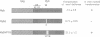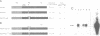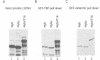Abstract
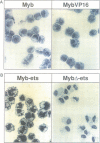
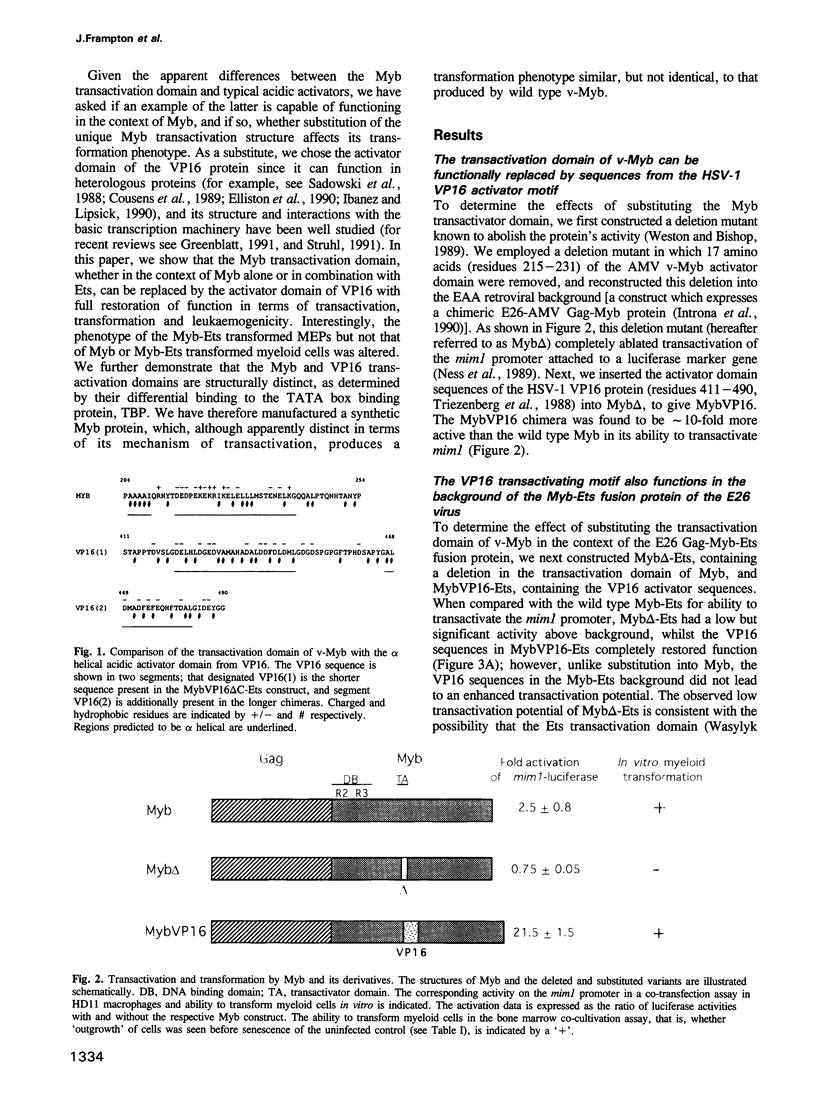
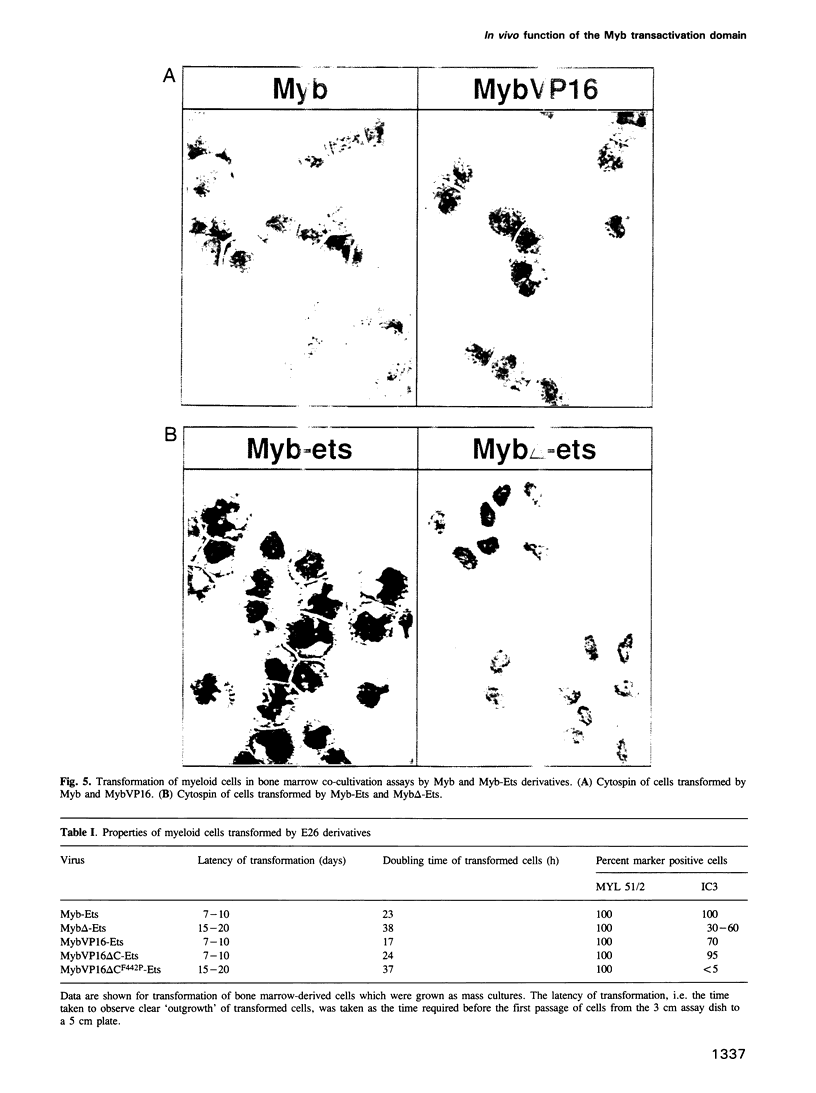
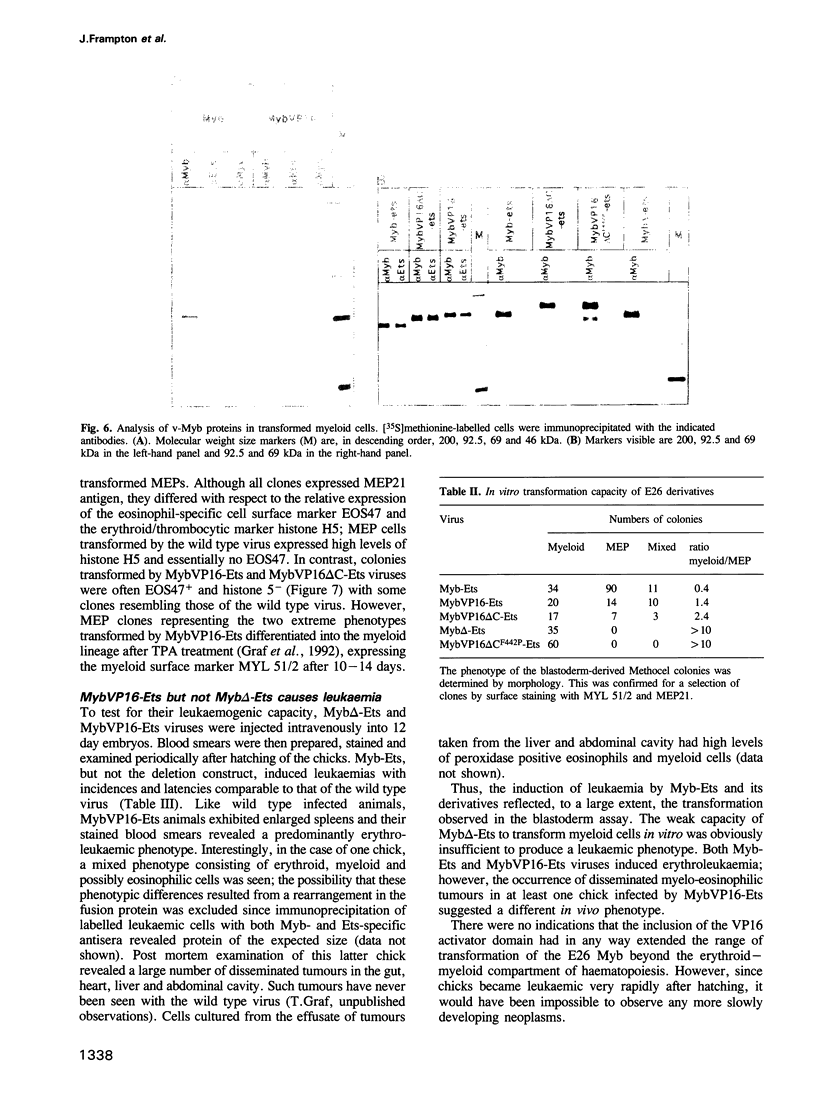
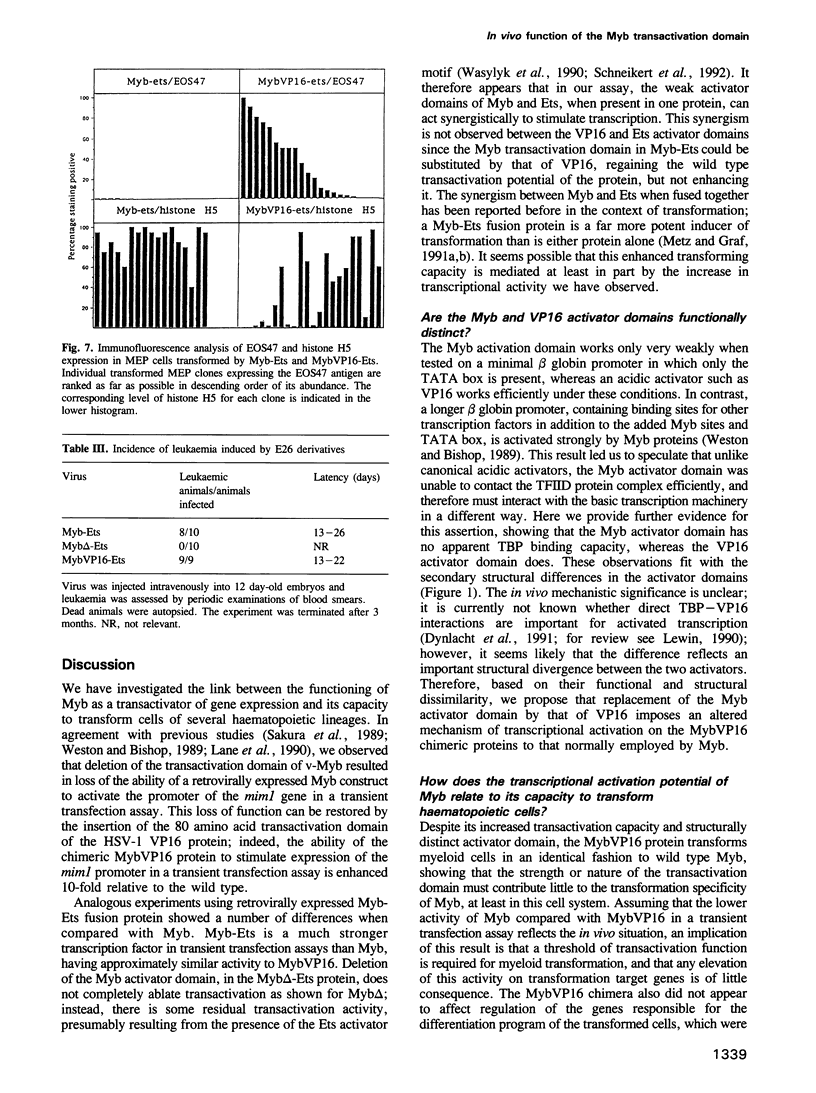
The v-myb-containing viruses AMV and E26 induce the proliferation of myelomonocytic cells. The E26 Myb protein, by virtue of its fusion to Ets, is also able to transform multipotent haematopoietic cells (MEPs). We have examined the biological effects of substituting the v-Myb transactivation domain with the strong acidic activator domain from the C-terminus of the HSV-1 VP16 protein. In the absence of Ets, deletion of the transactivation domain destroyed the ability of v-Myb to stimulate transcription and to transform cells, whilst the substitution of the VP16 transactivation domain into v-Myb resulted in a greatly enhanced transactivation potential and altered TATA box binding protein (TBP) binding properties. In spite of these functional differences, the v-Myb VP16 protein regained the ability to transform myeloid cells with the same characteristics as wild type v-Myb. A construct encoding v-Myb VP16 fused to v-Ets was still capable of inducing leukaemia and of transforming both myeloid cells and MEPs in vitro, although the latter cells exhibited an altered phenotype. Our results demonstrate that the transformation of myeloid cells by v-Myb is largely independent of the type and potency of the transactivation domain it contains, whereas transformation of MEPs by the Myb-Ets fusion protein has more stringent transactivation requirements of Myb.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., Doederlein G., Freudenstein C., Graf T. Erythroblast cell lines transformed by a temperature-sensitive mutant of avian erythroblastosis virus: a model system to study erythroid differentiation in vitro. J Cell Physiol Suppl. 1982;1:195–207. doi: 10.1002/jcp.1041130427. [DOI] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Biedenkapp H., Borgmeyer U., Sippel A. E., Klempnauer K. H. Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature. 