Abstract
Parkinson's disease is associated with mitochondrial decline in dopaminergic neurons of the substantia nigra. One of the genes linked with the onset of Parkinson's disease, DJ-1/PARK7, belongs to a novel glyoxalase family and influences mitochondrial activity. It has been assumed that glyoxalases fulfill this task by detoxifying aggressive aldehyde by-products of metabolism. Here we show that supplying either D-lactate or glycolate, products of DJ-1, rescues the requirement for the enzyme in maintenance of mitochondrial potential. We further show that glycolic acid and D-lactic acid can elevate lowered mitochondrial membrane potential caused by silencing PINK-1, another Parkinson's related gene, as well as by paraquat, an environmental toxin known to be linked with Parkinson's disease. We propose that DJ-1 and consequently its products are components of a novel pathway that stabilizes mitochondria during cellular stress. We go on to show that survival of cultured mesencephalic dopaminergic neurons, defective in Parkinson's disease, is enhanced by glycolate and D-lactate. Because glycolic and D-lactic acids occur naturally, they are therefore a potential therapeutic route for treatment or prevention of Parkinson's disease.
Keywords: Parkinson's disease, Glyoxalase, D-lactate, Glycolate, Mitochondrial membrane potential
INTRODUCTION
Parkinson's disease is caused by inexorable deterioration of dopaminergic neurons from the substantia nigra (Corti et al., 2011). Although little is known about the onset of Parkinson's disease, one clue is that a number of genes associated with it are linked to mitochondrial activity (Corti et al., 2011; Federico et al., 2012). One of such genes is DJ-1/PARK7, which was originally reported as an oncogene (Nagakubo et al., 1997), and associated with familial Parkinson's disease (Bonifati et al., 2003). DJ-1 deficiency was reported to lead to abnormal mitochondrial morphology and dynamics, increased sensitivity to oxidative stress, decreased mitochondrial membrane potential, and opening of the mitochondrial permeability transition pore (Irrcher et al., 2010; Giaime et al., 2012). DJ-1 protein exerts its neuroprotective function against oxidative stress primarily in mitochondria (Junn et al., 2009). Although DJ-1 is predicted and reported to have activity as protease and chaperon (Mizote et al., 1996; Bonifati et al., 2003; Shendelman et al., 2004; Gautier et al., 2012), it is unclear whether these activities contribute to mitochondrial fitness.
DJ-1 was recently reported to belong to a novel glyoxalase family (Lee et al., 2012). Glyoxalases are enzymes that can transform 2-oxoaldehydes glyoxal and methylglyoxal into corresponding 2-hydroxyacids glycolate and D-lactate, respectively. Glyoxal and methylglyoxal covalently react with proteins or lipids to form advanced glycation end-products (AGEs), which are implicated in neurodegenerative diseases including Parkinson's disease (Castellani et al., 1996; Li et al., 2012). So far, two systems of glyoxalases have been described: 1) Glutathione-dependent Glo I and Glo II systems (Thornalley, 2003) and 2) cofactor-independent Glo III system (DJ-1) (Misra et al., 1995; Lee et al., 2012). Because substrates of glyoxalases are aggressive aldehydes produced by oxidation of glucose during glycolysis (methylglyoxal) and peroxidation of fatty acids (glyoxal), it is assumed that the major function of glyoxalases is to detoxify aldehyde by-products of metabolism (Thornalley, 2003). However, this view has not always been prevalent. Glyoxalases, and their corresponding products (e.g. D-lactate) were considered major components of glycolysis (Ray and Ray, 1998). With the elucidation of the Embden–Meyerhof–Parnas pathway of glycolysis, production of D-lactate was considered an artifact of a biochemical procedure or an undesired side product of glycolysis. Thus, the cellular role of the products of glyoxalases remains unclear.
Here we show that in both HeLa cells and C. elegans, the products of DJ-1, glycolate and D-lactate, are required to maintain mitochondrial membrane potential. Remarkably, D-lactate and glycolate increase in vitro survival of primary dopaminergic neurons from Parkinson's model mice embryos. We propose that the products of the glyoxalases are components of a novel pathway that maintain high mitochondrial potential during cellular stress, and that production of glycolate and D-lactate is required to prevent degeneration of dopaminergic neurons in the substantia nigra.
RESULTS
Recently we reported that the Caenorhabditis elegans dauer larva, an arrested stage specialized for survival in adverse conditions, is resistant to severe desiccation (Erkut et al., 2011). However, this requires a preconditioning step at a mild desiccative environment (98% relative humidity) to prepare the organism for harsher desiccation conditions (60% relative humidity). We found that during preconditioning, glyoxalase genes djr-1.2 and glod-4 were very strongly upregulated (Erkut et al., 2013) (Fig. 1A,B; supplementary material Fig. S1). We asked whether glyoxalases are required to survive desiccation stress. To address this question, we first produced djr-1.1;djr-1.2 double mutant missing both DJ-1 homologs (ΔΔdjr), and djr-1.1;djr-1.2;glod-4 triple mutant defective additionally in Glo I/II system (Δglo) (see Materials and Methods). Dauer larvae of these mutants were first preconditioned, and then further desiccated at 60% relative humidity. Subsequently they were rehydrated and measured for their survival rate (Fig. 1A,C). The Δglo mutant showed significantly increased sensitivity to 60% relative humidity compared to wild type. For instance, 76% of wild-type dauers recovered from this stress, while only 22% of Δglo did. This result shows that glyoxalases are required for survival after desiccation stress.
Fig. 1. Glyoxalases are required for desiccation tolerance in the C. elegans dauer larva.
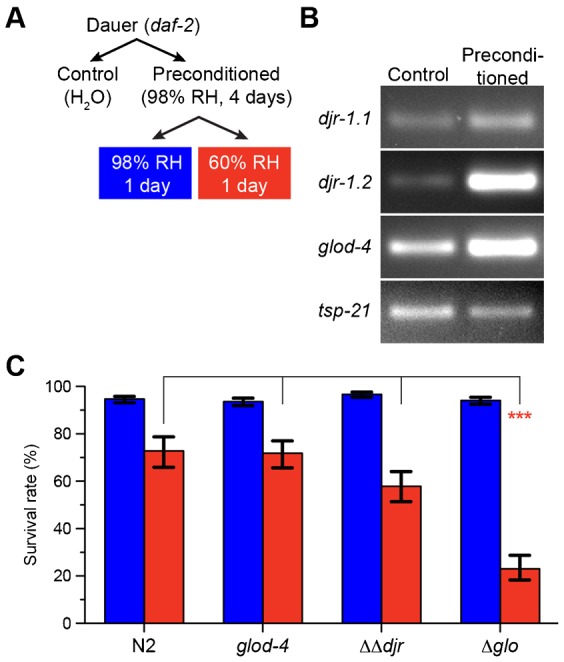
(A) Experimental procedure for preconditioning and further desiccation of C. elegans dauer larvae. RH, relative humidity. (B) Differential expression of djr-1.1, djr-1.2, and glod-4 genes in the C. elegans dauer larva upon preconditioning. Tsp-21 gene was used as the internal control. (C) Survival rates of desiccated dauer larvae. Columns show the estimated means of triplicates. Blue, 98% RH; Red, 60% RH. Error bars, standard error of the estimate. ***p<0.001.
