Abstract
Consistent with reports of cerebellar structural, functional, and neurochemical anomalies in schizophrenia, robust cerebellar-dependent delay eyeblink conditioning (dEBC) deficits have been observed in the disorder. Impaired dEBC is also present in schizotypal personality disorder, an intermediate phenotype of schizophrenia. The present work sought to determine whether dEBC deficits exist in nonpsychotic first-degree relatives of individuals with schizophrenia. A single-cue tone dEBC paradigm consisting of 10 blocks with 10 trials each (9 paired and 1 unpaired trials) was used to examine the functional integrity of cerebellar circuitry in schizophrenia participants, individuals with a first-degree relative diagnosed with schizophrenia, and healthy controls with no first-degree relatives diagnosed with schizophrenia. The conditioned stimulus (a 400ms tone) coterminated with the unconditioned stimulus (a 50ms air puff to the left eye) on paired trials. One relative and 2 healthy controls were removed from further analysis due to declining conditioned response rates, leaving 18 schizophrenia participants, 17 first-degree relatives, and 16 healthy controls. Electromyographic data were subsequently analyzed using growth curve models in hierarchical linear regression. Acquisition of dEBC conditioned responses was significantly impaired in schizophrenia and first-degree relative groups compared with controls. This finding that cerebellar-mediated associative learning deficits are present in first-degree relatives of individuals with schizophrenia provides evidence that dEBC abnormalities in schizophrenia may not be due to medication or course of illness effects. Instead, the present results are consistent with models of schizophrenia positing cerebellar-cortical circuit abnormalities and suggest that cerebellar abnormalities represent a risk marker for the disorder.
Key words: schizophrenia, eyeblink conditioning, cerebellum, relatives, associative learning, reflex conditioning, conditioned response, cognition, psychosis
Introduction
Motor abnormalities have long been observed in schizophrenia, even from its earliest conceptualization.1 The cerebellum has historically been identified as an integral structure for coordinated movement and motor learning, and accumulating evidence points to an important role in nonmotor psychological processes as well—including cognition.2–5 Motor dysmetria is commonly observed subsequent to cerebellar lesions. However, consistent with evidence of cerebellar contributions to nonmotor processes, cerebellar lesions can also result in cognitive and behavioral symptoms, including impaired visuospatial memory, blunted affect or disinhibited, contextually inappropriate behavior, impaired executive function, and inattention.6,7 These symptoms are remarkably similar to those observed in schizophrenia, contributing to theoretical evidence that the cerebellum may play a role in the disorder.
Empirical evidence of cerebellar dysfunction in schizophrenia has been revealed through postmortem and neuroimaging studies, which report reduced cerebellar volume in chronic,8–11 neuroleptic-naïve,12 adolescent,13 first-episode,14–16 and childhood-onset17 schizophrenia (but for exceptions see Cahn and colleagues18 and Levitt and colleagues19). Postmortem studies report reduced size and density of Purkinje cells in schizophrenia.20–22 Functional neuroimaging studies have found abnormal cerebellar blood flow at rest8,23,24 and during cognitive tasks25–27 in schizophrenia patients. Finally, cerebellar abnormalities are associated with clinical symptoms, cognitive deficits, and outcome measures in schizophrenia.10,28–30 For example, deficits in working memory and mental flexibility correlate with cerebellar volume,31 and fronto-cerebellar metabolic abnormalities are associated with anhedonia and ambivalence.32 Moreover, increased connectivity between frontal-parietal and cerebellar regions predicts better cognitive performance in controls and schizophrenia, and schizophrenia patients with improved connectivity have fewer disorganization symptoms.33
Our recent studies also indicate performance deficits in schizophrenia on a number of tasks linked to cerebellar function,34–36 most notably delay eyeblink conditioning (dEBC).37–40 Importantly, we have found significant dEBC associative learning deficits in schizotypal personality disorder.40 This finding is consistent with reports of cerebellar white and gray matter abnormalities in individuals at high genetic risk for schizophrenia.41 First-degree relatives of individuals with schizophrenia have also been found to have structural and functional cerebellar abnormalities. For example, the developmental trajectory of the cerebellum is altered in relatives of individuals diagnosed with schizophrenia.42 Functional connectivity of cerebellum to areas including hippocampus, inferior frontal gyrus, and insula are also significantly reduced in schizophrenia and sibling relatives compared with controls.43 Taken together, the foregoing evidence suggests that cerebellar abnormalities may serve as risk markers for schizophrenia.
dEBC provides a well-validated method to investigate the function of the cerebellum and related structures. In dEBC, a conditioned stimulus (ie, a tone) becomes associated with an unconditioned stimulus (ie, an air puff) after repeated paired presentations. Subjects demonstrate learning when an eyeblink (the conditioned response) occurs prior to the onset of the unconditioned stimulus. The neural circuits that underlie dEBC—where onset of the conditioned stimulus precedes that of the unconditioned stimulus, but they coterminate—are well characterized, and extensive evidence indicates that the cerebellum is essential to both the development and the manifestation of the eyeblink conditioned response.44,45 While additional cortical and subcortical brain areas modulate dEBC acquisition and response latency (ie, hippocampus, medial septum, and frontal cortex [see Christian and Thompson46 for review]), convincing evidence suggests that the cerebellum is the essential site of neuroplasticity underlying expression of the eyeblink conditioned response.47 Therefore, dEBC presents a useful method to assess the functional integrity of the cerebellum and related brain circuits.
This study set out to determine the extent to which cerebellar abnormalities may represent risk markers for schizophrenia by studying dEBC in nonpsychotic first-degree relatives of individuals with the disorder. The primary hypothesis was that schizophrenia and first-degree relative groups would show impaired dEBC compared with healthy comparison subjects.