1988 Oct 27;335(6193):835–837. doi: 10.1038/335835a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cousens D. J., Greaves R., Goding C. R., O'Hare P. The C-terminal 79 amino acids of the herpes simplex virus regulatory protein, Vmw65, efficiently activate transcription in yeast and mammalian cells in chimeric DNA-binding proteins. EMBO J. 1989 Aug;8(8):2337–2342. doi: 10.1002/j.1460-2075.1989.tb08361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress W. D., Triezenberg S. J. Critical structural elements of the VP16 transcriptional activation domain. Science. 1991 Jan 4;251(4989):87–90. doi: 10.1126/science.1846049. [DOI] [PubMed] [Google Scholar]
- Domenget C., Leprince D., Pain B., Peyrol S., Li R. P., Stehelin D., Samarut J., Jurdic P. The various domains of v-myb and v-ets oncogenes of E26 retrovirus contribute differently, but cooperatively, in transformation of hematopoietic lineages. Oncogene. 1992 Nov;7(11):2231–2241. [PubMed] [Google Scholar]
- Dynlacht B. D., Hoey T., Tjian R. Isolation of coactivators associated with the TATA-binding protein that mediate transcriptional activation. Cell. 1991 Aug 9;66(3):563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- Elliston J. F., Tsai S. Y., O'Malley B. W., Tsai M. J. Superactive estrogen receptors. Potent activators of gene expression. J Biol Chem. 1990 Jul 15;265(20):11517–11521. [PubMed] [Google Scholar]
- Frampton J., Gibson T. J., Ness S. A., Döderlein G., Graf T. Proposed structure for the DNA-binding domain of the Myb oncoprotein based on model building and mutational analysis. Protein Eng. 1991 Dec;4(8):891–901. doi: 10.1093/protein/4.8.891. [DOI] [PubMed] [Google Scholar]
- Frampton J., Leutz A., Gibson T., Graf T. DNA-binding domain ancestry. Nature. 1989 Nov 9;342(6246):134–134. doi: 10.1038/342134a0. [DOI] [PubMed] [Google Scholar]
- Frykberg L., Metz T., Brady G., Introna M., Beug H., Vennström B., Graf T. A point mutation in the DNA binding domain of the v-myb oncogene of E26 virus confers temperature sensitivity for transformation of myelomonocytic cells. Oncogene Res. 1988;3(4):313–322. [PubMed] [Google Scholar]
- Ghysdael J., Gegonne A., Pognonec P., Dernis D., Leprince D., Stehelin D. Identification and preferential expression in thymic and bursal lymphocytes of a c-ets oncogene-encoded Mr 54,000 cytoplasmic protein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1714–1718. doi: 10.1073/pnas.83.6.