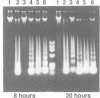
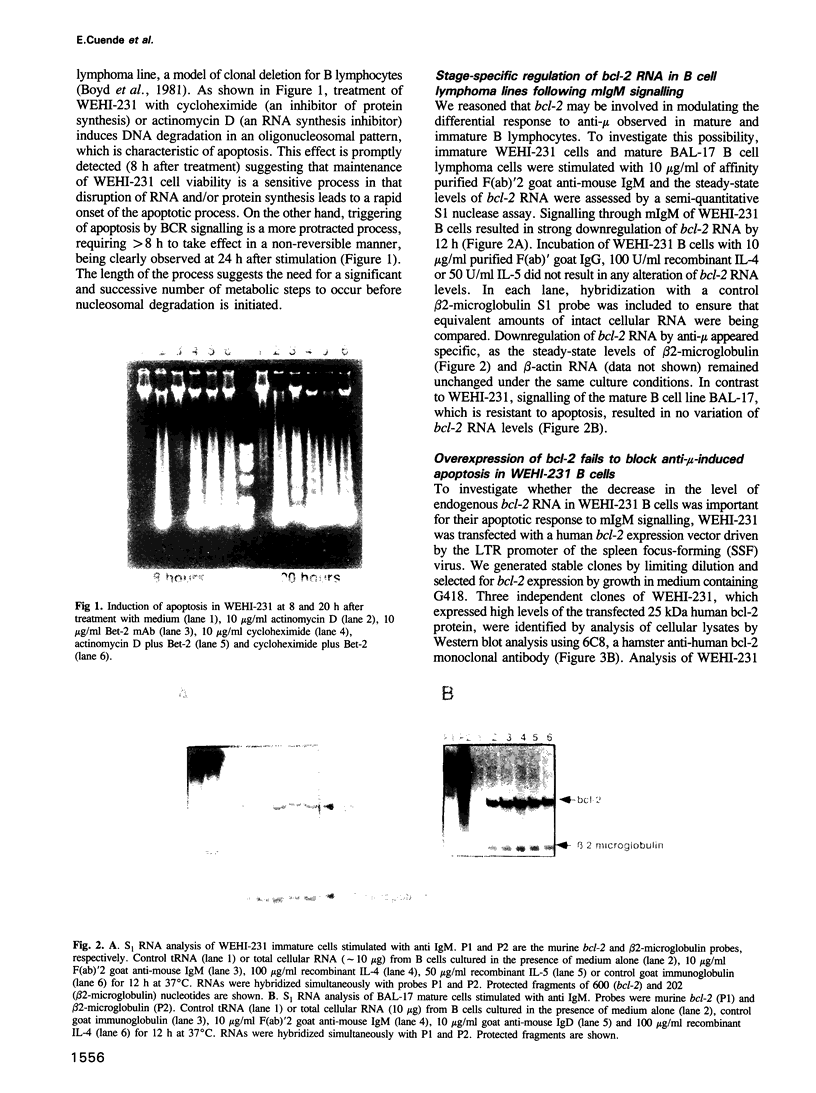
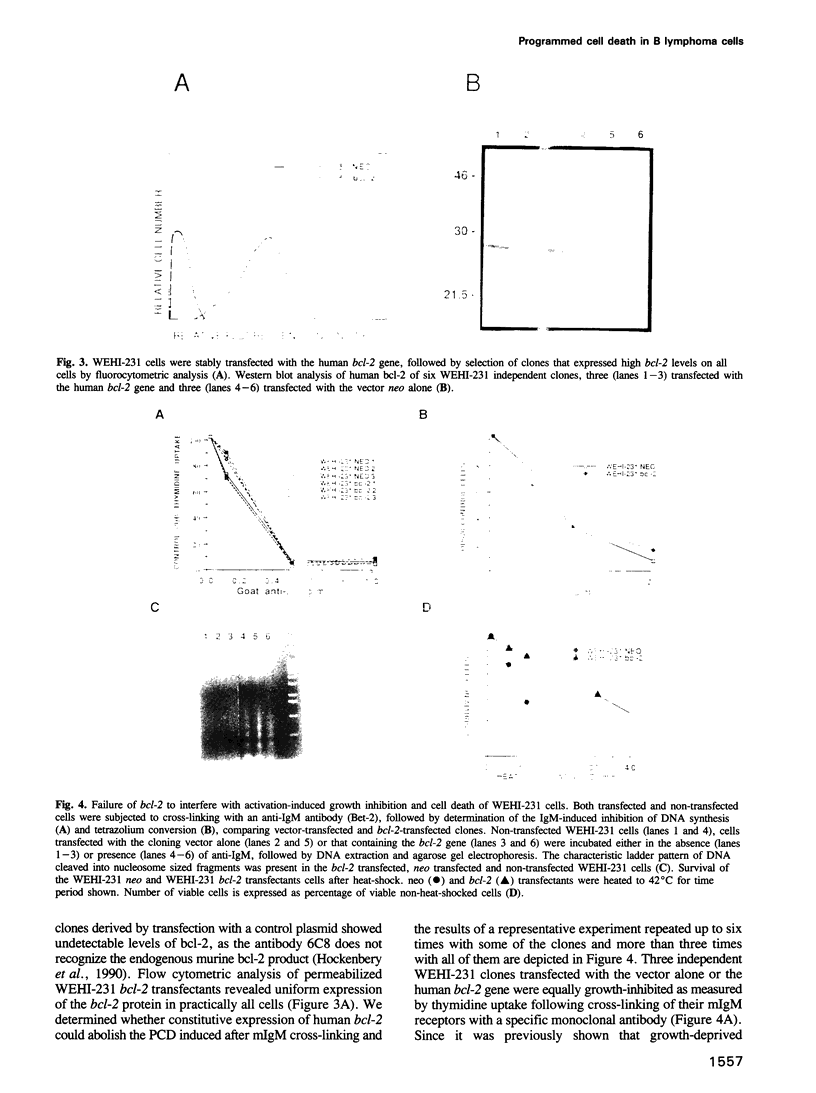
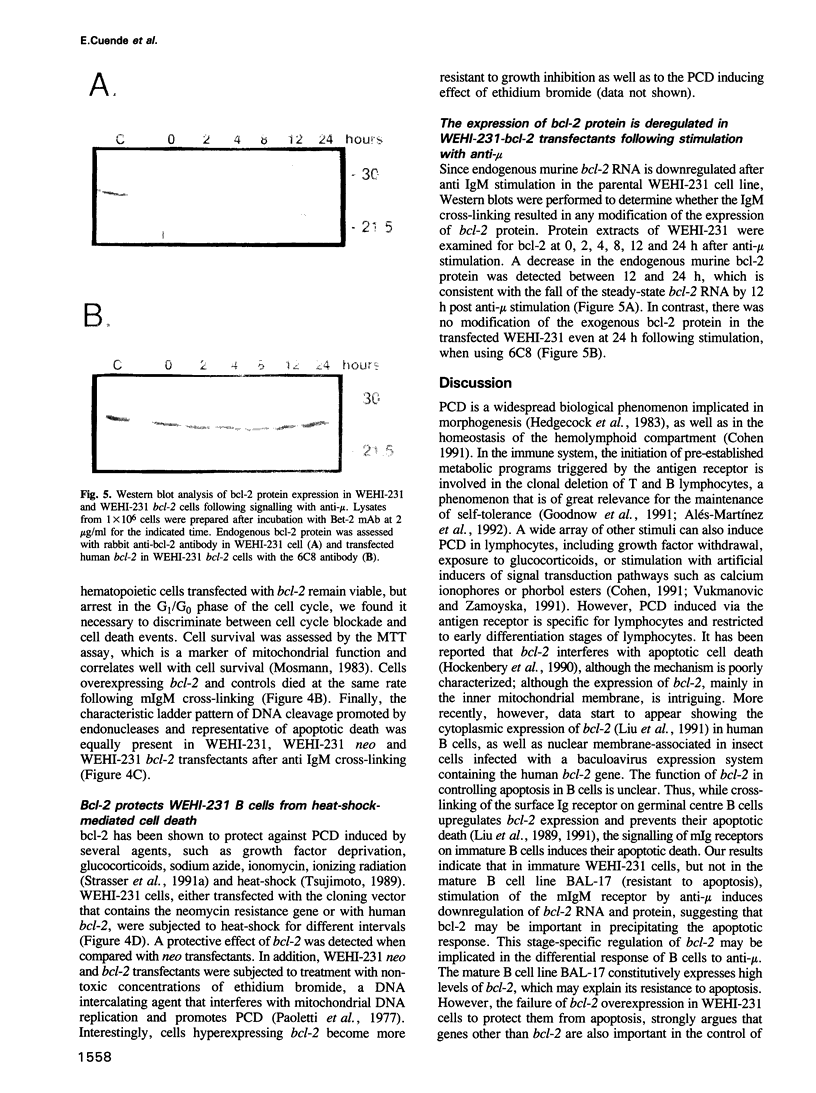
Abstract
Programmed cell death (PCD) or apoptosis is a common form of cellular demise during embryogenesis, tumorigenesis and clonal selection in the immune system. The bcl-2 proto-oncogene has been recently implicated as a potential physiological regulator of the PCD pathway. Gene transfer studies have shown that overexpression of bcl-2 blocks apoptosis mediated by several stimuli in cultured cell lines and promotes the survival of B and T lymphocytes in transgenic mice. However, it remains unclear whether under normal conditions bcl-2 is responsible for controlling cell death. We have investigated the role of bcl-2 in the antimembrane IgM (mIgM)-induced apoptotic death of WEHI-231 B cell lymphoma, a model that mimics clonal deletion of immature B cells by antigen. Signalling of mIgM receptors triggered downregulation of both bcl-2 RNA and protein, and induced apoptosis in WEHI-231 B cells. This effect appeared to be specific since (i) the levels of beta 2-microglobulin and beta-actin RNA remain unchanged and (ii) signalling of the apoptosis-resistant B cell lymphoma line BAL-17 with anti-mu was not associated with downregulation of bcl-2 RNA. However, stable expression of bcl-2 by transfection did not rescue WEHI-231 B cells from apoptosis, yet WEHI-231 cells overexpressing bcl-2 were more resistant to programmed cell death induced by heat-shock.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Robertson N. M., Fernandes T. F., Croce C. M., Litwack G. Overexpressed full-length human BCL2 extends the survival of baculovirus-infected Sf9 insect cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7295–7299. doi: 10.1073/pnas.89.16.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alés-Martínez J. E., Cuende E., Gaur A., Scott D. W. Prevention of B cell clonal deletion and anergy by activated T cells and their lymphokines. Semin Immunol. 1992 Jun;4(3):195–202. [PubMed] [Google Scholar]