Methods
Participants
Participants were 18 individuals (5 female) diagnosed with either schizophrenia (n = 14) or schizoaffective disorder (n = 4; schizophrenia group), 18 individuals (11 female) with a first-degree relative diagnosed with schizophrenia or schizoaffective disorder (first-degree relative group), and 18 control participants (10 female) with no personal or family history of schizophrenia spectrum diagnoses (control group). With the exception of 1 participant with schizophrenia who had a relative that was also included in the study, no other participants in the study were related to each other. The patient sample was recruited through outpatient and inpatient units at community and state hospitals. Healthy controls were recruited using fliers posted in the community and from newspaper advertisements. First-degree relatives were recruited using contact information obtained from a larger sample of schizophrenia patients who were willing to provide such information. The Family Interview for Genetic Studies48 was used to ascertain whether there were probable schizophrenia spectrum diagnoses in relatives of potential control participants. If a probable diagnosis was identified for a first-degree relative within the potential control participant’s family, the participant was excluded in cases where the diagnosis of the probable family member with the disorder could not be ruled out through an in-person diagnostic interview, eg, the probable family member with schizophrenia or schizoaffective disorder did not wish to participate in the study. The potential control participant’s group assignment was changed to first-degree relative if the relative was determined to meet diagnostic criteria for schizophrenia or schizoaffective disorder.
Of the 18 participants per group recruited for each study, data for 2 controls and 1 relative were excluded from the analysis because their data did not fit a positive linear growth model. (See data analysis section below for complete explanation).
Table 1 shows demographic, clinical, and medication information for the remaining 51 participants. The mean age of schizophrenia participants, controls, and relatives did not differ (F(2,48) = 0.022, P = .98), and sex was not significantly different across groups (χ2(2) = 4.17, P = .13). Education level was available for all except for 3 participants (1 in each group) and was found to differ across groups, F(2,45) = 11.31, P < .001. Bonferroni corrected comparisons showed that controls had more education than both the relative and schizophrenia groups.
Table 1.
Demographic, Clinical, and Medication Information
| Schizophrenia | Relatives | Controls | |
|---|---|---|---|
| Age (y); M (SD) | 36.0 (12) | 35.9 (13) | 36.8 (13) |
| Education Levela; M (SD) | 3.2 (1) | 3.1 (1) | 4.5 (1) |
| Sex (M:F) | 13:5 | 7:10 | 7:9 |
| PANSS total score; M (SD) | 61 (12) | — | — |
| Positive; M (SD) | 17 (5) | — | — |
| Negative; M (SD) | 15 (4) | — | — |
| General; M (SD) | 30 (7) | — | — |
| Past alcohol dependenceb | 7 | 3 | 0 |
| Past drug dependencec | 8 | 3 | 0 |
| Psychotropic medication | |||
| No medication | 2 | 16 | 18 |
| Atypical antipsychotic | 13 | 0 | 0 |
| Typical antipsychotic | 3 | 0 | 0 |
| Anticonvulsant | 2 | 0 | 0 |
| Antidepressant | 7 | 2 | 0 |
| Anticholinergic | 6 | 0 | 0 |
Note: PANSS, Positive and Negative Syndrome Scale.
aEducation level included self-report data on completion of grade school (1), junior high school (2), high school (3), some college (4), bachelor’s degree (5), master’s degree (6), and doctoral degree (7).
bFive schizophrenia patients and 1 relative met criteria for both past alcohol and other drug dependence.
cOther drug dependence included cannabis (n = 5 schizophrenia, n = 3 relatives) and cocaine (n = 4 schizophrenia).
Diagnostic status for the schizophrenia group was determined using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-IV Axis I Disorders (SCID-I)49 sections for mood disorders, psychotic disorders, and substance abuse disorders, as well as chart review. Kappa interrater reliability in our lab has been 0.95 for schizophrenia vs mood disordered, or other diagnoses in patients who have been prescreened for showing psychosis. Participants in the relative group were evaluated using the SCID-II for Axis II disorders50 and the SCID-I. Control participants were interviewed using the nonpatient version of SCID-I51 sections for mood, psychotic, and substance abuse and the SCID II to exclude psychiatric disorders. Participants with schizophrenia underwent symptom assessment using the Positive and Negative Syndrome Scale (PANSS).52 All assessments occurred within 30 days of dEBC testing, and all but 4 occurred within 7 days of testing (M = 5.6 days; SD = 6.6). The average PANSS total score indicated that the patient group as a whole fell in the mildly-to-moderately ill range (M = 61.3, SD = 11.8; see table 2).53
Table 2.
Parameter Estimates for the Hierarchical Linear Modeling Growth Curve Model for Percentage of Conditioned Responses
| Value (SE) | df | t Value | P Value | |
|---|---|---|---|---|
| R 2 = 0.75 | ||||
| Intercept | 48.9 (5.6) | 456 | 8.71 | .000 |
| SZ–HC | −12.8 (7.7) | 48 | −1.66 | .103 |
| Rel–HC | −13.1 (7.8) | 48 | −1.67 | .101 |
| Slope | 4.4 (0.6) | 456 | 7.50 | .000 |
| SZ–HC | −2.0 (0.8) | 456 | −2.54 | .012* |
| Rel–HC | −2.4 (0.8) | 456 | −2.95 | .003* |
Note: SZ = schizophrenia, Rel = relatives, HC = healthy controls.
*Indicates significance at P < .0125.
