Abstract
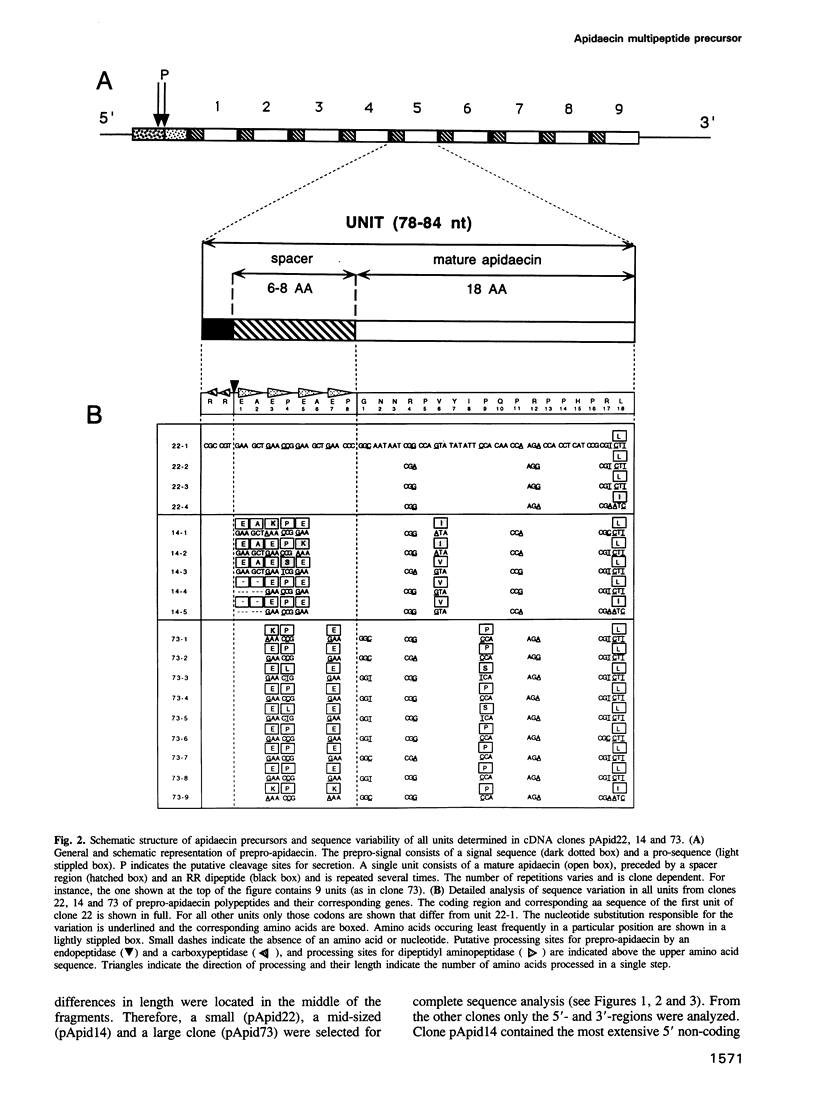
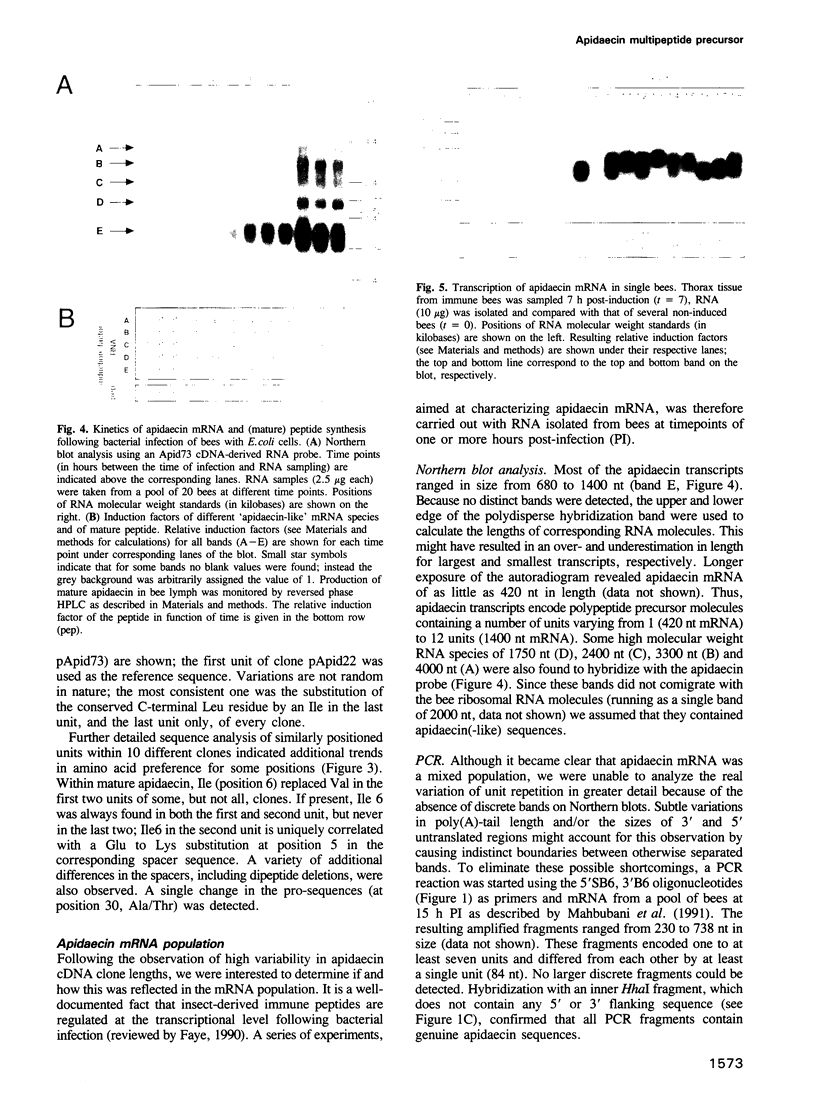
Apidaecins are the most prominent components of the honeybee humoral defense against microbial invasion. Our analysis of cDNA clones indicated that up to 12 of these short peptides (2 kDa) can be generated by processing of single precursor proteins; different isoforms are hereby linked in one promolecule. Assembly of the multipeptide precursors and the putative three-step maturation are strongly reminiscent of yeast alpha-mating factor. Bioactive apidaecins are flanked by the two 'processing' sequences, EAEPEAEP (or variants) and RR; joined together, they form a single unit that is repeated numerous times. The number of such repeats is variable and was reflected in the observed diversity of transcript lengths. Each such transcript is likely to be encoded by a different gene, forming a tight gene cluster. While transcriptional activation upon bacterial challenge is not exceptionally fast, the multigene and multipeptide precursor nature of the apidaecin genetic information allows for amplification of the response, resulting in a real overproduction of peptide antibiotic. Enhanced efficiency of the 'immune' response to bacterial infection through such a mechanism is, to our knowledge, unique among insects.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando K., Natori S. Molecular cloning, sequencing, and characterization of cDNA for sarcotoxin IIA, an inducible antibacterial protein of Sarcophaga peregrina (flesh fly). Biochemistry. 1988 Mar 8;27(5):1715–1721. doi: 10.1021/bi00405a050. [DOI] [PubMed] [Google Scholar]
- Boman H. C., Boman I. A., Andreu D., Li Z. Q., Merrifield R. B., Schlenstedt G., Zimmermann R. Chemical synthesis and enzymic processing of precursor forms of cecropins A and B. J Biol Chem. 1989 Apr 5;264(10):5852–5860. [PubMed] [Google Scholar]
- Boman H. G., Faye I., Gudmundsson G. H., Lee J. Y., Lidholm D. A. Cell-free immunity in Cecropia. A model system for antibacterial proteins. Eur J Biochem. 1991 Oct 1;201(1):23–31. doi: 10.1111/j.1432-1033.1991.tb16252.x. [DOI] [PubMed] [Google Scholar]
- Bulet P., Cociancich S., Dimarcq J. L., Lambert J., Reichhart J. M., Hoffmann D., Hetru C., Hoffmann J. A. Insect immunity. Isolation from a coleopteran insect of a novel inducible antibacterial peptide and of new members of the insect defensin family. J Biol Chem. 1991 Dec 25;266(36):24520–24525. [PubMed] [Google Scholar]
- Casteels P., Ampe C., Jacobs F., Vaeck M., Tempst P. Apidaecins: antibacterial peptides from honeybees. EMBO J. 1989 Aug;8(8):2387–2391. doi: 10.1002/j.1460-2075.1989.tb08368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels P., Ampe C., Riviere L., Van Damme J., Elicone C., Fleming M., Jacobs F., Tempst P. Isolation and characterization of abaecin, a major antibacterial response peptide in the honeybee (Apis mellifera). Eur J Biochem. 1990 Jan 26;187(2):381–386. doi: 10.1111/j.1432-1033.1990.tb15315.x. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson L., Russell V., Dunn P. E. A family of bacteria-regulated, cecropin D-like peptides from Manduca sexta. J Biol Chem. 1988 Dec 25;263(36):19424–19429. [PubMed] [Google Scholar]