How does DJ-1 contribute to survival under stress conditions? DJ-1 is reported to exert its neuroprotective function in mitochondria (Junn et al., 2009). Many genes involved in Parkinson's disease, among them DJ-1, have been linked to alterations in mitochondrial structure and function and an enhanced sensitivity to mitochondrial toxins like Complex-I inhibitors (Clark et al., 2006; Park et al., 2006; Irrcher et al., 2010; Kamp et al., 2010; Sai et al., 2012; Wang et al., 2012; Burchell et al., 2013). Thus we decided to test the structure and function of mitochondria in the absence of DJ-1 (ΔΔdjr) or complete glyoxalase activity (Δglo), as revealed by staining with MitoTracker CMXRos (Fig. 2A) (Pendergrass et al., 2004). In wild type (N2, top left), mitochondria exhibited elaborated networks, whose intensity of staining represent mitochondrial membrane potential (arrows, Fig. 2A) (Pendergrass et al., 2004). Single mutant glod-4 or ΔΔdjr greatly reduced networks. In the triple mutant (Δglo) the staining and thus the membrane potential of mitochondria was negligible, and only gut granules (arrowheads) were visible. Thus we conclude that glyoxalases are essential to maintain the mitochondrial structure, potential and function under desiccation stress.
Fig. 2. Glycolate and D-lactate are required for mitochondrial membrane potential.
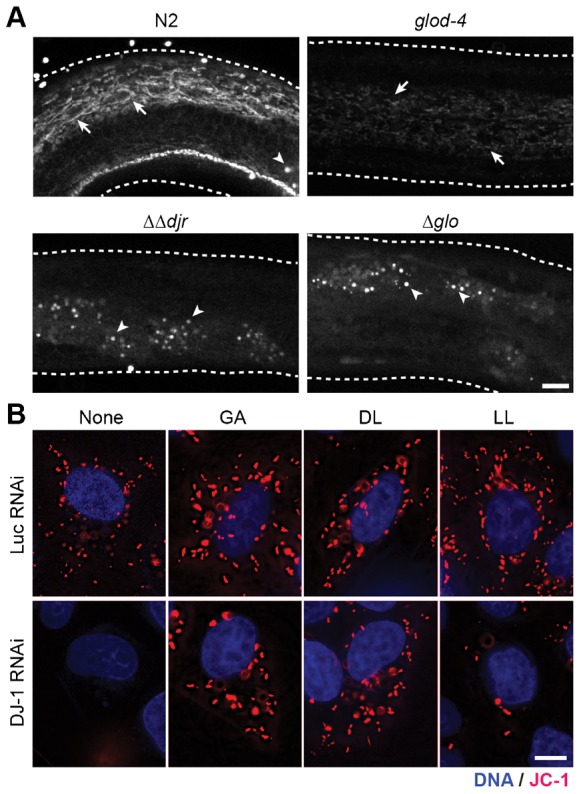
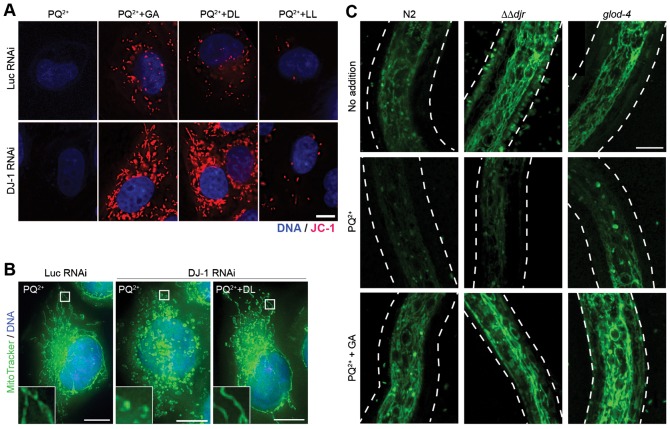
(A) Disruption of the mitochondrial network upon desiccation at 98% RH and rehydration in mutants for glyoxalases. Arrow, mitochondrial networks stained with MitoTracker. Arrowhead, non-specific staining of the gut granules. (B) JC-1 staining in live HeLa cells with 1 mM of glycolate (GA), D-lactate (DL), and L-lactate (LL). Red, JC-1; blue, DNA. For merged images and quantification, see Fig. 4. Scale bars: 10 µm.
One possibility to explain the data so far presented is that the lack of glyoxalases could lead to the build up of their substrates, toxic aldehydes, leading to phenotypic alteration. However, we propose another hypothesis: the defects may result not only from a build up of toxic aldehydes, but also from the lack of the enzymatic products themselves (α-hydroxyacids). To support this idea, we looked at the effects of the products of glyoxalases, D-lactic acid (DL) and glycolic acid (GA) (Thornalley, 2003; Lee et al., 2012), on the structure and activity of mitochondria. There is no easy way to supply these compounds to dauer larvae, because they do not feed. Therefore we decided to use HeLa cells as an alternative model for studying the role of glyoxalases in mitochondrial function.
In HeLa cells, DJ-1 RNAi specifically decreased expression of DJ-1 protein (supplementary material Fig. S2) and decreased mitochondrial membrane potential as probed with a J-aggregate forming lipophilic dye JC-1 (Fig. 2B) (Smiley et al., 1991), consistent with the previous reports (Larsen et al., 2011; Giaime et al., 2012; Heo et al., 2012). Remarkably, addition of 1 mM GA or DL but not L-lactate (LL) restored mitochondrial membrane potential, while these substances did not affect control Luciferase (Luc) RNAi treated cells (Fig. 2B). These results suggest that GA and DL, products of glyoxalases, are required for the activation or maintenance of mitochondrial membrane potential.
Paraquat is an environmental poison known to affect mitochondria (Palmeira et al., 1995); it has been implicated in the onset of Parkinson's disease, and has been shown to decrease mitochondrial membrane potential (McCarthy et al., 2004; Mitsopoulos and Suntres, 2011). In our assay, paraquat indeed decreased mitochondrial membrane potential in both control and DJ-1 RNAi cells as tested by JC-1 (Fig. 3A). Remarkably, addition of GA or DL, but not LL, restored mitochondrial membrane potential of the paraquat-treated cells. In addition to affecting mitochondrial potential, paraquat also induces change in mitochondrial structure (Ueda et al., 1985). Thus we imaged structure of mitochondria in the presence of paraquat with MitoTracker, as it robustly stained mitochondria in HeLa. Although DJ-1 RNAi in HeLa cells did not produce an altered mitochondrial structure (supplementary material Fig. S3), together with a low dose of paraquat in DJ-1 RNAi cells, mitochondria became more circular (Fig. 3B, insets). This circular phenotype was also rescued by the addition of DL and GA (Fig. 3B, insets; supplementary material Fig. S3).