Exclusion criteria for all participants included a history of neurological or cardiovascular disease, clinically documented hearing loss, head injury resulting in loss of consciousness, electroconvulsive therapy, diagnosis of alcohol or other substance dependence within 3 months, and intelligence quotient (IQ) below 70. Control participants were excluded if they had a history of psychotic or mood disorder. Recruiting for this study occurred within the context of a larger effort in which approximately 30% of participants interviewed as potential controls were excluded using these criteria. Of the remaining 70%, approximately 8% were enrolled as first-degree relatives based on information obtained during interviews. Family members were not excluded for a diagnosis of schizotypal personality disorder (n = 1), schizoid personality disorder (n = 1), depression (n = 2), dysthymia (n = 1), or past alcohol or other substance dependence (n = 6), because these disorders may reflect expression of risk factors also associated with schizophrenia. Ten schizophrenia patients met criteria for past alcohol or other substance dependence. The Indiana University Human Subjects Institutional Review Board approved all study procedures, and written informed consent was obtained from all participants.
Eyeblink Conditioning Procedure
Participants completed a single-cue tone dEBC task. The conditioned stimulus was a 400ms, 1000 Hz (80 dB sound pressure level) tone, which, on paired trials, coterminated with a 50ms air puff to the eye, the unconditioned stimulus. Subjects were presented with 8 unconditioned stimulus-alone trials (intertrial interval = 15 s), followed by 10 blocks of conditioning trials (mean intertrial interval = 15 s; range = 10–20 s). Each trial block consisted of 9 paired trials, in which the tone and air puff were presented together, and 1 tone-alone trial. To maintain the participants’ attention throughout the experiment, neutral photographs selected from the International Affective Picture System54 were presented (2-s duration) between each trial, and participants rated the pleasantness of the images by pressing a response pad button. In addition, participants were observed via a closed circuit monitor to ensure that their eyes remained open. The experiment was briefly suspended if signs of fatigue were observed so that the examiner could interact with the participant.
Procedure
Two bipolar eletromyographic electrodes (4mm Ag/Ag–Cl) were placed within 1cm below the left eyelid, centered under the pupil, and placed 1cm apart. These recorded eyeblinks from the orbicularis palpebrarum muscle of the eye. A ground electrode was placed on the forehead. The inside corner of the left eye was presented with an unconditioned stimulus air puff (50ms, 10 lb psi at source) delivered via copper tubing (fused to the rim of lens-less glasses) connected to a regulator delivering air via plastic tubing (120 in.). The conditioned stimulus tone was delivered via ear inserts (E-A-RLINK—Aearo Company Auditory Systems). Electromyographic recordings were made continuously (2.5 KHz A/D rate; high-pass filter = 1 Hz; low-pass filter = 500 Hz; gain = 1000) throughout the experiment and stored offline.
Data Processing
Continuous data files for each subject were divided into 1086ms epochs starting 500ms prior to conditioned stimulus onset. After a 28 Hz (6 dB/octave) high-pass filter was applied, the data were rectified and smoothed using a 41-point Gaussian weighted moving average. Data were entered into DataMunch, a Matlab computer program purposely written for EBC data analysis37,39,40,55–58 for further analysis of the 90 paired trials. Alpha responses, which are reflexive, nonassociative orienting electromyographic responses to the tone conditioned stimulus, were assessed between 25 and 100ms after conditioned stimulus onset. On a subject-by-subject basis, responses were recorded as blinks if the amplitude exceeded five standard deviations above the baseline (baseline window for each trial = 125ms prior to conditioned stimulus onset). The analysis incorporated a “bad trial” window that was used to exclude trials where electromyogram activity was increased immediately before (−75ms) and shortly after conditioned stimulus onset (+25ms). If a participant exhibits electromyographic blink activity during this interval, it is unlikely that a conditioned response can be emitted immediately thereafter. That is, spontaneous blinks occurring during this “bad trial” window may interfere with the subsequent execution of a conditioned response. Blinks recorded in this window are considered spontaneous blinks because they occur too early in reference to conditioned stimulus onset to be considered either tone related or conditioning related. Accordingly, the number of “bad trials” can be used as a rough index of spontaneous blink rate. The average number of “bad trials” rejected from analysis did not differ between groups, F(2,48) = 1.27, P = .29.
Conditioned responses were recorded if the blink occurred between 100 and 350ms after conditioned stimulus onset, which corresponded to a period beginning 250ms before the onset of the unconditioned stimulus. The onset latency was calculated as the point in time when the conditioned response exceeded 0.5 SD from the baseline.
Hierarchical Linear Modeling
Electromyographic data were analyzed using growth curve models in hierarchical linear modeling (HLM). HLM is superior to repeated measures ANOVA because it takes into account the dependence of nested (multilevel) data. In HLM, the best-fitting line through each individual’s trajectory is identified while taking into account the trajectories of other members of the sample. In this way, using multiple iterations, HLM increases the accuracy of the fit and decreases measurement error for both the population and for each individual. While heterogeneity of variance and violations of sphericity are treated as nuisance factors to be eliminated in traditional statistical approaches, including ANOVA, HLM has fewer assumptions than ANOVA and can accommodate unequal variances. This point is important because heterogeneity of variance can be potentially meaningful, and ignoring it can obscure significant interaction effects.59
Statistical Analysis
For each individual, the percentage of conditioned re sponses was calculated for each of the 10 blocks of the experiment, and the best-fitting line was generated for these 10 data points, producing 54 lines—one line for each individual initially included in the study. Three individuals (2 controls and 1 relative) were then excluded from further analysis because they displayed negative learning curves, operationally defined as a decrease in conditioned responding of more than 20% from the first to the last block of the experiment using the fitted values of the model. (A figure including best-fitting line for all participants, as well as complete parameter estimates and significance tests with these 3 outliers included in the model, can be found in the online supplementary material.) Therefore, 51 participants were included in the final analysis.