- Dimarcq J. L., Zachary D., Hoffmann J. A., Hoffmann D., Reichhart J. M. Insect immunity: expression of the two major inducible antibacterial peptides, defensin and diptericin, in Phormia terranovae. EMBO J. 1990 Aug;9(8):2507–2515. doi: 10.1002/j.1460-2075.1990.tb07430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmochowska A., Dignard D., Henning D., Thomas D. Y., Bussey H. Yeast KEX1 gene encodes a putative protease with a carboxypeptidase B-like function involved in killer toxin and alpha-factor precursor processing. Cell. 1987 Aug 14;50(4):573–584. doi: 10.1016/0092-8674(87)90030-4. [DOI] [PubMed] [Google Scholar]
- Dorsett D., Viglianti G. A., Rutledge B. J., Meselson M. Alteration of hsp82 gene expression by the gypsy transposon and suppressor genes in Drosophila melanogaster. Genes Dev. 1989 Apr;3(4):454–468. doi: 10.1101/gad.3.4.454. [DOI] [PubMed] [Google Scholar]
- Faye I. Acquired immunity in insects: the recognition of nonself and the subsequent onset of immune protein genes. Res Immunol. 1990 Nov-Dec;141(9):927–932. doi: 10.1016/0923-2494(90)90195-5. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Imai J., Fujiwara M., Yaeshima T., Kawashima T., Kobayashi K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J Biol Chem. 1990 Jul 5;265(19):11333–11337. [PubMed] [Google Scholar]
- Fuller R. S., Brake A., Thorner J. Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+-dependent serine protease. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1434–1438. doi: 10.1073/pnas.86.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson G. H., Lidholm D. A., Asling B., Gan R., Boman H. G. The cecropin locus. Cloning and expression of a gene cluster encoding three antibacterial peptides in Hyalophora cecropia. J Biol Chem. 1991 Jun 25;266(18):11510–11517. [PubMed] [Google Scholar]
- Hoffmann D., Hoffmann J. A. Cellular and molecular aspects of insect immunity. Res Immunol. 1990 Nov-Dec;141(9):895–896. doi: 10.1016/0923-2494(90)90189-6. [DOI] [PubMed] [Google Scholar]
- Julius D., Blair L., Brake A., Sprague G., Thorner J. Yeast alpha factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell. 1983 Mar;32(3):839–852. doi: 10.1016/0092-8674(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Kainz P., Schmiedlechner A., Strack H. B. In vitro amplification of DNA fragments greater than 10 kb. Anal Biochem. 1992 Apr;202(1):46–49. doi: 10.1016/0003-2697(92)90203-j. [DOI] [PubMed] [Google Scholar]