Fig. 3. Glycolate and D-lactate rescue the stress-induced mitochondrial defects.
(A) JC-1 staining in live esiRNA-transfected HeLa cells treated with 50 µM paraquat (PQ2+) and 1 mM of glycolate (GA), D-lactate (DL), and L-lactate (LL). Red, JC-1; blue, DNA. For merged images, see Fig. 4A. (B) Mitochondria of paraquat-treated HeLa cells. Green, MitoTracker; Blue, DNA. Inset, 4× magnification of the boxed area. (C) Mitochondria in worm glyoxalase mutant larvae. Wild type (N2), DJ-1 mutant (ΔΔdjr) and GLOD-4 mutant (glod-4) were treated with PQ2+ with or without 10 mM GA, and stained with MitoTracker (green). Dashed lines show the outlines of larvae. Scale bars: 10 µm.
Increasing doses of paraquat resulted in shorter lengths of wild-type, ΔΔdjr and glod-4 mutant reproductive larvae of C. elegans, suggesting inhibition of development or growth of worms (supplementary material Fig. S4A,B). ΔΔdjr mutant exhibited slightly increased sensitivity to paraquat (supplementary material Fig. S4C). The decreased viability was rescued by addition of GA (supplementary material Fig. S4C). Similar to human cells, paraquat disrupted mitochondrial membrane potential in reproductive larvae of C. elegans, which was rescued by the addition of GA (Fig. 3C).
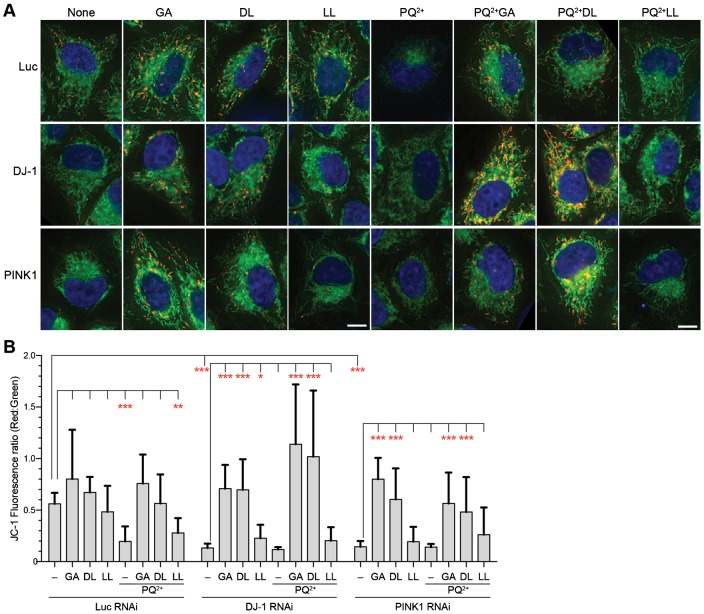
We wanted to test whether GA and DL can rescue a mitochondrial defect caused by loss of other Parkinson's genes, i.e. PINK1 and Parkin. These genes are genetically and functionally related to DJ-1 (Exner et al., 2007; Hao et al., 2010; Irrcher et al., 2010). Because Parkin was not detected in HeLa (Matsuda et al., 2010), PINK1 was investigated. The protein was downregulated by RNAi (supplementary material Fig. S5). PINK1 RNAi decreased mitochondrial membrane potential, as reported previously (Exner et al., 2007). Remarkably, GA and DL rescued this defect (Fig. 4A,B). In addition, the substances restored mitochondrial membrane potential of paraquat-treated PINK1 RNAi cells.
Fig. 4. Glycolate and D-lactate rescue lowered mitochondrial membrane potential caused by silencing PINK1.
(A) Merged images of JC-1 fluorescence in live esiRNA-transfected HeLa cells. Blue, DNA; Green and red, mitochondria with low and high membrane potentials, respectively. Scale bars: 10 µm. (B) The ratio of JC-1 fluorescence intensities in the individual cells. The column and the bar represent the mean and SD, respectively. n≧6. *p<0.05; **p<0.01; ***p<0.001.
So far, we have shown that the addition of products of glyoxalases can rescue lowered mitochondrial membrane potential caused by downregulation of DJ-1 or PINK1 or by environmental stress. But are GA and DL produced endogenously? We asked whether activation of DJ-1, or glyoxylases in general, during stress leads to a bulk accumulation of DL or GA. For this purpose, we looked for both these compounds in C. elegans as well as in HeLa cells. By using a chiral column in an LC-MS application, we could separate D- and L-lactate very effectively (arrows in supplementary material Fig. S6A). In dauer larvae before preconditioning, almost all of lactate was present as the L-stereoisomer (supplementary material Fig. S6A). Preconditioning did not increase D-lactate to any significant extent. Interestingly, the amount of L-lactate decreased 3-fold, perhaps due to entry of the metabolite into gluconeogenesis. We could not detect any GA in the same amount of extract, while an increased amount of trehalose was detected upon preconditioning, consistent with a previous report (supplementary material Fig. S6B,C) (Erkut et al., 2011). Thus we conclude that either these substances are transient intermediates of a pathway needed for the activation of mitochondria, or they are produced in very small amounts and act as signaling molecules.
We have shown that the products of glyoxalases are required for maintenance of mitochondrial potential in HeLa cells and C. elegans. Because toxins that affect mitochondrial function can hasten Parkinson's disease (Bové et al., 2005), we wondered whether GA and DL would protect neurons against mitochondrial damage. We generated embryonic mesencephalic primary neuron cultures and analyzed the survival of tyrosine hydroxylase-positive (TH+) neurons by immunostaining. Strikingly the in vitro survival of dopaminergic neurons was stimulated by GA, and DL, but not by LL (Fig. 5A; supplementary material Fig. S7A). Furthermore, GA and DL significantly rescued the toxic effect of paraquat on the TH+ neurons (Fig. 5B). We also investigated the effect of these substances on dopaminergic neurons from DJ-1 knock out mice (supplementary material Fig. S7B). Similarly, GA and DL could stimulate neuronal survival. These results stress the importance of GA and DL in survival of dopaminergic neurons.
Fig. 5. Glycolate and D-lactate support viability of dopaminergic neurons in vitro.