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Buckmaster C., Ramsay R. G. Activation of c-myb by carboxy-terminal truncation: relationship to transformation of murine haemopoietic cells in vitro. EMBO J. 1989 Jun;8(6):1777–1783. doi: 10.1002/j.1460-2075.1989.tb03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., McNagny K., Brady G., Frampton J. Chicken "erythroid" cells transformed by the Gag-Myb-Ets-encoding E26 leukemia virus are multipotent. Cell. 1992 Jul 24;70(2):201–213. doi: 10.1016/0092-8674(92)90096-u. [DOI] [PubMed] [Google Scholar]
- Graf T. Myb: a transcriptional activator linking proliferation and differentiation in hematopoietic cells. Curr Opin Genet Dev. 1992 Apr;2(2):249–255. doi: 10.1016/s0959-437x(05)80281-3. [DOI] [PubMed] [Google Scholar]
- Graf T., von Kirchbach A., Beug H. Characterization of the hematopoietic target cells of AEV, MC29 and AMV avian leukemia viruses. Exp Cell Res. 1981 Feb;131(2):331–343. doi: 10.1016/0014-4827(81)90236-6. [DOI] [PubMed] [Google Scholar]
- Greenblatt J. Roles of TFIID in transcriptional initiation by RNA polymerase II. Cell. 1991 Sep 20;66(6):1067–1070. doi: 10.1016/0092-8674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Grässer F. A., Graf T., Lipsick J. S. Protein truncation is required for the activation of the c-myb proto-oncogene. Mol Cell Biol. 1991 Aug;11(8):3987–3996. doi: 10.1128/mcb.11.8.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeier C., Walker S., Caswell R., Kouzarides T., Sinclair J. The human cytomegalovirus 80-kilodalton but not the 72-kilodalton immediate-early protein transactivates heterologous promoters in a TATA box-dependent mechanism and interacts directly with TFIID. J Virol. 1992 Jul;66(7):4452–4456. doi: 10.1128/jvi.66.7.4452-4456.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. L., Ramsay R. G., Kanei-Ishii C., Ishii S., Gonda T. J. Transformation by carboxyl-deleted Myb reflects increased transactivating capacity and disruption of a negative regulatory domain. Oncogene. 1991 Sep;6(9):1549–1553. [PubMed] [Google Scholar]
- Ibanez C. E., Lipsick J. S. Structural and functional domains of the myb oncogene: requirements for nuclear transport, myeloid transformation, and colony formation. J Virol. 1988 Jun;62(6):1981–1988. doi: 10.1128/jvi.62.6.1981-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez C. E., Lipsick J. S. trans activation of gene expression by v-myb. Mol Cell Biol. 1990 May;10(5):2285–2293. doi: 10.1128/mcb.10.5.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles C. J., Shales M., Cress W. D., Triezenberg S. J., Greenblatt J. Reduced binding of TFIID to transcriptionally compromised mutants of VP16. Nature. 1991 Jun 13;351(6327):588–590. doi: 10.1038/351588a0. [DOI] [PubMed] [Google Scholar]
