2001;98:10439–44. doi: 10.1073/pnas.181219298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crnic LS, Pennington B. In: Progress in Infancy Research. Rovee-Collier C, Lipsitt LP, Hayne H, editors. Vol. 1. Lawrence Erlbaum Associates, Inc; Mahwah: 2000. pp. 69–111. [Google Scholar]
- Das I, Reeves R. The use of mouse models to understand and improve cognitive deficits in Down syndrome. Dis Model Mech. 2011;4:596–606. doi: 10.1242/dmm.007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, et al. Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA. 1999;281:1401–6. doi: 10.1001/jama.281.15.1401. [DOI] [PubMed] [Google Scholar]
- Davison JM, Mellott TJ, Kovacheva VP, Blusztajn JK. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J Biol Chem. 2002;284:1982–9. doi: 10.1074/jbc.M807651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–55. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- Detopoulou P, et al. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. 2008;87:424–30. doi: 10.1093/ajcn/87.2.424. [DOI] [PubMed] [Google Scholar]
- Escorihuela RM, et al. A behavioral assessment of Ts65Dn mice: a putative Down syndrome model. Neurosci Lett. 1995;199:143–6. doi: 10.1016/0304-3940(95)12052-6. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Garner CC. Episodic-like memory in Ts65Dn, a mouse model of Down syndrome. Behav Brain Res. 2008;188:233–7. doi: 10.1016/j.bbr.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodale V, et al. The cholinergic system in Down’s syndrome. J Intellect Disabil. 2006;10:261–74. doi: 10.1177/1744629506067615. [DOI] [PubMed] [Google Scholar]
- Fuchs C, et al. Early-occurring proliferation defects in peripheral tissues of the Ts65Dn mouse model of Down syndrome are associated with patched1 over expression. Lab Invest. 2012;92:1648–60. doi: 10.1038/labinvest.2012.117. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, et al. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci. 2007;25:2473–82. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm AC, Sanders LA, Crnic LS. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down’s syndrome. Exp Neurol. 2000;161:647–63. doi: 10.1006/exnr.1999.7289. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, et al. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Gwee MC, Sim MK. Free choline concentration and cephalin-N-methyltransferase activity in the maternal and foetal liver and placenta of pregnant rats. Clin Exp Pharmacol Physiol. 1978;5:649–53. doi: 10.1111/j.1440-1681.1978.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Gwee MC, Sim MK. Changes in the concentration of free choline and cephalin-N-methyltransferase activity of the rat material and fetal liver and placeta during gestation and of the maternal and neonatal liver in the early postpartum period. Clin Exp Pharmacol Physiol. 1979;6:259–65. doi: 10.1111/j.1440-1681.1979.tb01247.x. [DOI] [PubMed] [Google Scholar]
- Hempstead BL. Dissecting the diverse actions of pro- and mature neurotrophins. Curr Alzheimer Res. 2006;3:19–24. doi: 10.2174/156720506775697061. [DOI] [PubMed] [Google Scholar]
- Holmes GL, et al. Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Res. 2002;48:3–13. doi: 10.1016/s0920-1211(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, et al. Mouse model of neurodegeneration: atrophy of basal forebrain cholinergic neurons in trisomy 16 transplants. Proc Natl Acad Sci U S A. 1992;89:1383–7. doi: 10.1073/pnas.89.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, et al. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc Natl Acad Sci U S A. 1996;93:13333–8. doi: 10.1073/pnas.93.23.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MD, Gottschall PE. Altered synaptic marker abundance in the hippocampal stratum oriens of Ts65Dn mice is associated with exuberant expression of versican. ASN Neuro. 2012;4 doi: 10.1042/AN20110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CL, Bimonte HA, Granholm AC. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav Brain Res. 2003;138:121–31. doi: 10.1016/s0166-4328(02)00275-9. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Crnic LS. Age-related deficits in context discrimination learning in Ts65Dn mice that model Down syndrome and Alzheimer’s disease. Behav Neurosci. 2001;115:1239–46. [PubMed] [Google Scholar]
- Hyde LA, Frisone DF, Crnic LS. Ts65Dn mice, a model for Down syndrome, have deficits in context discrimination learning suggesting impaired hippocampal function. Behav Brain Res. 2001;118:53–60. doi: 10.1016/s0166-4328(00)00313-2. [DOI] [PubMed] [Google Scholar]