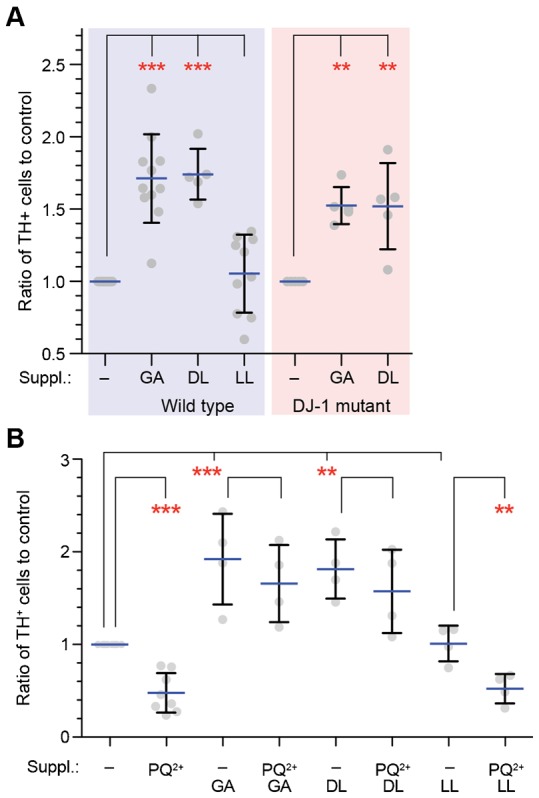
(A) Viability of the dopaminergic neurons in vitro from wild-type and DJ-1 mutant mice embryos in the presence or absence of 10 mM glycolate (GA), D-lactate (DL), or L-lactate (LL). Points show the ratio of tyrosine hydroxylase-positive (TH+) cells to the control (no supplement). Horizontal blue bars and error bars represent the mean and SD, respectively. **p<0.01; ***p<0.001. (B) Viability of the dopaminergic neurons in the presence of 12.5 µM paraquat (PQ2+) in the absence or presence of 10 mM GA, DL, or LL. Primary neurons isolated from wild-type E14.5 embryos were cultured in vitro in the presence of the indicated supplements and PQ2+. Points show the ratio of TH+ cells to the control. Horizontal blue bars and error bars represent the mean and SD, respectively.
DISCUSSION
In this paper, we have shown that the products of glyoxalases, glycolate and D-lactate, are required to maintain mitochondrial membrane potential. Maintenance of mitochondrial membrane potential is associated with response to desiccation stress in worms, and importantly, the survival of dopaminergic neurons in mammals. Our data therefore highlight an understudied aspect of the Embden–Meyerhof glycolytic pathway: A small fraction of triose-phosphate is converted into methylglyoxal, which is further transformed into D-lactate by glyoxalases (Thornalley, 2003). It has so far been thought that glyoxalases protect cells by removing products of glycolysis or lipid oxidation. Thus, our data allow us to suggest the idea that glyoxalases have two functions: on one hand they detoxify chemically aggressive aldehydes and on the other hand they produce compounds necessary for maintaining mitochondrial potential.
DJ-1 is a member of a new class of glutathione-independent glyoxalases that have recently been identified, and studies of their enzymology are just beginning. In vitro enzyme assays using NMR have shown that both human and C. elegans DJ-1 can produce glycolic acid and lactate, but they did not distinguish between L- and D-lactate (Lee et al., 2012). More recently, studies on the biochemical activity of DJ-1 expressed in vitro, using enzyme-coupled assays, suggest that it has only a weak activity as a methylglyoxalase (Hasim et al., 2014). Indeed, our measurements of D-lactate and glycolate (supplementary material Fig. S6) show that they do not accumulate in high amounts, which further suggests that they do not function in high concentration. However, it is also possible that these glyoxalases use other substrates more efficiently. Further studies will be required to understand the in vivo regulation and activity of the DJ-1 family, but this will require improved methods to detect flux through the glyoxalase pathway in vivo. However, the facts that D-lactate and glycolic acid have such an effect on mitochondria, and rescue the DJ-1 phenotype, strongly suggest that these substances are important products of the DJ-1 glyoxalase in vivo.
We do not yet understand how D-lactate and glycolate increase or maintain mitochondrial potential. For instance it is quite possible that these products are further processed. We know, however, that GA and DL restore mitochondrial membrane potential in cells depleted of DJ-1, PINK1 or treated with paraquat. Thus, GA and DL are core compounds in a general pathway that maintains mitochondrial potential. Because both paraquat and loss of DJ-1 are thought to increase permeability of the inner mitochondrial membrane (Costantini et al., 1995; Giaime et al., 2012), one possibility is that GA and DL decrease permeability of the mitochondrial membrane under stressed conditions. Understanding this molecular mechanism is an avenue for future investigation.
It seems likely that the symptoms of Parkinson's disease, neuronal cell death in the substantia nigra, arise from an increased sensitivity of dopaminergic neurons to diminished mitochondrial membrane potential (Corti et al., 2011; Federico et al., 2012). A decline in mitochondrial activity would therefore tend to exacerbate this problem. Indeed, recent experiments in C. elegans show that mitochondria are required for survival of neurons (Rawson et al., 2014). Further investigation of the phenotypes we observe should clarify which aspects of cellular metabolism require mitochondrial potential. Our data show that there is no reduction in ATP levels in DJ-1 mutant conditions, suggesting that it is not an energy requirement (data not shown). Rather, other mitochondrial metabolism cycles, such as one-carbon metabolism (Tibbetts and Appling, 2010; Locasale, 2013) may be involved. Other pathways, such as the glyoxylate shunt in worms, may also play a role.
Therapeutic routes for Parkinson's disease have so far been symptomatic and intractable. It has been shown that environmental toxins that affect mitochondria are strongly linked to the appearance of Parkinson's disease (Song et al., 2004; Freire and Koifman, 2012) and impairment of the mitochondrial function is a common feature of both idiopathic and genetic Parkinson's disease (Schapira et al., 1989; Clark et al., 2006; Park et al., 2006; Irrcher et al., 2010; Kamp et al., 2010; Pan-Montojo et al., 2012; Wang et al., 2012; Braidy et al., 2014; Burchell et al., 2013). Our discovery that the production of molecules from endogenous enzymatic pathways can protect neurons, offers a potential therapeutic direction that could include preventive strategies. Both products of glyoxalases exist in many natural products. Thus, providing neurons with these substances might protect them against metabolic or environmental stress. Because many diseases are associated with a decline in mitochondrial activity (Schapira, 2012), the products of glyoxalases could have a general role in protecting cells from decline.
MATERIALS AND METHODS
Chemicals
Glucose (Merck), glycolic acid (15451, ACROS Organics) neutralized with NaOH to pH = 7.4, and sodium D-lactate (71716, Sigma), glyoxal (128465, Sigma), paraquat (sc-257968, SantaCruz biotechnologies or 36541 Fluka® from Sigma–Aldrich) were used.
Cell culture, RNAi, worm strains
HeLa cells were maintained in DMEM (Life Technologies) supplemented with 10% fetal bovine serum (FBS), 2 mM GlutaMAX, 100 unit/ml penicillin, 100 µg/ml streptomycin at 37°C in a 5% CO2 environment. HeLa cells were RNAi-transfected with 10 nM endoribonuclease-digested small interfering RNA (MISSION esiRNA, Sigma) using Oligofectamine RNAi MAX reagent (Invitrogen). Cells were assayed after 48 hours from RNAi.