- Introna M., Golay J., Frampton J., Nakano T., Ness S. A., Graf T. Mutations in v-myb alter the differentiation of myelomonocytic cells transformed by the oncogene. Cell. 1990 Dec 21;63(6):1289–1297. doi: 10.1016/0092-8674(90)90424-d. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Klempnauer K. H., Arnold H., Biedenkapp H. Activation of transcription by v-myb: evidence for two different mechanisms. Genes Dev. 1989 Oct;3(10):1582–1589. doi: 10.1101/gad.3.10.1582. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Beug H., Doederlein G., Graf T. Detection of avian hematopoietic cell surface antigens with monoclonal antibodies to myeloid cells. Their distribution on normal and leukemic cells of various lineages. Exp Cell Res. 1983 Feb;143(2):383–394. doi: 10.1016/0014-4827(83)90065-4. [DOI] [PubMed] [Google Scholar]
- Lane T., Ibanez C., Garcia A., Graf T., Lipsick J. Transformation by v-myb correlates with trans-activation of gene expression. Mol Cell Biol. 1990 Jun;10(6):2591–2598. doi: 10.1128/mcb.10.6.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leprince D., Gegonne A., Coll J., de Taisne C., Schneeberger A., Lagrou C., Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983 Nov 24;306(5941):395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Leutz A., Beug H., Graf T. Purification and characterization of cMGF, a novel chicken myelomonocytic growth factor. EMBO J. 1984 Dec 20;3(13):3191–3197. doi: 10.1002/j.1460-2075.1984.tb02278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Commitment and activation at pol II promoters: a tail of protein-protein interactions. Cell. 1990 Jun 29;61(7):1161–1164. doi: 10.1016/0092-8674(90)90675-5. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Ha I., Maldonado E., Reinberg D., Green M. R. Binding of general transcription factor TFIIB to an acidic activating region. Nature. 1991 Oct 10;353(6344):569–571. doi: 10.1038/353569a0. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part II. Myb. Genes Dev. 1990 Dec;4(12B):2235–2241. doi: 10.1101/gad.4.12b.2235. [DOI] [PubMed] [Google Scholar]