- Iivonen H, et al. Hypothermia in mice tested in Morris water maze. Behav Brain Res. 2003;141:207–13. doi: 10.1016/s0166-4328(02)00369-8. [DOI] [PubMed] [Google Scholar]
- Isacson O, et al. Alzheimer’s disease and Down’s syndrome: roles of APP, trophic factors and ACh. Trends Neurosci. 2002;25:79–84. doi: 10.1016/s0166-2236(02)02037-4. [DOI] [PubMed] [Google Scholar]
- Keeler C. Retinal degeneration in the mouse is rodless retina. J Hered. 1966;57:47–50. doi: 10.1093/oxfordjournals.jhered.a107462. [DOI] [PubMed] [Google Scholar]
- Kelley CM, et al. Maternal choline supplementation differentially alters the basal forebrain cholinergic system of young-adult Ts65Dn and disomic mice. J Comp Neurol. 2014a;522:1390–410. doi: 10.1002/cne.23492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley CM, et al. Sex differences in the cholinergic basal forebrain in the Ts65Dn mouse model of Down syndrome and Alzheimer’s disease. Brain Pathol. 2014b;24:33–44. doi: 10.1111/bpa.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockrow J, et al. Effects of long-term memantine on memory and neuropathology in Ts65Dn mice, a model for Down syndrome. Behav Brain Res. 2011;221:610–22. doi: 10.1016/j.bbr.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, Yates PO, Marcyniuk B. Changes in nerve cells of the nucleus basalis of Meynert in Alzheimer’s disease and their relationship to ageing and to the accumulation of lipofuscin pigment. Mech Ageing Dev. 1984;25:189–204. doi: 10.1016/0047-6374(84)90140-4. [DOI] [PubMed] [Google Scholar]
- Mann DM, et al. The topography of plaques and tangles in Down’s syndrome patients of different ages. Neuropathol Appl Neurobiol. 1986;12:447–57. doi: 10.1111/j.1365-2990.1986.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Mann DM. Alzheimer’s disease and Down’s syndrome. Histopathology. 1988;13:125–37. doi: 10.1111/j.1365-2559.1988.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Mann DM, Esiri MM. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down’s syndrome. J Neurol Sci. 1989;89:169–79. doi: 10.1016/0022-510x(89)90019-1. [DOI] [PubMed] [Google Scholar]
- McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci Biobehav Rev. 2006;30:696–712. doi: 10.1016/j.neubiorev.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci. 1989;103:1234–41. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Res Dev Brain Res. 1999;118:51–9. doi: 10.1016/s0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–99. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Meck WH, et al. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci. 2007;1:7. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci. 2008 doi: 10.3389/neuro.07.007.2007. eCollection 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AL. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:185–201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Moon J, et al. Perinatal choline supplementation improves cognitive functioning and emotion regulation in the Ts65Dn mouse model of Down syndrome. Behav Neurosci. 2010;124:346–61. doi: 10.1037/a0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Ma SY, Cochran EJ, Bennett DA, Beckett LA, Jaffar S, Saragovi HU, Kordower Loss of nucleus basalis neurons containing trkA immunoreactivity in individuals with mild cognitive impairment and early Alzheimer’s disease. J Comp Neurol. 2000;427:19–30. doi: 10.1002/1096-9861(20001106)427:1<19::aid-cne2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, et al. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 2003;26:233–42. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Nag N, Berger-Sweeney JE. Postnatal dietary choline supplementation alters behavior in a mouse model of Rett syndrome. Neurobio Dis. 2007;26:473–480. doi: 10.1016/j.nbd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J Neurochem. 2004;89:1252–1259. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline de ciency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LE, et al. A chromosome 21 critical region does not cause specific Down syndrome phenotypes. Science. 2004;306:687–90. doi: 10.1126/science.1098992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LE, et al. Trisomy for the Down syndrome ‘critical region’ is necessary but not sufficient for brain phenotypes of trisomic mice. Hum Mol Genet. 2007;16:774–82. doi: 10.1093/hmg/ddm022. [DOI] [PubMed] [Google Scholar]