To model learning curves of conditioned responses, we used the lme function of the nlme package60 in R 3.0 (R Development Core Team, 2009) for growth curve modeling in HLM. In this study, HLM accounted for the dependency of data within individuals due to the repeated measures. Models used maximum likelihood estimation, except when testing whether effects should be fixed or random, in which case restricted maximum likelihood was used as suggested by Singer and Willett.61 We examined linear and nonlinear forms of change with nested model comparisons using the likelihood ratio test. Model fit was examined with pseudo-R 2,61 which was calculated by the squared correlation between the model’s fitted and observed values, representing the proportion of variance in the outcome explained by the predictors.
After examining various forms of change, we settled on linear trajectories with random intercepts and slopes, which fit the data well. Linear growth curves were fit to each participant’s learning curve across blocks and estimated whether groups differed in two parameters: intercepts and slopes. The intercepts reflected model-fitted performance during the first block and tested whether groups differed in their initial values at block 1. Slopes reflected change across blocks and tested whether groups differed in their learning rates.
The intercepts and slopes of the schizophrenia and relatives groups were compared with the control group, resulting in 4 separate statistical tests of between-group differences. Results with P < .05 are reported, but only those that survived Bonferroni-corrected alpha levels of P < .0125 (P < .05/4 comparisons) are deemed significant. The same analysis procedures were then applied to each of the following dEBC measures as secondary analyses: conditioned response onset latency, conditioned response amplitude, unconditioned response amplitude, and unconditioned response peak latency.
Results
Baseline Unconditioned Response Amplitude
In order to rule out blink performance differences between groups as a source of differences in percentage of conditioned responses, responses to 8 solitary unconditioned stimuli presented prior to the conditioning phase of the procedure were analyzed. Neither the average peak unconditioned response amplitudes (F(2,48) = 0.60, P = .56) nor latencies (F(2,48) = 0.93, P = .40) were significantly different across diagnostic categories, suggesting that differences in conditioned response rates between groups are unlikely to be due to deficits in sensorimotor processing or motor responding in the schizophrenia and first-degree relative groups.
Conditioned Responses
Percentage of Conditioned Responses.
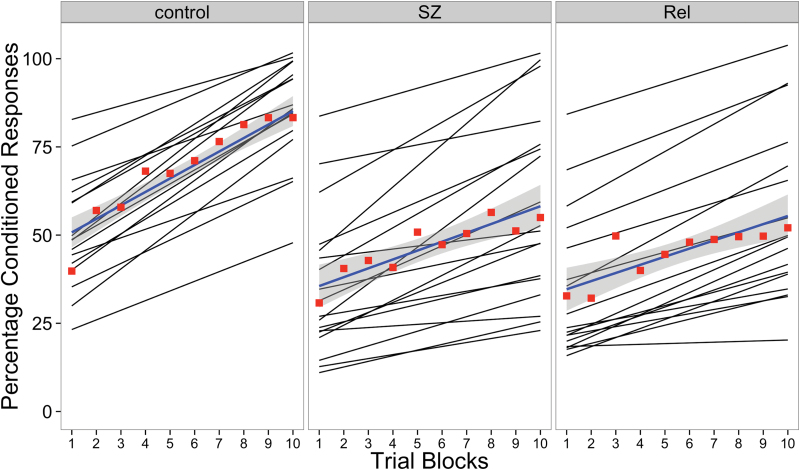
The model fit the data well, with a pseudo-R 2 = 0.75. Individual fitted lines and the average line fit for each group can be seen in figure 1, along with the 10 block averages for each group. Collapsing across groups, performance improved on average as the experiment progressed, t(456) = 7.50, P < 0.001, SE = 0.58. Although there were no significant differences between groups at block 1, the rate of learning varied between groups. Specifically, both the schizophrenia (t(456) = −2.54, P = .012, SE = 0.80) and relatives (t(456) = −2.95, P = .003, SE = 0.81) groups had significantly smaller increases compared with controls, indicating impaired acquisition of dEBC associative learning. Complete information regarding parameter estimates and model fit can be found in table 2.
Fig. 1.
Fitted lines for the percentage of conditioned responses across the 10 blocks of the experiment for each individual (black lines) with group averages for each block (red squares) and group averaged line fits.
Conditioned Response Onset Latency and Amplitude.
Neither the schizophrenia group nor the relatives group differed from controls on conditioned response onset latency or amplitude with respect to the intercept or slope. Moreover, collapsing across groups, neither onset latency nor amplitude showed a significant effect of slope, indicating that neither measure systematically changed on average as the experiment progressed.
Unconditioned Responses (Paired Trials)
Unconditioned Response Latency and Amplitude.
When the slopes for unconditioned response latency and amplitude for both the schizophrenia and relatives groups were compared to controls, no significant differences emerged. Likewise, no significant differences between groups were found with respect to the intercept for unconditioned response latency. The schizophrenia group had a higher intercept compared with controls on unconditioned response amplitude, indicating larger amplitude responses from the start of the experiment, but this difference did not survive Bonferroni correction (P = .018 > .0125). Collapsing across groups, the unconditioned response latencies became longer on average as the experiment progressed (t(456) = −2.26, P = .024, SE = 0.75). Similarly, unconditioned response amplitude decreased significantly over time (t(456) = −2.90, P = .004, SE = 1.37).
Discussion
The present results illustrate a striking dEBC associative learning deficit in first-degree relatives of schizophrenia patients that is remarkably similar in magnitude to individuals who have been diagnosed with the disorder. This finding adds to a growing literature documenting cognitive and learning deficits in first-degree relatives of individuals with schizophrenia, but extends previous findings by demonstrating an impairment on a fundamental form of cerebellar-dependent associative learning—dEBC.