- McNagny K. M., Lim F., Grieser S., Graf T. Cell surface proteins of chicken hematopoietic progenitors, thrombocytes and eosinophils detected by novel monoclonal antibodies. Leukemia. 1992 Oct;6(10):975–984. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz T., Graf T. Fusion of the nuclear oncoproteins v-Myb and v-Ets is required for the leukemogenicity of E26 virus. Cell. 1991 Jul 12;66(1):95–105. doi: 10.1016/0092-8674(91)90142-l. [DOI] [PubMed] [Google Scholar]
- Metz T., Graf T. v-myb and v-ets transform chicken erythroid cells and cooperate both in trans and in cis to induce distinct differentiation phenotypes. Genes Dev. 1991 Mar;5(3):369–380. doi: 10.1101/gad.5.3.369. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Moscovici M. G., Jurdic P., Samarut J., Gazzolo L., Mura C. V., Moscovici C. Characterization of the hemopoietic target cells for the avian leukemia virus E26. Virology. 1983 Aug;129(1):65–78. doi: 10.1016/0042-6822(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Mándi Y., Veromaa T., Baranji K., Miczák A., Béládi I., Toivanen P. Granulocyte-specific monoclonal antibody inhibiting cytotoxicity reactions in the chicken. Immunobiology. 1987 May;174(3):292–299. doi: 10.1016/s0171-2985(87)80004-9. [DOI] [PubMed] [Google Scholar]
- Ness S. A., Beug H., Graf T. v-myb dominance over v-myc in doubly transformed chick myelomonocytic cells. Cell. 1987 Oct 9;51(1):41–50. doi: 10.1016/0092-8674(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Ness S. A., Marknell A., Graf T. The v-myb oncogene product binds to and activates the promyelocyte-specific mim-1 gene. Cell. 1989 Dec 22;59(6):1115–1125. doi: 10.1016/0092-8674(89)90767-8. [DOI] [PubMed] [Google Scholar]
- Nunn M. F., Seeburg P. H., Moscovici C., Duesberg P. H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983 Nov 24;306(5941):391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Harosi F. I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975 Jan;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., McKnight J. L., Jenkins F. J., Roizman B. Nucleotide sequence and predicted amino acid sequence of a protein encoded in a small herpes simplex virus DNA fragment capable of trans-inducing alpha genes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5870–5874. doi: 10.1073/pnas.82.17.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Beug H., Kornfeld S., Graf T. Transformation of both erythroid and myeloid cells by E26, an avian leukemia virus that contains the myb gene. Cell. 1982 Dec;31(3 Pt 2):643–653. doi: 10.1016/0092-8674(82)90320-8. [DOI] [PubMed] [Google Scholar]
