Abstract
Background
Pneumococcal infections are a significant cause of morbidity and mortality, and young infants are particularly vulnerable to infection. Maternal immunization can protect infants, but there are limited data on the duration of pneumococcal vaccine antibody in pregnant women. We report on maternal antibody concentrations one year after immunization with 23-valent pneumococcal polysaccharide (23vPPS) vaccine.
Method
The Mother's Gift study randomly assigned 340 pregnant Bangladeshi mothers between ages 18 and 36 to receive either inactivated influenza vaccine (Fluarix®) or the 23vPPS vaccine (Pneumovax®) during the third trimester. Sera were collected before immunization, at delivery, and at one year post-delivery. We determined anti-capsular IgG antibody to 9 pneumococcal serotypes by a multiplex Luminex ELISA. We report antibody geometric mean concentrations (GMCs) for 9 serotypes, 12 month/delivery geometric mean ratios (GMRs) and proportions seroprotected (>0.35 mcg/mL) in 23vPPS vaccine recipients and controls at delivery and at 12 months.
Results
Among pneumococcal vaccinees, GMCs remained stable, with an overall 12 month/delivery GMR of 0.83 (95% CI, 0.75–0.92). In the control group, GMCs increased with a mean ratio of 1.98 (95% CI, 1.81–2.17; P < 0.0001). GMCs in these vaccinees did not decline significantly in the 12 months after antenatal immunization.
Conclusion
GMCs in these adult vaccinees and controls did not decline significantly in the 12 months after antenatal immunization. Interestingly, mothers who did not receive 23vPPS in pregnancy show a substantial increase of GMC for most serotypes in the first year after immunization. Further studies are needed to determine the need for repeat doses of 23vPPS vaccine in subsequent pregnancies more than a year later.
Keywords: Pneumococcal vaccine, Maternal immunization, Antibody titers
1. Background
Streptococcus pneumoniae remains an important cause of pneumonia, meningitis, and bacteremia, especially in resource-limited countries. The World Health Organization estimates an annual mortality secondary to pneumococcal disease of 1.6 million people, and children less than two years of age and elderly people carry the major burden of disease [1]. Rates of pneumococcal disease are particularly high in young infants. Some regions demonstrate invasive pneumococcal incidence rates of up to 363 cases per 100,000 children 2–5 months of age [2]. In Burkina Faso and Togo, 35% of acute bacterial meningitis cases in infants less than one year of age were due to S. pneumoniae [3]. Similar proportions of pneumococcal meningitis cases affect infants in East Africa and Mozambique [4]. Unfortunately, although pneumococcal disease is frequent in this young age group, a recent report of vaccine trials of conjugate pneumococcal vaccine given in infancy show that there is no reduction of pneumococcal disease in vaccinees before six months of age [2]. The strategy of maternal antepartum immunization has been suggested as an approach to protect young infants from pneumococcal disease, similar to the strategy of maternal antepartum tetanus immunization to prevent tetanus in the new-born and young infant, followed by active immunization of the infant [5].
Maternal immunization can protect young infants against tetanus and influenza [6], but there are limited data on pneumococcal immunization in pregnant women. A few studies have demonstrated that maternal immunization with pneumococcal vaccine can provide increased infant antibody concentrations and decreased nasophargyngeal colonization of infants [5,7,8], though a study from Brazil did not demonstrate a decrease in infant colonization with pneumococcus after maternal immunization [9]. Maternal immunization with the polysaccharide pneumococcal vaccine increases pneumococcal antibody concentrations in breast milk [5,10], but there are limited data on duration of elevated maternal pneumococcal serum antibody levels in vaccinated pregnant women. It is not clear if pneumococcal immunization is needed with each pregnancy to assure that antibodies are transferred to the neonate. This would be important information to have, because pneumococcal immunization could be added to routine antepartum tetanus toxoid programs. Santosham et al. reported that women immunized before pregnancy did not have significantly elevated concentrations of pneumococcal antibody at delivery, and their infants had pneumococcal antibody concentrations similar to those of infants born to unimmunized mothers [11]. We investigated maternal pneumococcal antibody concentrations for 12 months after delivery among Asian women immunized with 23vPPS vaccine during the third trimester, in order to define the duration of likely passive protection in young infants and need for re-vaccination of mothers.
2. Methods
2.1. Study design
We conducted a prospective, individually randomized, double-blinded, parallel group trial to assess antibody concentrations in South Asian women who were vaccinated with either 23vPPS vaccine or inactivated trivalent influenza vaccine (control) during the third trimester and were followed for one year after delivery. Detailed clinical methods and statistical analyses for the trial have been described [6]. The current analysis reports the levels of anti-capsular IgG antibodies to 9 pneumococcal serotypes.
This study was conducted using sera obtained from pregnant women in the Mother's Gift study (ClinicalTrials.gov number, NCT00142389) [6]. Briefly, we recruited mothers at three clinics in Dhaka, Bangladesh, during the third trimester of pregnancy. After obtaining written informed consent, we randomly assigned 340 pregnant women aged 18–36 to receive either 23vPPS or influenza vaccine (control) during the third trimester of pregnancy.