- Kockum K., Faye I., Hofsten P. V., Lee J. Y., Xanthopoulos K. G., Boman H. G. Insect immunity. Isolation and sequence of two cDNA clones corresponding to acidic and basic attacins from Hyalophora cecropia. EMBO J. 1984 Sep;3(9):2071–2075. doi: 10.1002/j.1460-2075.1984.tb02093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G., Haiml L., Suchanek G. Stepwise cleavage of the pro part of promelittin by dipeptidylpeptidase IV. Evidence for a new type of precursor--product conversion. Eur J Biochem. 1980 Oct;111(1):49–58. doi: 10.1111/j.1432-1033.1980.tb06073.x. [DOI] [PubMed] [Google Scholar]
- Kylsten P., Samakovlis C., Hultmark D. The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection. EMBO J. 1990 Jan;9(1):217–224. doi: 10.1002/j.1460-2075.1990.tb08098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladendorff N. E., Kanost M. R. Bacteria-induced protein P4 (hemolin) from Manduca sexta: a member of the immunoglobulin superfamily which can inhibit hemocyte aggregation. Arch Insect Biochem Physiol. 1991;18(4):285–300. doi: 10.1002/arch.940180410. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Boman A., Sun C. X., Andersson M., Jörnvall H., Mutt V., Boman H. G. Antibacterial peptides from pig intestine: isolation of a mammalian cecropin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9159–9162. doi: 10.1073/pnas.86.23.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Selsted M. E. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991 Jan 25;64(2):229–230. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- Mahbubani M. H., Bej A. K., Miller R. D., Atlas R. M., DiCesare J. L., Haff L. A. Detection of bacterial mRNA using polymerase chain reaction. Biotechniques. 1991 Jan;10(1):48–49. [PubMed] [Google Scholar]
- Masters D. B., Griggs C. T., Berde C. B. High sensitivity quantification of RNA from gels and autoradiograms with affordable optical scanning. Biotechniques. 1992 Jun;12(6):902-6, 908-11. [PubMed] [Google Scholar]
- Matsumoto N., Okada M., Takahashi H., Ming Q. X., Nakajima Y., Nakanishi Y., Komano H., Natori S. Molecular cloning of a cDNA and assignment of the C-terminal of sarcotoxin IA, a potent antibacterial protein of Sarcophaga peregrina. Biochem J. 1986 Nov 1;239(3):717–722. doi: 10.1042/bj2390717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K., Natori S. Molecular cloning of cDNA for sapecin and unique expression of the sapecin gene during the development of Sarcophaga peregrina. J Biol Chem. 1988 Nov 15;263(32):17117–17121. [PubMed] [Google Scholar]
- Poulter L., Terry A. S., Williams D. H., Giovannini M. G., Moore C. H., Gibson B. W. Levitide, a neurohormone-like peptide from the skin of Xenopus laevis. Peptide and peptide precursor cDNA sequences. J Biol Chem. 1988 Mar 5;263(7):3279–3283. [PubMed] [Google Scholar]
- Reichhart J. M., Essrich M., Dimarcq J. L., Hoffmann D., Hoffmann J. A., Lagueux M. Insect immunity. Isolation of cDNA clones corresponding to diptericin, an inducible antibacterial peptide from Phormia terranovae (Diptera). Transcriptional profiles during immunization. Eur J Biochem. 1989 Jun 15;182(2):423–427. doi: 10.1111/j.1432-1033.1989.tb14848.x. [DOI] [PubMed] [Google Scholar]