- Ramsay R. G., Ishii S., Gonda T. J. Increase in specific DNA binding by carboxyl truncation suggests a mechanism for activation of Myb. Oncogene. 1991 Oct;6(10):1875–1879. [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988 Oct 6;335(6190):563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Sakura H., Kanei-Ishii C., Nagase T., Nakagoshi H., Gonda T. J., Ishii S. Delineation of three functional domains of the transcriptional activator encoded by the c-myb protooncogene. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5758–5762. doi: 10.1073/pnas.86.15.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneikert J., Lutz Y., Wasylyk B. Two independent activation domains in c-Ets-1 and c-Ets-2 located in non-conserved sequences of the ets gene family. Oncogene. 1992 Feb;7(2):249–256. [PubMed] [Google Scholar]
- Stringer K. F., Ingles C. J., Greenblatt J. Direct and selective binding of an acidic transcriptional activation domain to the TATA-box factor TFIID. Nature. 1990 Jun 28;345(6278):783–786. doi: 10.1038/345783a0. [DOI] [PubMed] [Google Scholar]
- Strum K. Acid connections. Curr Biol. 1991 Jun;1(3):188–191. doi: 10.1016/0960-9822(91)90230-t. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Lai J. S., Herr W. Promoter-selective activation domains in Oct-1 and Oct-2 direct differential activation of an snRNA and mRNA promoter. Cell. 1992 Feb 21;68(4):755–767. doi: 10.1016/0092-8674(92)90150-b. [DOI] [PubMed] [Google Scholar]
- Triezenberg S. J., Kingsbury R. C., McKnight S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988 Jun;2(6):718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Flores P., Begue A., Leprince D., Stehelin D. The c-ets proto-oncogenes encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature. 1990 Jul 12;346(6280):191–193. doi: 10.1038/346191a0. [DOI] [PubMed] [Google Scholar]
- Weston K., Bishop J. M. Transcriptional activation by the v-myb oncogene and its cellular progenitor, c-myb. Cell. 1989 Jul 14;58(1):85–93. doi: 10.1016/0092-8674(89)90405-4. [DOI] [PubMed] [Google Scholar]
- Weston K. Extension of the DNA binding consensus of the chicken c-Myb and v-Myb proteins. Nucleic Acids Res. 1992 Jun 25;20(12):3043–3049. doi: 10.1093/nar/20.12.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]