All C. elegans strains were maintained on NGM agar plates seeded with Escherichia coli NA22 at 15°C (Brenner, 1974). Mutant strains djr-1.1(tm918), djr-1.2(tm951) and glod-4(tm1266) were obtained from National Bioresource Project, Japan. Wild-type (N2) and daf-2(e1370) mutant strains were obtained from Caenorhabditis Genetics Center, USA. All mutants were outcrossed at least twice with the wild type to eliminate background mutations.
Preparation, culture and treatment of primary mesencephalic dopaminergic neurons from mouse embryos
Primary mesencephalic neuronal cell cultures were prepared as previously described (Gille et al., 2004). Briefly, brain mesencephalons from E14.5 C57JBL6 or DJ-1 embryos were dissected under the microscope and digested with Trypsin-EDTA (Sigma–Aldrich). The trypsin reaction was stopped by adding the basic medium (BM) containing Neurobasal A medium (Gibco), 1 mg/ml penicillin/streptomycin, 10% (v/v) fetal calf serum (Invitrogen) and 2 mM L-Glutamine and cells were mechanically dissociated using a fire-polished Pasteur pipette. Medium was fully replaced by centrifuging for 5 minutes at 1200 rpm, aspiring the supernatant and adding 8 ml of the fresh BM to the pellet. Concentration of cells in the medium was estimated and cells were plated in a volume of 250 µl in 4-well plates (176740, Nunc, Thermo Scientific) or 35 µl in μ-clear 96-well plates (Greiner) coated with poly-D-lysine (Sigma–Aldrich) at a concentration of 2×106 cells per ml. The same volume of medium containing the different treatment substances was added 4 hours after plating to obtain the following treatment concentrations: control, 10 mM GA, 10 mM DL and 10 mM LL. 24 hours later 1/3 of the medium was replaced with fresh BM. On DIV3 (day-in-vitro-culture 3) half of the medium was replaced with B27 medium containing Neurobasal A medium, 1 mg/ml penicillin/streptomycin, 2 mM L-Glutamine (Sigma–Aldrich) and B-27 supplement (Life Technologies) and on DIV5 all medium was replaced by B27 medium. On DIV7 cell were either fixed using Accustain® (Sigma–Aldrich) for 30 minutes or PQ2+ treated at a concentration of 12.5 µM for 72 hours more and fixed. All animal experiments were performed in accordance with German animal welfare legislation.
Immunocytology of mesencephalic cell cultures
Accustain® fixed neuronal cell cultures were washed 3× 10 minutes in phosphate buffered saline (PBS), blocked using a blocking solution (BS) (0.2% Triton X-100 in PBS and 5% donkey serum (DS)) for 1 hour at RT, and incubated with mouse anti-TH (1:500, Millipore), chicken anti-βIII-tubulin (1:500, Millipore) and rabbit anti-TOM20 (1:200, FL-145, Santa Cruz Biotechnology) or rabbit anti-NeuN (1:500, Millipore) primary antibodies in BS overnight at 4°C. On the next day cells were washed 4× 10 minutes with PBS, incubated in donkey Alexa® 488 anti-rabbit, donkey Alexa® 555 anti-mouse (Life Technologies) and donkey Alexa® 647-anti-chicken (Jackson Immunoresearch) secondary antibodies for 1 hour at RT, washed 4×10 minutes with PBS, incubated with Hoechst33342 for 10 minutes and washed once more in PBS.
Generation of C. elegans multiple mutant strains
djr-1.1 and djr-1.2 males and hermaphrodites were first crossed reciprocally. L4 hermaphrodites from the F1 generation were singled out and let lay eggs for 2 days. Subsequently, the adults were lysed and genotyped individually.
One adult was put in 100 µl lysis buffer (1× PCR buffer and 200 ng/µl proteinase-K in water), snap-frozen in liquid nitrogen and incubated for 1 hour at 65°C. Then, the enzyme was denatured at 98°C for 15 minutes. Genotyping PCR was performed in 1× PCR buffer with MgCl2, 200 µM dNTP mix, 400 nM of each primer, 0.02 U Taq polymerase and 5 µl of gDNA from the lysis of an adult hermaphrodite using the primers listed in supplementary material Table S1. PCR conditions were the following: Initial denaturation at 94°C for 10 minutes, amplification in 30 cycles of 94°C for 30 seconds, 62°C for 25 seconds and 72°C for 30 seconds, final extension at 72°C for 10 minutes.
Populations arising from an individual heterozygous for both alleles were selected and L4 hermaphrodites were singled out for one more round of genotyping as described above. Finally, 3 lines homozygous for both alleles were found. One of these lines was selected to be used in subsequent experiments. We named this double mutant ΔΔdjr.
As a next step, ΔΔdjr mutants were crossed with glod-4(tm1266) mutants. The genotyping and selection of homozygous triple mutants were done similarly, using the primers listed in supplementary material Table S1. PCR conditions were the same as above, except that the annealing temperature was increased to 65°C. Finally, two lines homozygous for all 3 alleles were obtained. One of these lines was used in experiments. For convenience, we named this triple mutant Δglo.
Desiccation and measurement of desiccation tolerance in dauers
N2, glod-4, ΔΔdjr and Δglo dauers were generated on agarose plates by sterol depletion/lophenol substitution (Matyash et al., 2004). daf-2 dauers were generated in liquid culture by growing at 25°C. Worms were preconditioned at 98% RH for 4 days and/or desiccated at 60% RH for 1 day in controlled humidity chambers (Erkut et al., 2013). Survival rate of different strains were calculated as the percentage of survivors in the total population after rehydration.
Measurement of desiccation-induced gene expression
Total RNA was extracted from daf-2 dauers before and after preconditioning in 4 biological replicates. 500 ng of total RNA was reverse transcribed using 250 ng oligo(dT)12–18 primer (Invitrogen, CA), 5 nmol dNTP and 200 U SuperScript III reverse transcriptase (Invitrogen, CA) at 50°C for 1 h according to the protocol supplied by the manufacturer. PCR was performed in 1× PCR buffer with MgCl2, 200 µM dNTP, 500 nM of forward and reverse primers, and 0.05 U Taq polymerase, using 0.1 µl of the cDNA product. PCR conditions were the following: 94°C for 10 minutes, 30 cycles of 94°C for 20 seconds, 61.5°C for 25 seconds and 72°C for 15 seconds, finally 72°C for 5 minutes. The primer sequences are presented in supplementary material Table S1. PCR products are separated on a 3% agarose gel and visualized using ethidium bromide.
Paraquat treatment of HeLa cells and worm larvae
For experiments with paraquat (PQ2+) in cells, 50 µM PQ2+ and 1 mM GA/DL/LL were added to HeLa cells and incubated for 24 hours. The media and the supplements were replaced 1 hour before fixation or assay.
