Abstract
In this issue of Blood, Hervier et al has identified that cooccurrence of Langerhans cell histiocytosis (LCH) and Erdheim-Chester disease (ECD) in the same patient is not a rare event.1 Mixed histiocytosis (MH) highlights existence of a link between distinct groups of histiocytic disorders and suggests presence of a common progenitor cell. Today, histiocytic disorders are classified into 3 groups based on clinical presentation and gross anatomic findings.
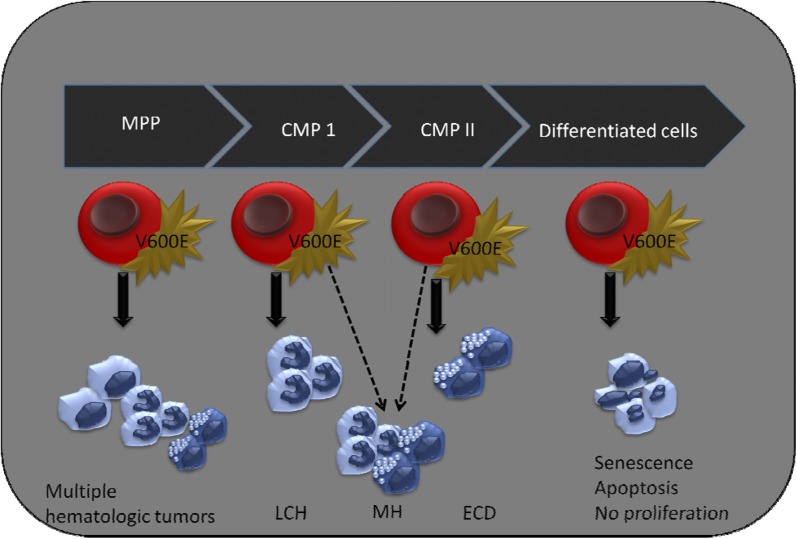
Cell of origin in histiocytic disorders. Expression of BRAFV600E in terminally differentiated myeloid cells does not lead to cellular proliferation, whereas early expression of mutant BRAF (in MPP) gives rise to multiple hematologic tumors (Badalian-Very, unpublished data). Somatic mutation of BRAFV600E in LCH and ECD is an early event that occurs in bone marrow progenitor cells. The cell of origin of both LCH and ECD is a matter of debate, but progenitor cells that give rise both to dendritic cells and macrophages is a strong candidate. Concomitant presence of LCH and ECD, better known as MH, also points to a biological link between these so-called distinct histiocytic disorders and suggest a common precursor cell for these diseases. Further studies are required to determine the additional factors that influence clinical presentation and disease outcome of mixed histiocytic disorders. CMP, common myeloid progenitor; MPP, multipotent progenitor.
Until recently, little attention was paid to the underlying genetic structure of this heterogeneous group of disorders, and no efforts were directed in reevaluating disease classification based on molecular/genetic background and disease pathomechanism. The momentum for reclassification of histiocytic disorders was already initiated with the discovery of somatic BRAFV600E mutation in LCH and ECD.2,3 This discovery was suggestive of the neoplastic nature of these disorders, but additional studies were required to prove that BRAFV600E is not merely associated with LCH and ECD as a passenger mutation. It has been demonstrated that BRAFV600E is indeed a driver mutation in both diseases, and blocking BRAF kinase could dramatically improve the disease outcome in patients harboring this mutation.4,5 To date, clinical significance of BRAFV600E mutation is limited to therapy of patients harboring this activating mutation. Little is known about the diagnostic and prognostic value of BRAFV600E.
Currently, 2 fundamental questions exist in the field: (1) cell of origin and (2) clinical and biological significance of BRAFV600E mutation in histiocytic disorders.