- Reichhart J. M., Meister M., Dimarcq J. L., Zachary D., Hoffmann D., Ruiz C., Richards G., Hoffmann J. A. Insect immunity: developmental and inducible activity of the Drosophila diptericin promoter. EMBO J. 1992 Apr;11(4):1469–1477. doi: 10.1002/j.1460-2075.1992.tb05191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K., Egger R., Kreil G. Sequence of preprocaerulein cDNAs cloned from skin of Xenopus laevis. A small family of precursors containing one, three, or four copies of the final product. J Biol Chem. 1986 Mar 15;261(8):3676–3680. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Singh A., Chen E. Y., Lugovoy J. M., Chang C. N., Hitzeman R. A., Seeburg P. H. Saccharomyces cerevisiae contains two discrete genes coding for the alpha-factor pheromone. Nucleic Acids Res. 1983 Jun 25;11(12):4049–4063. doi: 10.1093/nar/11.12.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. C., Asling B., Faye I. Organization and expression of the immunoresponsive lysozyme gene in the giant silk moth, Hyalophora cecropia. J Biol Chem. 1991 Apr 5;266(10):6644–6649. [PubMed] [Google Scholar]
- Sun S. C., Faye I. Cecropia immunoresponsive factor, an insect immunoresponsive factor with DNA-binding properties similar to nuclear-factor kappa B. Eur J Biochem. 1992 Mar 1;204(2):885–892. doi: 10.1111/j.1432-1033.1992.tb16708.x. [DOI] [PubMed] [Google Scholar]
- Sun S. C., Lindström I., Lee J. Y., Faye I. Structure and expression of the attacin genes in Hyalophora cecropia. Eur J Biochem. 1991 Feb 26;196(1):247–254. doi: 10.1111/j.1432-1033.1991.tb15811.x. [DOI] [PubMed] [Google Scholar]
- Sures I., Crippa M. Xenopsin: the neurotensin-like octapeptide from Xenopus skin at the carboxyl terminus of its precursor. Proc Natl Acad Sci U S A. 1984 Jan;81(2):380–384. doi: 10.1073/pnas.81.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry A. S., Poulter L., Williams D. H., Nutkins J. C., Giovannini M. G., Moore C. H., Gibson B. W. The cDNA sequence coding for prepro-PGS (prepro-magainins) and aspects of the processing of this prepro-polypeptide. J Biol Chem. 1988 Apr 25;263(12):5745–5751. [PubMed] [Google Scholar]
- Walldorf U., Richter S., Ryseck R. P., Steller H., Edström J. E., Bautz E. K., Hovemann B. Cloning of heat-shock locus 93D from Drosophila melanogaster. EMBO J. 1984 Nov;3(11):2499–2504. doi: 10.1002/j.1460-2075.1984.tb02163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker C., Reichhart J. M., Hoffmann D., Hultmark D., Samakovlis C., Hoffmann J. A. Insect immunity. Characterization of a Drosophila cDNA encoding a novel member of the diptericin family of immune peptides. J Biol Chem. 1990 Dec 25;265(36):22493–22498. [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]