Worms were treated with 200 µM PQ with or without 10 mM GA, and compared to worms that are not treated with either PQ or GA. Subsequently, they were stained and imaged for mitochondrial organization and activity.
Mitochondrial live staining of worm larva
Mitochondrial staining was performed as previously described (Yang and Hekimi, 2010). Briefly, Mitotracker Deep Red and CMXROS (M22426, M7512, Life Technologies) were dissolved in DMSO at a concentration of 5 mM and kept at −20°C as a stock solution. On the day of microscopy worms were incubated in a 1:1000 diluted Mitotracker for 45 minutes at room temperature. Worms were then paralyzed with 1 mM Levamizol (Sigma–Aldrich), placed on slides covered with a thin layer of NGM medium on top of which the coverslip (22×22 mm, Menzel–Glaser no. 1) was fixed using nail polish.
Light microscopy, and image analysis
To image mitochondria of HeLa cells, MitoTracker Red CMXRos was added at 150 nM and fixed with 3% (v/w) paraformaldehyde in PBS, 1 mM MgCl2, and 5 mM EGTA. DNA was counter-stained by 1 µg/ml Hoechst33342. As MitoTracker Red CMXRos robustly stained mitochondria in HeLa, JC-1 (Santa Cruz Biotechnology, 10 µg/ml) was used to visualize mitochondrial membrane potentials in live cells, with 100 ng/ml Hoechst33342. They were imaged on the DeltaVision system (Applied Precision Inc., Issaquah, WA, USA) using a 60× objective (PlanApo N, NA = 1.42, Olympus) equipped with a CO2 supply, and deconvolved and maximally projected images were used for the analysis. Due to high background of MitoTracker in the center of the cell, mitochondria in a 15×15-µm area in the periphery were manually annotated on ImageJ software. Total integrated intensity of green- and red-fluorescent JC-1 in the individual cells was measured to obtain the fluorescence ratio. All JC-1 images were adjusted for the contrast in the same way on ImageJ.
Microscopy images from live paralyzed worm larvae stained with Mitotracker were taken using a confocal microscope (LSM510, Zeiss, Germany). Samples were excited using a 514 (Mitotracker CMXRos) or a 647 (Mitotracker Deep Red) nm lasers and two channels, one BP505-550 or LP650 and the BF channel were used to acquire the images. Gain was maintained between 450 and 515 for all samples to ensure the detection of signal intensity differences.
Counting of dopaminergic neurons
Dopaminergic TH+ neurons were observed using an inverted fluorescence microscope (Axiovert 200M, Zeiss) under a 10× objective (PlanApo, NA = 0.45). The diameter of every well was scanned in two perpendicular directions (i.e. top to bottom and left to right) and total TH+ neurons were counted for every well.
Immunoblotting
EsiRNAi-transfected HeLa and mouse brains were lysed in a lysis buffer (50 mM HEPES pH = 7.5, 150 mM KCl, 1 mM MgCl2, 10% glycerol, 0.1% NP-40) with a protease inhibitor cocktail (Complete, Roche), resolved in SDS-PAGE, and transferred onto a nitrocellulose membrane. In immunoblotting, the following primary antibodies were used: human DJ-1 (FL-189, SantaCruz, 1:200 dilution); mouse DJ-1 (HPA004190, Sigma, 1:250); PINK1 (38CT20.8.5, Thermo, 1:500); alpha-tubulin (DM1A, Sigma, 1:2000). Horseradish peroxidase-conjugated anti-IgG antibodies (Bio-Rad, 1:2000) were used for the secondary antibody. Chemiluminescence by ECL reagent was developed on a Hyperfilm (GE Healthcare).
Liquid chromatography mass spectrometry (LC-MS) analysis of alpha-hydroxy acids
daf-2 dauers directly collected from the liquid culture or preconditioned at 98% RH for 4 days were homogenized and extracted according to Bligh's and Dyer's method (Bligh and Dyer, 1959). The aqueous phases were dried and dissolved again in 50% methanol (v/v) using volumes calculated according to total soluble protein amounts. HeLa cells harvested directly or treated with GA were also extracted by the same method. The final volume of their aqueous fractions was normalized according to the number of cells.
Separation and detection of glycolic and lactic acids were performed by normal-phase LC-MS using an Agilent G1312A pump equipped with an Agilent Autosampler G1329A. Separation employed a Cogent Diamond Hydride column (25 cm×4.6 mm i.d., 4 µm, Microsolv) coupled on-line to a Waters/Micromass LCT time of flight (TOF) mass spectrometer equipped with electrospray ionization (ESI). Alpha-hydroxy acid species were separated by isocratic elution. An aqueous formic acid (0.1%; v/v), including 10 mM ammonium formate/acetonitrile mixture (30:70; v/v) was used. The column was operated at 40°C and flow rate was set to 1 ml/minute with a split to 100 µl/minute into the mass spectrometer. 5 µl of each sample were injected into the column.
For the separation of D- and L-lactic acid enantiomers, we employed chiral chromatography by using an Astec CHIROBIOTIC R chiral column (25 cm×4.6 mm i.d., 5 µm, Supelco), as reported previously (Henry et al., 2012). Isocratic elution was performed with the mobile phase 15% (v/v) 30 mM ammonium acetate in H2O (adjusted with acetic acid to pH 3) and 85% (v/v) acetonitrile. The column was operated at 5°C with a split to 50 µl/minute into the same mass spectrometer.
The mass spectrometer was operated with a spray voltage of 2.5 kV and a source temperature of 140°C in negative and positive ion mode. Nitrogen was used as the cone and nebulizing gas at flow rates of approximately 40 and 500 L/h, respectively. Positive and negative ion full scan mass spectra were acquired from the m/z range of 60–1000 mass units in a scan time of 1 second. The system was operated and the resulting data were processed by MassLynx (Version 4.1) software (Waters).
Statistics and graph representation
Statistical differences between the tested treatments were determined by ANOVA followed by the Tukey's honestly significant differences post-hoc test. Survival rates were compared using beta regression (Erkut et al., 2013). Data expressed in percentages were first transformed by Tukey's double arcsine function (Freeman and Tukey, 1950) to achieve normal distribution prior to ANOVA. Statistical analysis and graphs were done on a Prism software version 5 (GraphPad Inc.) and an R environment.
Supplementary Material
Acknowledgments
We thank Suzanne Eaton and Marino Zerial at the Max Planck Institute for Molecular Cell Biology and Genetics for critical reading of the manuscript and constructive suggestions. We thank Frank Buchholz and Mirko Theis for esiRNA, Zoltan Maliga, John Asara and Min Yuan for helpful discussions, Marc Bickle for suggestions on mitochondria annotation, Ina Poser for technical help, and Human Protein Atlas for the DJ-1 antibody.