The observed dEBC abnormalities in first-degree relatives and in schizophrenia may arise from aberrant cerebellar processing. Specifically, on the basis of an extensive animal and human literature, these deficits implicate anomalies both in the cerebellar cortex and deep interpositus nucleus and, perhaps, in the function of the Purkinje cells that project from the cortex to the deep nuclei of the cerebellum in both individuals with schizophrenia and their first-degree relatives. Animal studies have shown that the critical association between the conditioned and unconditioned stimuli occurs in the anterior interpositus nucleus,62–64 one of the deep cerebellar nuclei. The cortex of the cerebellum is thought to control the expression of conditioned responses and modulate both their timing and amplitude.45,65,66 The anterior lobe, through Purkinje cells projecting to the interpositus, appears to play a critical role in response timing, delaying the onset of conditioned responses until just prior to the unconditioned stimulus onset.67,68 Findings from these animal studies are supported by studies in humans suggesting that abnormalities in cerebellar structure are associated with dEBC performance.69,70
Our findings are consistent with reports of cerebellar neurotransmitter, neurochemical, and genetic abnormalities in schizophrenia patients46,53,71–73 and with previous reports of structural cerebellar anomalies in family members of individuals with schizophrenia.42 Taken with the foregoing evidence of critical cerebellar involvement in EBC, the finding that a similar pattern of dEBC deficit occurs in first-degree relatives of individuals with schizophrenia suggests that these abnormalities may be a core feature of schizophrenia.
An advantage of the cross-sectional design of this study is that any lingering questions about the origins of dEBC abnormalities observed in schizophrenia can now be more directly addressed. Because dEBC deficits are observed in first-degree relatives they are unlikely to be associated with the onset of schizophrenia, but instead may represent risk factors. Investigations such as the present one that examined nonpsychotic first-degree relatives are powerful tools in the identification of risk markers of schizophrenia for several reasons. First, familial studies indicate that first-degree relatives have elevated risk for schizophrenia spectrum disorders.74 Second, first-degree relatives share genetic (and some environmental) risk factors with affected probands without exhibiting psychotic disorder and are estimated to have at least 10-fold greater risk for developing the disorder.75–77 Finally, compared with individuals with schizophrenia, first-degree relatives have far less exposure to psychotropic medications, especially antipsychotic drugs. Therefore, the observation of dEBC deficits in schizophrenia and in first-degree relatives strengthens the conclusion that dEBC deficits observed in schizophrenia in this study (and others)37–40,78 are unlikely to be due psychotropic medications. This point is important because a recent review79 urged caution in ascribing dEBC abnormalities to illness mechanisms and argued that antipsychotic medication could account for conditioning deficits. The present findings of dEBC deficits in relatives also suggest that impaired dEBC in schizophrenia is unlikely to be due to course of illness variables.
Taken together with findings from this study, given that (1) schizophrenia is highly heritable,80 (2) first-degree relatives are at substantially higher risk for the onset of schizophrenia,74 (3) cerebellar abnormalities have been found using a wide variety of methodologies and paradigms and at multiple levels of analysis in schizophrenia (see Andreasen and Pierson81 for review), and (4) first-degree relatives show alterations in the cerebellum,42,43,82,83 these findings converge on the conclusion that dEBC abnormalities may represent a risk factor for schizophrenia.
Conclusions, Limitations, and Future Directions
While it seems likely that the observed deficits in dEBC are related to a failure in first-degree relative and schizophrenia to acquire a cerebellar-mediated conditioned response, a distributed network of brain regions participate in this form of learning. Therefore, neuroimaging methods can more definitively identify the extent to which these deficits are uniquely related to cerebellar alterations, and such studies are currently underway in our laboratory.
General intelligence has also been linked to cerebellar structure and function. For example, there is evidence linking cerebellar volume to IQ84–86 and our own data have shown an association between IQ and dEBC that was evident in controls but not in individuals with schizophrenia.87 Reduced IQ is commonly found in schizophrenia,88 and nonpsychotic relatives from multiplex families also reportedly have lower IQ compared with healthy control samples.89 Therefore, the present findings suggest that a careful study of the relationship between cognition, symptom profiles, genetic loading for schizophrenia, and cerebellar measures is warranted.
The findings reported here are important because they suggest cerebellar abnormalities may represent potential risk markers of schizophrenia although it has not previously been commonly considered as such. In the context of the present findings and given the known role of the cerebellum in motor coordination and motor learning, it is worth noting that early motor abnormalities have convincingly been associated with worsening of prodromal symptoms in high-risk groups90,91 and the subsequent development of schizophrenia spectrum disorders.92–94 Early evidence suggests that consideration of novel treatment strategies focused on improving cerebellar function could represent a novel therapeutic approach. For example, we and others have shown the efficacy of pharmacological55 and repetitive transcranial magnetic stimulation95 targeting of cerebellar-dependent deficits. Our laboratory has studies underway that are aimed at characterizing the sensitivity of an ensemble of candidate biomarkers to cerebellar abnormalities in schizophrenia. This biomarker validation will provide an essential framework for translational research and intervention development.
Supplementary Material
Supplementary material is available at http://schizophrenia bulletin.oxfordjournals.org.
Funding
National Institute of Mental Health (R01 MH074983B, PI: W.H.; R21 MH091774-01, PIs: B.F.O. and S.M.).