- Reeves RH, et al. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–84. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- Roper RJ, John STJK, Phillip J, Lawler A, Reeves RH. Perinatal loss of Ts65Dn Down syndrome mice. Genetics. 2006;172:437–43. doi: 10.1534/genetics.105.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda N, Florez J, Martinez-Cue C. Mouse models of Down syndrome as a tool to unravel the causes of mental disabilities. Neural Plast. 2012;2012:584071. doi: 10.1155/2012/584071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye DB, et al. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13:627–43. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Sago H, et al. Genetic dissection of region associated with behavioral abnormalities in mouse models for Down syndrome. Pediatr Res. 2000;48:606–13. doi: 10.1203/00006450-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Loy R, Williams CL. Prenatal choline supplementation increases NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Res. 2002;7:9–16. doi: 10.1016/s0006-8993(02)02900-1. [DOI] [PubMed] [Google Scholar]
- Salehi A, et al. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Sendera TJ, et al. Reduction in TrkA-immunoreactive neurons is not associated with an overexpression of galaninergic fibers within the nucleus basalis in Down’s syndrome. J Neurochem. 2000;74:1185–96. doi: 10.1046/j.1471-4159.2000.741185.x. [DOI] [PubMed] [Google Scholar]
- Seo H, Isacson O. Abnormal APP, cholinergic and cognitive function in Ts65Dn Down’s model mice. Exp Neurol. 2005;193:469–80. doi: 10.1016/j.expneurol.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Stasko MR, Costa AC. Experimental parameters affecting the Morris water maze performance of a mouse model of Down syndrome. Behav Brain Res. 2004;154:1–17. doi: 10.1016/j.bbr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Stasko MR, Scott-McKean JJ, Costa AC. Hypothermic responses to 8-OH-DPAT in the Ts65Dn mouse model of Down syndrome. Neuroreport. 2006;17:837–41. doi: 10.1097/01.wnr.0000220129.78726.bb. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Adams CE, Yonchek J, Hickel C, Danielson J, Kisley MA. Permanent improvement in deficient sensory inhibition in DBA/2 mice with increased perinatal choline. Psychopharmacology (Berl) 2008;198:413–20. doi: 10.1007/s00213-008-1170-3. [DOI] [PubMed] [Google Scholar]
- Sturgeon X, Gardiner KJ. Transcript catalogs of human chromosome 21 and orthologous chimpanzee and mouse regions. Mamm Genome. 2011;22:261–71. doi: 10.1007/s00335-011-9321-y. [DOI] [PubMed] [Google Scholar]
- Tees RC. The influences of rearing environment and neonatal choline dietary supplementation on spatial learning and memory in adult rats. Behav Brain Res. 1999;105:173–88. doi: 10.1016/s0166-4328(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Tees RC, Mohammadi E. The effects of neonatal choline dietary supplementation on adult spatial and configural learning and memory in rats. Dev Psychobio. 1999;35:226–40. [PubMed] [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol. 2009;31:303–11. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez R, et al. Maternal choline supplementation improves spatial learning and adult hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiol Dis. 2013;58:92–101. doi: 10.1016/j.nbd.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse PJ, et al. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–9. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Williams CL, et al. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998;794:225–38. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, et al. Alzheimer’s disease in Down’s syndrome: clinicopathologic studies. Neurology. 1985a;35:957–61. doi: 10.1212/wnl.35.7.957. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985b;17:278–82. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Wolfinger RDOCM. Generalized Linear Mixed Models: a Pseudo-likelihood Approach. Journal of Statistical Computation and Simulation. 1993;48 [Google Scholar]
- Wong-Goodrich SJ, et al. Spatial memory and hippocampal plasticity are differentially sensitive to the availability of choline in adulthood as a function of choline supply in utero. Brain Res. 2008;1237:153–66. doi: 10.1016/j.brainres.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr. 2012;95:1060–71. doi: 10.3945/ajcn.111.022772. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, et al. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr. 1995;125:3049–54. doi: 10.1093/jn/125.12.3049. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Niculescu MD. Perinatal choline influences brain structure and function. Nutr Rev. 2006;64:197–203. doi: 10.1111/j.1753-4887.2006.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Epigenetic mechanisms for nutrition determinants of later health outcomes. J Am Coll Nutr. 2009;89:1488S–1493S. doi: 10.3945/ajcn.2009.27113B. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.