Footnotes
Author Contributions: Y.T., C.E., F.P.-M., D.J.M., A.A.H. and T.V.K. conceived and designed the experiments. Y.T., C.E., F.P.-M., S.B. and M.P.S. performed the experiments. Y.T., C.E., F.P.-M., W.W., A.A.H. and T.V.K. interpreted and analyzed the data. Y.T., C.E., F.P.-M., A.A.H. and T.V.K. wrote the paper.
Competing interests: The authors have no competing interests to declare.
Funding
This work was supported by the Max Planck Society (to A.A.H. and T.V.K.).
References
- Bligh E. G., Dyer W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- Bonifati V., Rizzu P., van Baren M. J., Schaap O., Breedveld G. J., Krieger E., Dekker M. C., Squitieri F., Ibanez P., Joosse M. et al. (2003). Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259 10.1126/science.1077209 [DOI] [PubMed] [Google Scholar]
- Bové J., Prou D., Perier C., Przedborski S. (2005). Toxin-induced models of Parkinson's disease. NeuroRx 2, 484–494 10.1602/neurorx.2.3.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidy N., Gai W. P., Xu Y. H., Sachdev P., Guillemin G. J., Jiang X. M., Ballard J. W., Horan M. P., Fang Z. M., Chong B. H. et al. (2014). Alpha-synuclein transmission and mitochondrial toxicity in primary human foetal enteric neurons in vitro. Neurotox. Res. 25, 170–182 10.1007/s12640-013-9420-5 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell V. S., Nelson D. E., Sanchez-Martinez A., Delgado-Camprubi M., Ivatt R. M., Pogson J. H., Randle S. J., Wray S., Lewis P. A., Houlden H. et al. (2013). The Parkinson's disease-linked proteins Fbxo7 and Parkin interact to mediate mitophagy. Nat. Neurosci. 16, 1257–1265 10.1038/nn.3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani R., Smith M. A., Richey P. L., Perry G. (1996). Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res. 737, 195–200 10.1016/0006-8993(96)00729-9 [DOI] [PubMed] [Google Scholar]
- Clark I. E., Dodson M. W., Jiang C., Cao J. H., Huh J. R., Seol J. H., Yoo S. J., Hay B. A., Guo M. (2006). Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441, 1162–1166 10.1038/nature04779 [DOI] [PubMed] [Google Scholar]
- Corti O., Lesage S., Brice A. (2011). What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol. Rev. 91, 1161–1218 10.1152/physrev.00022.2010 [DOI] [PubMed] [Google Scholar]
- Costantini P., Petronilli V., Colonna R., Bernardi P. (1995). On the effects of paraquat on isolated mitochondria. Evidence that paraquat causes opening of the cyclosporin A-sensitive permeability transition pore synergistically with nitric oxide. Toxicology 99, 77–88 10.1016/0300-483X(94)02997-9 [DOI] [PubMed] [Google Scholar]
- Erkut C., Penkov S., Khesbak H., Vorkel D., Verbavatz J. M., Fahmy K., Kurzchalia T. V. (2011). Trehalose renders the dauer larva of Caenorhabditis elegans resistant to extreme desiccation. Curr. Biol. 21, 1331–1336 10.1016/j.cub.2011.06.064 [DOI] [PubMed] [Google Scholar]
- Erkut C., Vasilj A., Boland S., Habermann B., Shevchenko A., Kurzchalia T. V. (2013). Molecular strategies of the Caenorhabditis elegans dauer larva to survive extreme desiccation. PLoS ONE 8, e82473 10.1371/journal.pone.0082473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner N., Treske B., Paquet D., Holmström K., Schiesling C., Gispert S., Carballo-Carbajal I., Berg D., Hoepken H. H., Gasser T. et al. (2007). Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J. Neurosci. 27, 12413–12418 10.1523/JNEUROSCI.0719-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico A., Cardaioli E., Da Pozzo P., Formichi P., Gallus G. N., Radi E. (2012). Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 322, 254–262 10.1016/j.jns.2012.05.030 [DOI] [PubMed] [Google Scholar]
- Freeman M. F., Tukey J. W. (1950). Transformations related to the angular and the square root. Ann. Math. Statist. 21, 607–611 10.1214/aoms/1177729756 [DOI] [Google Scholar]
- Freire C., Koifman S. (2012). Pesticide exposure and Parkinson's disease: epidemiological evidence of association. Neurotoxicology 33, 947–971 10.1016/j.neuro.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Gautier V., Le H. T., Malki A., Messaoudi N., Caldas T., Kthiri F., Landoulsi A., Richarme G. (2012). YajL, the prokaryotic homolog of the Parkinsonism-associated protein DJ-1, protects cells against protein sulfenylation. J. Mol. Biol. 421, 662–670 10.1016/j.jmb.2012.01.047 [DOI] [PubMed] [Google Scholar]
- Giaime E., Yamaguchi H., Gautier C. A., Kitada T., Shen J. (2012). Loss of DJ-1 does not affect mitochondrial respiration but increases ROS production and mitochondrial permeability transition pore opening. PLoS ONE 7, e40501 10.1371/journal.pone.0040501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille G., Hung S. T., Reichmann H., Rausch W. D. (2004). Oxidative stress to dopaminergic neurons as models of Parkinson's disease. Ann. N. Y. Acad. Sci. 1018, 533–540 10.1196/annals.1296.066 [DOI] [PubMed] [Google Scholar]
- Hao L. Y., Giasson B. I., Bonini N. M. (2010). DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc. Natl. Acad. Sci. USA 107, 9747–9752 10.1073/pnas.0911175107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasim S., Hussin N. A., Alomar F., Bidasee K. R., Nickerson K. W., Wilson M. A. (2014). A glutathione-independent glyoxalase of the DJ-1 superfamily plays an important role in managing metabolically generated methylglyoxal in Candida albicans. J. Biol. Chem. 289, 1662–1674 10.1074/jbc.M113.505784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry H., Marmy Conus N., Steenhout P., Béguin A., Boulat O. (2012). Sensitive determination of D-lactic acid and L-lactic acid in urine by high-performance liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 26, 425–428 10.1002/bmc.1681 [DOI] [PubMed] [Google Scholar]
- Heo J. Y., Park J. H., Kim S. J., Seo K. S., Han J. S., Lee S. H., Kim J. M., Park J. I., Park S. K., Lim K. et al. (2012). DJ-1 null dopaminergic neuronal cells exhibit defects in mitochondrial function and structure: involvement of mitochondrial complex I assembly. PLoS ONE 7, e32629 10.1371/journal.pone.0032629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrcher I., Aleyasin H., Seifert E. L., Hewitt S. J., Chhabra S., Phillips M., Lutz A. K., Rousseaux M. W., Bevilacqua L., Jahani-Asl A. et al. (2010). Loss of the Parkinson's disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 19, 3734–3746 10.1093/hmg/ddq288 [DOI] [PubMed] [Google Scholar]