Supplementary Material
Acknowledgments
We would like to thank the participants and clinical research team at Larue D. Carter Memorial Hospital and the Indiana University Neuroscience Clinical Research Center for their support. We are especially grateful to Colleen Merrill for her assistance in assessment and diagnosis of participants and in collecting EBC data. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Kraepelin E. Dementia Praecox and Paraphrenia. Edinburgh: Living-stone; 1919. [Google Scholar]
- 2. Ivry RB, Keele SW. Timing of functions of the cerebellum. J Cogn Neurosci. 1989;1:136–162 [DOI] [PubMed] [Google Scholar]
- 3. Katz DB, Steinmetz JE. Psychological functions of the cerebellum. Behav Cogn Neurosci Rev. 2002;1:229–241 [DOI] [PubMed] [Google Scholar]
- 4. Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16:444–447 [DOI] [PubMed] [Google Scholar]
- 5. Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378 [DOI] [PubMed] [Google Scholar]
- 6. Schmahmann JD, Sherman JC. Cerebellar cognitive affective syndrome. Int Rev Neurobiol. 1997;41:433–440 [DOI] [PubMed] [Google Scholar]
- 7. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579 [DOI] [PubMed] [Google Scholar]
- 8. Loeber RT, Sherwood AR, Renshaw PF, Cohen BM, Yurgelun-Todd DA. Differences in cerebellar blood volume in schizophrenia and bipolar disorder. Schizophr Res. 1999;37:81–89 [DOI] [PubMed] [Google Scholar]
- 9. Molina V, Martín C, Ballesteros A, de Herrera AG, Hernández-Tamames JA. Optimized voxel brain morphometry: association between brain volumes and the response to atypical antipsychotics. Eur Arch Psychiatry Clin Neurosci. 2011;261:407–416 [DOI] [PubMed] [Google Scholar]
- 10. Nopoulos PC, Ceilley JW, Gailis EA, Andreasen NC. An MRI study of cerebellar vermis morphology in patients with schizophrenia: evidence in support of the cognitive dysmetria concept. Biol Psychiatry. 1999;46:703–711 [DOI] [PubMed] [Google Scholar]
- 11. Volz H, Gaser C, Sauer H. Supporting evidence for the model of cognitive dysmetria in schizophrenia—a structural magnetic resonance imaging study using deformation-based morphometry. Schizophr Res. 2000;46:45–56 [DOI] [PubMed] [Google Scholar]
- 12. Ichimiya T, Okubo Y, Suhara T, Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biol Psychiatry. 2001;49:20–27 [DOI] [PubMed] [Google Scholar]
- 13. Henze R, Brunner R, Thiemann U, et al. Gray matter alterations in first-admission adolescents with schizophrenia. J Neuroimaging. 2011;21:241–246 [DOI] [PubMed] [Google Scholar]
- 14. Bottmer C, Bachmann S, Pantel J, et al. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2005;140:239–250 [DOI] [PubMed] [Google Scholar]
- 15. Kaspárek T, Marecek R, Schwarz D, et al. Source-based morphometry of gray matter volume in men with first-episode schizophrenia. Hum Brain Mapp. 2010;31:300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rasser PE, Schall U, Peck G, et al. Cerebellar grey matter deficits in first-episode schizophrenia mapped using cortical pattern matching. Neuroimage. 2010;53:1175–1180 [DOI] [PubMed] [Google Scholar]
- 17. Jacobsen LK, Giedd JN, Berquin PC, et al. Quantitative morphology of the cerebellum and fourth ventricle in childhood-onset schizophrenia. Am J Psychiatry. 1997;154:1663–1669 [DOI] [PubMed] [Google Scholar]
- 18. Cahn W, Hulshoff Pol HE, Bongers M, et al. Brain morphology in antipsychotic-naïve schizophrenia: a study of multiple brain structures. Br J Psychiatry Suppl. 2002;43:s66–s72 [DOI] [PubMed] [Google Scholar]
- 19. Levitt JJ, McCarley RW, Nestor PG, et al. Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: clinical and cognitive correlates. Am J Psychiatry. 1999;156:1105–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maloku E, Covelo IR, Hanbauer I, et al. Lower number of cerebellar Purkinje neurons in psychosis is associated with reduced reelin expression. Proc Natl Acad Sci U S A. 2010;107:4407–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reyes MG, Gordon A. Cerebellar vermis in schizophrenia. Lancet. 1981;2:700–701 [DOI] [PubMed] [Google Scholar]
- 22. Tran KD, Smutzer GS, Doty RL, Arnold SE. Reduced Purkinje cell size in the cerebellar vermis of elderly patients with schizophrenia. Am J Psychiatry. 1998;155:1288–1290 [DOI] [PubMed] [Google Scholar]
- 23. Steinberg JL, Devous MD, Sr, Moeller FG, Paulman RG, Raese JD, Gregory RR. Cerebellar blood flow in schizophrenic patients and normal control subjects. Psychiatry Res. 1995;61:15–31 [DOI] [PubMed] [Google Scholar]
- 24. Volkow ND, Levy A, Brodie JD, et al. Low cerebellar metabolism in medicated patients with chronic schizophrenia. Am J Psychiatry. 1992;149:686–688 [DOI] [PubMed] [Google Scholar]
- 25. Andreasen NC, O’Leary DS, Cizadlo T, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996;93:9985–9990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crespo-Facorro B, Paradiso S, Andreasen NC, et al. Recalling word lists reveals “cognitive dysmetria” in schizophrenia: a positron emission tomography study. Am J Psychiatry. 1999;156:386–392 [DOI] [PubMed] [Google Scholar]
- 27. Kim JJ, Mohamed S, Andreasen NC, et al. Regional neural dysfunctions in chronic schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000;157:542–548 [DOI] [PubMed] [Google Scholar]
- 28. Ho BC, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry. 2004;55:1146–1153 [DOI] [PubMed] [Google Scholar]
- 29. Potkin SG, Alva G, Fleming K, et al. A PET study of the pathophysiology of negative symptoms in schizophrenia. Positron emission tomography. Am J Psychiatry. 2002;159:227–237 [DOI] [PubMed] [Google Scholar]