- Basten A., Brink R., Peake P., Adams E., Crosbie J., Hartley S., Goodnow C. C. Self tolerance in the B-cell repertoire. Immunol Rev. 1991 Aug;122:5–19. doi: 10.1111/j.1600-065x.1991.tb00593.x. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Schrader J. W. The regulation of growth and differentiation of a murine B cell lymphoma. II. The inhibition of WEHI 231 by anti-immunoglobulin antibodies. J Immunol. 1981 Jun;126(6):2466–2469. [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Cohen J. J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- Dive C., Hickman J. A. Drug-target interactions: only the first step in the commitment to a programmed cell death? Br J Cancer. 1991 Jul;64(1):192–196. doi: 10.1038/bjc.1991.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis H. M., Horvitz H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986 Mar 28;44(6):817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Fine S. M., Unanue E. R., Chaplin D. D. Expression of membrane interleukin 1 by fibroblasts transfected with murine pro-interleukin 1 alpha cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5649–5653. doi: 10.1073/pnas.85.15.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C. C., Brink R., Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991 Aug 8;352(6335):532–536. doi: 10.1038/352532a0. [DOI] [PubMed] [Google Scholar]
- Gurfinkel N., Unger T., Givol D., Mushinski J. F. Expression of the bcl-2 gene in mouse B lymphocytic cell lines is differentiation stage specific. Eur J Immunol. 1987 Apr;17(4):567–570. doi: 10.1002/eji.1830170421. [DOI] [PubMed] [Google Scholar]
- Hasbold J., Klaus G. G. Anti-immunoglobulin antibodies induce apoptosis in immature B cell lymphomas. Eur J Immunol. 1990 Aug;20(8):1685–1690. doi: 10.1002/eji.1830200810. [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Sulston J. E., Thomson J. N. Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science. 1983 Jun 17;220(4603):1277–1279. doi: 10.1126/science.6857247. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Kung J. T., Sharrow S. O., Sieckmann D. G., Lieberman R., Paul W. E. A mouse IgM allotypic determinant (Igh-6.5) recognized by a monoclonal rat antibody. J Immunol. 1981 Sep;127(3):873–876. [PubMed] [Google Scholar]