- Junn E., Jang W. H., Zhao X., Jeong B. S., Mouradian M. M. (2009). Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J. Neurosci. Res. 87, 123–129 10.1002/jnr.21831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp F., Exner N., Lutz A. K., Wender N., Hegermann J., Brunner B., Nuscher B., Bartels T., Giese A., Beyer K. et al. (2010). Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J. 29, 3571–3589 10.1038/emboj.2010.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen N. J., Ambrosi G., Mullett S. J., Berman S. B., Hinkle D. A. (2011). DJ-1 knock-down impairs astrocyte mitochondrial function. Neuroscience 196, 251–264 10.1016/j.neuroscience.2011.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Song J., Kwon K., Jang S., Kim C., Baek K., Kim J., Park C. (2012). Human DJ-1 and its homologs are novel glyoxalases. Hum. Mol. Genet. 21, 3215–3225 10.1093/hmg/dds155 [DOI] [PubMed] [Google Scholar]
- Li J., Liu D., Sun L., Lu Y., Zhang Z. (2012). Advanced glycation end products and neurodegenerative diseases: mechanisms and perspective. J. Neurol. Sci. 317, 1–5 10.1016/j.jns.2012.02.018 [DOI] [PubMed] [Google Scholar]
- Locasale J. W. (2013). Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 10.1038/nrc3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N., Sato S., Shiba K., Okatsu K., Saisho K., Gautier C. A., Sou Y. S., Saiki S., Kawajiri S., Sato F. et al. (2010). PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 189, 211–221 10.1083/jcb.200910140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash V., Entchev E. V., Mende F., Wilsch-Bräuninger M., Thiele C., Schmidt A. W., Knölker H. J., Ward S., Kurzchalia T. V. (2004). Sterol-derived hormone(s) controls entry into diapause in Caenorhabditis elegans by consecutive activation of DAF-12 and DAF-16. PLoS Biol. 2, e280 10.1371/journal.pbio.0020280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S., Somayajulu M., Sikorska M., Borowy-Borowski H., Pandey S. (2004). Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol. Appl. Pharmacol. 201, 21–31 10.1016/j.taap.2004.04.019 [DOI] [PubMed] [Google Scholar]
- Misra K., Banerjee A. B., Ray S., Ray M. (1995). Glyoxalase III from Escherichia coli: a single novel enzyme for the conversion of methylglyoxal into D-lactate without reduced glutathione. Biochem. J. 305, 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsopoulos P., Suntres Z. E. (2011). Protective effects of liposomal n-acetylcysteine against paraquat-induced cytotoxicity and gene expression. J. Toxicol. 2011, 808967 10.1155/2011/808967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizote T., Tsuda M., Nakazawa T., Nakayama H. (1996). The thiJ locus and its relation to phosphorylation of hydroxymethylpyrimidine in Escherichia coli. Microbiology 142, 2969–2974 10.1099/13500872-142-10-2969 [DOI] [PubMed] [Google Scholar]
- Nagakubo D., Taira T., Kitaura H., Ikeda M., Tamai K., Iguchi-Ariga S. M., Ariga H. (1997). DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem. Biophys. Res. Commun. 231, 509–513 10.1006/bbrc.1997.6132 [DOI] [PubMed] [Google Scholar]
- Palmeira C. M., Moreno A. J., Madeira V. M. (1995). Mitochondrial bioenergetics is affected by the herbicide paraquat. Biochim. Biophys. Acta 1229, 187–192 10.1016/0005-2728(94)00202-G [DOI] [PubMed] [Google Scholar]
- Pan-Montojo F., Schwarz M., Winkler C., Arnhold M., O'Sullivan G. A., Pal A., Said J., Marsico G., Verbavatz J. M., Rodrigo-Angulo M. et al. (2012). Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2, 898 10.1038/srep00898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Lee S. B., Lee S., Kim Y., Song S., Kim S., Bae E., Kim J., Shong M., Kim J. M. et al. (2006). Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441, 1157–1161 10.1038/nature04788 [DOI] [PubMed] [Google Scholar]
- Pendergrass W., Wolf N., Poot M. (2004). Efficacy of MitoTracker Green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry 61A, 162–169 10.1002/cyto.a.20033 [DOI] [PubMed] [Google Scholar]
- Rawson R. L., Yam L., Weimer R. M., Bend E. G., Hartwieg E., Horvitz H. R., Clark S. G., Jorgensen E. M. (2014). Axons degenerate in the absence of mitochondria in C. elegans. Curr. Biol. 24, 760–765 10.1016/j.cub.2014.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray M., Ray S. (1998). Methylglyoxal: from a putative intermediate of glucose breakdown to its role in understanding that excessive ATP formation in cells may lead to malignancy. Curr. Sci. 75, 103–113. [Google Scholar]
- Sai Y., Zou Z., Peng K., Dong Z. (2012). The Parkinson's disease-related genes act in mitochondrial homeostasis. Neurosci. Biobehav. Rev. 36, 2034–2043 10.1016/j.neubiorev.2012.06.007 [DOI] [PubMed] [Google Scholar]
- Schapira A. H. (2012). Mitochondrial diseases. Lancet 379, 1825–1834 10.1016/S0140-6736(11)61305-6 [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Dexter D., Jenner P., Clark J. B., Marsden C. D. (1989). Mitochondrial complex I deficiency in Parkinson's disease. Lancet 333, 1269 10.1016/S0140-6736(89)92366-0 [DOI] [PubMed] [Google Scholar]
- Shendelman S., Jonason A., Martinat C., Leete T., Abeliovich A. (2004). DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2, e362 10.1371/journal.pbio.0020362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley S. T., Reers M., Mottola-Hartshorn C., Lin M., Chen A., Smith T. W., Steele G. D., Jr, Chen L. B. (1991). Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. USA 88, 3671–3675 10.1073/pnas.88.9.3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D. D., Shults C. W., Sisk A., Rockenstein E., Masliah E. (2004). Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp. Neurol. 186, 158–172 10.1016/S0014-4886(03)00342-X [DOI] [PubMed] [Google Scholar]
- Thornalley P. J. (2003). Glyoxalase I – structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 31, 1343–1348 10.1042/BST0311343 [DOI] [PubMed] [Google Scholar]
- Tibbetts A. S., Appling D. R. (2010). Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 30, 57–81 10.1146/annurev.nutr.012809.104810 [DOI] [PubMed] [Google Scholar]
- Ueda T., Hirai K., Ogawa K. (1985). Effects of paraquat on the mitochondrial structure and Ca-ATPase activity in rat hepatocytes. J. Electron Microsc. (Tokyo) 34, 85–91. [PubMed] [Google Scholar]
- Wang X., Yan M. H., Fujioka H., Liu J., Wilson-Delfosse A., Chen S. G., Perry G., Casadesus G., Zhu X. (2012). LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum. Mol. Genet. 21, 1931–1944 10.1093/hmg/dds003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Hekimi S. (2010). A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 8, e1000556 10.1371/journal.pbio.1000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.