- 30. Wassink TH, Andreasen NC, Nopoulos P, Flaum M. Cerebellar morphology as a predictor of symptom and psychosocial outcome in schizophrenia. Biol Psychiatry. 1999;45:41–48 [DOI] [PubMed] [Google Scholar]
- 31. Segarra N, Bernardo M, Valdes M, et al. Cerebellar deficits in schizophrenia are associated with executive dysfunction. Neuroreport. 2008;19:1513–1517 [DOI] [PubMed] [Google Scholar]
- 32. Park KM, Kim JJ, Seok JH, Chun JW, Park HJ, Lee JD. Anhedonia and ambivalence in schizophrenic patients with fronto-cerebellar metabolic abnormalities: a fluoro-d-glucose positron emission tomography study. Psychiatry Investig. 2009;6:72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;69:967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carroll CA, Boggs J, O’Donnell BF, Shekhar A, Hetrick WP. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67:150–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain Cogn. 2009;70:181–190 [DOI] [PubMed] [Google Scholar]
- 36. Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009;71:345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bolbecker AR, Mehta CS, Edwards CR, Steinmetz JE, O’Donnell BF, Hetrick WP. Eye-blink conditioning deficits indicate temporal processing abnormalities in schizophrenia. Schizophr Res. 2009;111:182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bolbecker AR, Steinmetz AB, Mehta CS, et al. Exploration of cerebellar-dependent associative learning in schizophrenia: effects of varying and shifting interstimulus interval on eyeblink conditioning. Behav Neurosci. 2011;125:687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brown SM, Kieffaber PD, Carroll CA, et al. Eyeblink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain Cogn. 2005;58:94–108 [DOI] [PubMed] [Google Scholar]
- 40. Forsyth JK, Bolbecker AR, Mehta CS, et al. Cerebellar-dependent eyeblink conditioning deficits in schizophrenia spectrum disorders. Schizophr Bull. 2012;38:751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marcelis M, Suckling J, Woodruff P, Hofman P, Bullmore E, van Os J. Searching for a structural endophenotype in psychosis using computational morphometry. Psychiatry Res. 2003;122:153–167 [DOI] [PubMed] [Google Scholar]
- 42. Greenstein D, Lenroot R, Clausen L, et al. Cerebellar development in childhood onset schizophrenia and non-psychotic siblings. Psychiatry Res. 2011;193:131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Collin G, Hulshoff Pol HE, Haijma SV, Cahn W, Kahn RS, van den Heuvel MP. Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psychiatry. 2011;2:73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181 [DOI] [PubMed] [Google Scholar]
- 45. Steinmetz JE. Brain substrates of classical eyeblink conditioning: a highly localized but also distributed system. Behav Brain Res. 2000;110:13–24 [DOI] [PubMed] [Google Scholar]
- 46. Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455 [DOI] [PubMed] [Google Scholar]
- 47. Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234 [DOI] [PubMed] [Google Scholar]
- 48. Maxwell ME. Manual for the Family Interview for Genetic Studies (FIGS). Bethesda, MD: National Institute of Mental Health; 1992. [Google Scholar]
- 49. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM- IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 50. First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. SCID-II Personality Questionnaire. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 51. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders—Nonpatient Version 2.0. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 52. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 53. Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res. 2005;79:231–238 [DOI] [PubMed] [Google Scholar]
- 54. Lang PJ, Greenwald MK. The International Affective Picture System Standardization Procedure and Initial Group Results for Affective Judgements: Technical Reports 1A and 1B . Gainseville, FL: Center for Research in Psychophysiology, University of Florida; 1988. [Google Scholar]
- 55. Bolbecker AR, Hetrick WP, Johannesen JK, O’Donnell BF, Steinmetz JE, Shekhar AS. Secretin effects on cerebellar-dependent motor learning in schizophrenia. Am J Psychiatry. 2009;166:460–466 [DOI] [PubMed] [Google Scholar]
- 56. Bolbecker AR, Mehta C, Johannesen JK, et al. Eyeblink conditioning anomalies in bipolar disorder suggest cerebellar dysfunction. Bipolar Disord. 2009;11:19–32 [DOI] [PubMed] [Google Scholar]
- 57. Steinmetz AB, Edwards CR, Steinmetz JE, Hetrick WP. Comparison of auditory and visual conditioning stimuli in delay eyeblink conditioning in healthy young adults. Learn Behav. 2009;37:349–356 [DOI] [PubMed] [Google Scholar]
- 58. Steinmetz AB, Skosnik PD, Edwards CR, Bolbecker AR, Steinmetz JE, Hetrick WP. Evaluation of bidirectional interstimulus interval (ISI) shift in auditory delay eye-blink conditioning in healthy humans. Learn Behav. 2011;39:358–370 [DOI] [PubMed] [Google Scholar]
- 59. Bryk AS, Raudenbush SW. Heterogeneity of variance in experimental studies: A challenge to conventional interpretations. Psychol Bull. 1988;104:396–404 [Google Scholar]
- 60. Pinheiro J, Bates D, DebRoy S, Sarkar D; Team Rc. NLME: Linear and Nonlinear Mixed Effects Models; R Package Version 3; 2009. [Google Scholar]
- 61. Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford; 2003. [Google Scholar]
- 62. Lavond DG. Role of the nuclei in eyeblink conditioning. Ann N Y Acad Sci. 2002;978:93–105 [DOI] [PubMed] [Google Scholar]
- 63. Steinmetz JE, Logue SF, Steinmetz SS. Rabbit classically conditioned eyelid responses do not reappear after interpositus nucleus lesion and extensive post-lesion training. Behav Brain Res. 1992;51:103–114 [DOI] [PubMed] [Google Scholar]