- Liu Y. J., Joshua D. E., Williams G. T., Smith C. A., Gordon J., MacLennan I. C. Mechanism of antigen-driven selection in germinal centres. Nature. 1989 Dec 21;342(6252):929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Mason D. Y., Johnson G. D., Abbot S., Gregory C. D., Hardie D. L., Gordon J., MacLennan I. C. Germinal center cells express bcl-2 protein after activation by signals which prevent their entry into apoptosis. Eur J Immunol. 1991 Aug;21(8):1905–1910. doi: 10.1002/eji.1830210819. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Lennon S. V., Bonham A. M., Cotter T. G. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990 Sep 15;145(6):1859–1867. [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Nunez G., Platt F. M., Hockenberry D., London L., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2-immunoglobulin transgene expands a resting but responsive immunoglobulin M and D-expressing B-cell population. Mol Cell Biol. 1990 May;10(5):1901–1907. doi: 10.1128/mcb.10.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi J., Tsang W., Morrison S. L., Beaven M. A., Paul W. E. Membrane IgM, IgD, and IgG act as signal transmission molecules in a series of B lymphomas. J Immunol. 1986 Oct 1;137(7):2162–2167. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Negrini M., Silini E., Kozak C., Tsujimoto Y., Croce C. M. Molecular analysis of mbcl-2: structure and expression of the murine gene homologous to the human gene involved in follicular lymphoma. Cell. 1987 May 22;49(4):455–463. doi: 10.1016/0092-8674(87)90448-x. [DOI] [PubMed] [Google Scholar]
- Nemazee D., Russell D., Arnold B., Haemmerling G., Allison J., Miller J. F., Morahan G., Buerki K. Clonal deletion of autospecific B lymphocytes. Immunol Rev. 1991 Aug;122:117–132. doi: 10.1111/j.1600-065x.1991.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Nuñez G., Hockenbery D., McDonnell T. J., Sorensen C. M., Korsmeyer S. J. Bcl-2 maintains B cell memory. Nature. 1991 Sep 5;353(6339):71–73. doi: 10.1038/353071a0. [DOI] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Osmond D. G. Proliferation kinetics and the lifespan of B cells in central and peripheral lymphoid organs. Curr Opin Immunol. 1991 Apr;3(2):179–185. doi: 10.1016/0952-7915(91)90047-5. [DOI] [PubMed] [Google Scholar]