Despite the existing comments in the literature, pointing to near-mature cells as a cell of origin both for LCH and ECD, there were numerous facts suggesting that these disorders are initiated in bone marrow progenitor cells. First, it is of note that LCH and ECD primarily affect bones in 70% and 90% of cases, respectively. It is rather uncommon to have such a tropism in dissemination of any neoplasm that overwhelmingly would dominate the primary site of origin. Second, pathologic cells in both diseases suffer maturation arrest, suggesting that a progenitor cell should be primarily affected.6,7 Third, the concomitant occurrence of histiocytic disorders that was sporadically reported in the literature were pointing to an early abnormal event in myeloid linage. Although the presence of MH was documented in the literature, the orphan nature of these diseases had created serious complications in conducting comprehensive and conclusive studies. Hervier et al interrogated mixed disorders and discovered that once BRAFV600E mutation is present in MH, it occurs in both LCH and ECD. This observation is not only suggestive of a common progenitor cell for these diseases, but also suggestive of the fact that BRAFV600E mutation is an early event. A CMP cell that gives rise to dendritic cells and monocyte/macrophages would be a reasonable candidate, and it would be worthwhile to conduct future studies and determine the effect of somatic BRAFV600E mutations on development and differentiation of this cell8 (see figure).
Mixed histiocytic disorders uncover the prognostic value of BRAFV600E. The current article has interrogated the existing cases of MH both in the literature and in the French national database and it clearly demonstrates that the occurrence of MH is more frequent than what is generally accepted. These data suggest that one-fifth of ECD cases were previously diagnosed as LCH. It is of note that 69% of the patients with MH harbored the BRAFV600E mutation in comparison with 57% and 54% in isolated LCH and ECD, respectively. This is of significance because the presence of BRAFV600E in pathologic cells is predictive of developing secondary histiocytic disorders. It must be emphasized that there was no correlation between the presence of BRAFV600E mutation and disease extent in LCH or ECD despite the direct correlation between BRAFV600E and cooccurrence of these diseases in the same patients. Therefore, in the presence of BRAFV600E, clinical follow-up should be undertaken more vigorously because a reasonable proportion of patients harboring the mutation are destined to develop additional histiocytic disorders. Consequently, appropriate biomarkers for disease follow-up are urgently needed.
Mixed histiocytic disorders impose additional challenges in clinics because the response rates to traditional therapeutic regimens in these disorders are significantly lower compared with isolated disease. Also, there is no treatment of concomitant LCH and ECD, and mortality rate in these patients is relatively high. Because of the high incidence of BRAFV600E in MH, tyrosine kinase inhibitors (vemurafenib) could be used as the gold-standard treatment. Furthermore, pan MEK inhibitors could be beneficial for patients demonstrating limited response to RAF inhibitors. Further studies are necessary to fully characterize mixed histiocytic disorders and determine the additional factors that could play a role in initiation and progression of these disorders. Eventually, multicenter studies are necessary to evaluate the efficacy of vemurafenib on overall survival of patients with MH.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Hervier B, Haroche J, Arnaud L, et al. Association of both Langerhans cell histiocytosis and Erdheim-Chester disease linked to the BRAFV600E mutation. Blood. 2014;124(7):1119–1126. doi: 10.1182/blood-2013-12-543793. [DOI] [PubMed] [Google Scholar]
- 2.Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116(11):1919–1923. doi: 10.1182/blood-2010-04-279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700–2703. doi: 10.1182/blood-2012-05-430140. [DOI] [PubMed] [Google Scholar]
- 4.Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121(9):1495–1500. doi: 10.1182/blood-2012-07-446286. [DOI] [PubMed] [Google Scholar]
- 5.Berres ML, Lim KP, Peters T, et al. BRAF-V600E expression in precursor versus differentiated dendritic cells defines clinically distinct LCH risk groups. J Exp Med. 2014;211(4):669–683. doi: 10.1084/jem.20130977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badalian-Very G, Vergilio JA, Fleming M, Rollins BJ. Pathogenesis of Langerhans cell histiocytosis. Annu Rev Pathol. 2013;8:1–20. doi: 10.1146/annurev-pathol-020712-163959. [DOI] [PubMed] [Google Scholar]
- 7.Allen CE, Li L, Peters TL, et al. Cell specific gene expression in Langerhans cell histiocytosis reveals a distinct profile compared to epithermal Langerhans cells. J Immunol. 2010;184(8):4557–4567. doi: 10.4049/jimmunol.0902336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]