- 64. Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. I. Lesions of the cerebellar nuclei. Exp Brain Res. 1985;60:87–98 [DOI] [PubMed] [Google Scholar]
- 65. Garcia KS, Mauk MD. Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology. 1998;37:471–480 [DOI] [PubMed] [Google Scholar]
- 66. Steinmetz JE, Kim J, Thompson RF. Biological models of associative learning. In: Gallagher M, Nelson R, eds. Handbook of Psychology, Volume 3 Biological Psychology. New York: Wiley; 2002. [Google Scholar]
- 67. Garcia KS, Steele PM, Mauk MD. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. J Neurosci. 1999;19:10940–10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993;13:1708–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Edwards CR, Newman S, Bismark A, et al. Cerebellum volume and eyeblink conditioning in schizophrenia. Psychiatry Res. 2008;162:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gerwig M, Hajjar K, Dimitrova A, et al. Timing of conditioned eyeblink responses is impaired in cerebellar patients. J Neurosci. 2005;25:3919–3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Guidotti A, Auta J, Davis JM, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069 [DOI] [PubMed] [Google Scholar]
- 72. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33; quiz 34–57 [PubMed] [Google Scholar]
- 73. Fatemi SH, Hossein Fatemi S, et al. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67kDa and Reelin proteins in cerebellum. Schizophr Res. 2005;72:109–122 [DOI] [PubMed] [Google Scholar]
- 74. Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. 2004;161:398–413 [DOI] [PubMed] [Google Scholar]
- 75. Kendler KS, Gardner CO. The risk for psychiatric disorders in relatives of schizophrenic and control probands: a comparison of three independent studies. Psychol Med. 1997;27:411–419 [DOI] [PubMed] [Google Scholar]
- 76. McGue M, Gottesman II, Rao DC. The transmission of schizophrenia under a multifactorial threshold model. Am J Hum Genet. 1983;35:1161–1178 [PMC free article] [PubMed] [Google Scholar]
- 77. Zerbin-Rüdin E. [What are the implications of the current findings in twins for schizophrenia research?]. Dtsch Med Wochenschr. 1967;92:2121–2122 [DOI] [PubMed] [Google Scholar]
- 78. Hofer E, Doby D, Anderer P, Dantendorfer K. Impaired conditional discrimination learning in schizophrenia. Schizophr Res. 2001;51:127–136 [DOI] [PubMed] [Google Scholar]
- 79. Lubow RE. Classical eyeblink conditioning and schizophrenia: a short review. Behav Brain Res. 2009;202:1–4 [DOI] [PubMed] [Google Scholar]
- 80. Wray NR, Gottesman Using summary data from the danish national registers to estimate heritabilities for schizophrenia, bipolar disorder, and major depressive disorder. Front Genet. 2012;3:118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fusar-Poli P, Perez J, Broome M, et al. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31:465–484 [DOI] [PubMed] [Google Scholar]
- 83. MacDonald AW, 3rd, Thermenos HW, Barch DM, Seidman LJ. Imaging genetic liability to schizophrenia: systematic review of fMRI studies of patients’ nonpsychotic relatives. Schizophr Bull. 2009;35:1142–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Frangou S, Chitins X, Williams SC. Mapping IQ and gray matter density in healthy young people. Neuroimage. 2004;23:800–805 [DOI] [PubMed] [Google Scholar]
- 85. Paradiso S, Andreasen NC, O’Leary DS, Arndt S, Robinson RG. Cerebellar size and cognition: correlations with IQ, verbal memory and motor dexterity. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:1–8 [PubMed] [Google Scholar]
- 86. Taki Y, Hashizume H, Sassa Y, et al. Correlation among body height, intelligence, and brain gray matter volume in healthy children. Neuroimage. 2012;59:1023–1027 [DOI] [PubMed] [Google Scholar]
- 87. Bolbecker AR, Mehta CS, Edwards CR, Steinmetz JE, O’Donnell BF, Hetrick WP. Eye-blink conditioning deficits indicate temporal processing abnormalities in schizophrenia. Schizophr Res. 2009;111:182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tamminga CA, Buchanan RW, Gold JM. The role of negative symptoms and cognitive dysfunction in schizophrenia outcome. Int Clin Psychopharmacol. 1998;13(suppl 3):S21–S26 [DOI] [PubMed] [Google Scholar]
- 89. Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT. Neuropsychologic functioning among the nonpsychotic relatives of schizophrenic patients: the effect of genetic loading. Biol Psychiatry. 2000;48:120–126 [DOI] [PubMed] [Google Scholar]
- 90. Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch Gen Psychiatry. 2008;65:165–171 [DOI] [PubMed] [Google Scholar]
- 91. Mittal VA, Walker EF. Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. J Abnorm Psychol. 2007;116:796–803 [DOI] [PubMed] [Google Scholar]
- 92. Mittal VA, Walker EF, Bearden CE, et al. Markers of basal ganglia dysfunction and conversion to psychosis: neurocognitive deficits and dyskinesias in the prodromal period. Biol Psychiatry. 2010;68:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ridler K, Veijola JM, Tanskanen P, et al. Fronto-cerebellar systems are associated with infant motor and adult executive functions in healthy adults but not in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:15651–15656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sanches M, Roberts RL, Sassi RB, et al. Developmental abnormalities in striatum in young bipolar patients: a preliminary study. Bipolar Disord. 2005;7:153–158 [DOI] [PubMed] [Google Scholar]
- 95. Demirtas-Tatlidede A, Freitas C, Cromer JR, et al. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res. 2010;124:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.