- Paoletti J., Magee B. B., Magee P. T. The structure of chromatin: interaction of ethidium bromide with native and denatured chromatin. Biochemistry. 1977 Feb 8;16(3):351–357. doi: 10.1021/bi00622a002. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Seidman J. G. Structure of wild-type and mutant mouse beta 2-microglobulin genes. Cell. 1982 Jun;29(2):661–669. doi: 10.1016/0092-8674(82)90182-9. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Owen J. J., Cooper M. D., Lawton A. R., 3rd, Megson M., Gathings W. E. Differences in susceptibility of mature and immature mouse B lymphocytes to anti-immunoglobulin-induced immunoglobulin suppression in vitro. Possible implications for B-cell tolerance to self. J Exp Med. 1975 Nov 1;142(5):1052–1064. doi: 10.1084/jem.142.5.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. W., Livnat D., Pennell C. A., Keng P. Lymphoma models for B cell activation and tolerance. III. Cell cycle dependence for negative signalling of WEHI-231 B lymphoma cells by anti-mu. J Exp Med. 1986 Jul 1;164(1):156–164. doi: 10.1084/jem.164.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Siegel R. M., Katsumata M., Miyashita T., Louie D. C., Greene M. I., Reed J. C. Inhibition of thymocyte apoptosis and negative antigenic selection in bcl-2 transgenic mice. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7003–7007. doi: 10.1073/pnas.89.15.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases by glutathione-affinity chromatography. Methods Enzymol. 1981;77:235–237. doi: 10.1016/s0076-6879(81)77031-9. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Bath M. L., Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990 Nov 22;348(6299):331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Finger L. R., Yunis J., Nowell P. C., Croce C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y. Stress-resistance conferred by high level of bcl-2 alpha protein in human B lymphoblastoid cell. Oncogene. 1989 Nov;4(11):1331–1336. [PubMed] [Google Scholar]
- Vukmanović S., Zamoyska R. Anti-CD3-induced cell death in T cell hybridomas: mitochondrial failure and DNA fragmentation are distinct events. Eur J Immunol. 1991 Feb;21(2):419–424. doi: 10.1002/eji.1830210225. [DOI] [PubMed] [Google Scholar]