The randomization sequence was computer-generated, stratified according to clinic, and blocked in groups of four; sequentially numbered opaque envelopes with data regarding assignments to study groups were provided to each clinic. Mothers, families, and study staff who collected data were unaware of the study-group assignments. Blood was collected from mothers before immunization, at delivery, and at approximately one year post delivery.
The project protocol was reviewed and approved by the institutional review boards at the International Centre for Diarrheal Disease Research, Bangladesh, and the Bloomberg School of Public Health at Johns Hopkins University, Baltimore. Use of study vaccines was approved by the Directorate of Drug Administration, the Government of the People's Republic of Bangladesh.
2.2. Study vaccines
Mothers were randomly assigned to receive one dose of the 23-valent polysaccharide pneumococcal vaccine, Pneumovax® (lot number, 0987N; Merck & Co., Inc.) or the inactivated influenza virus vaccine, Fluarix® (lot number, AFLUA004BC; GlaxoSmithKline Biologicals). Fluarix® contained the WHO-recommended influenza antigens for the southern hemisphere: A/New Caledonia (H1N1), A/Fujian (H3N2), and B/Hong Kong. All study vaccines were purchased from the manufacturers. The Pneumovax® was given subcutaneously with a 0.5 cm insulin needle, while the Fluarix® was given intramuscularly with a 1.5 cm needle. All women received tetanus toxoid vaccines at the time of vaccine administration. There were no cold chain or storage effects, as all vaccines were stored and administered in the clinic. All vaccine recipients were healthy, pregnant Bangladeshi women with no underlying diseases and no history of drug or tobacco use.
2.3. Antibody assay
Blood samples were collected by venipuncture from all participants at birth, and at one year post delivery. Anti-capsular IgG antibodies to 9 of the 23 serotypes were determined by a multiplex Luminex enzyme-linked immunosorbent assay (ELISA) [12].
2.4. Statistical analysis
We calculated geometric mean concentrations (GMC) and geometric mean ratios (GMR) for sixty 23vPPS recipients and sixty controls (influenza vaccine recipients) for 9 selected serotypes. As a way of summarizing change in antibody concentrations over time, GMRs represent the ratio of GMCs of antibodies at delivery to GMCs 12 months after delivery. For a comparison of pneumococcal vaccinees and controls, we used the Student's t test to compare the antibody GMC and the 12 month/delivery GMR. We also compared the proportions of pneumococcal vaccinees and controls with antibody levels ≥0.35 mcg/mL, which may correlate with protection against pneumococcal disease [13], for each serotype at delivery and 12 months post-partum using two-sided chi-square and Fisher's exact tests.
3. Results
3.1. Antibody concentrations
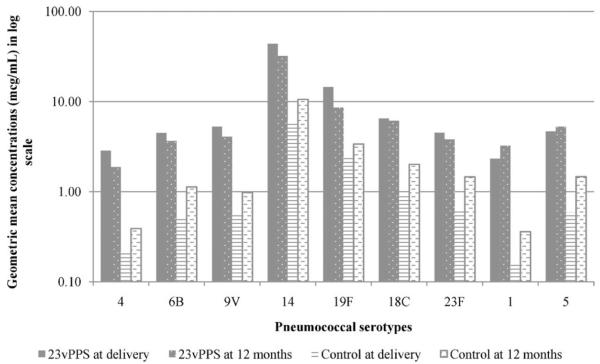
Antibody concentrations before immunization were similar among both groups (P ≥ 0.4) but significantly different at delivery (P < 0.0001) and at 12 months post-delivery (P ≤ 0.0013). Among pneumococcal vaccinees, there was an overall slight decrease in antibody concentrations between delivery and 12 months postpartum. Antibody concentrations against serotypes 1 and 5 increased between delivery and 12 months (Fig. 1) with GMRs of 1.32 and 1.09, respectively. Antibodies to the other seven serotypes decreased during the one-year time period with a mean 12-month to delivery GMR for all 9 serotypes of 0.83 (95% CI, 0.75–0.92). Among the mothers who did not receive pneumococcal vaccine, GMCs for all serotypes doubled (Fig. 1), with a mean 12-month to delivery GMR of 1.98 (P < 0.0001 compared to pneumococcal vaccine group; 95% CI, 1.81–2.17). The rises in antibody levels in the control group were statistically significant (P < 0.05) for all serotypes (Table 1).
Fig. 1.
Geometric mean concentrations in pneumococcal vaccinees and controls (influenza vaccinees) at delivery vs. 12 months post-delivery. Abbreviations: 23vPPS, 23-valent pneumococcal polysaccharide.
Table 1.
12 Month/delivery geometric mean ratios of antibody titers and seroprotection at delivery vs. 12 months post-delivery by pneumococcal serotype.
| Percent of mothers with titers >0.35 mcg/mL |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 12 month/delivery geometric mean ratios |
Delivery |
12 months post-delivery |
|||||||
| Serotype | 23vPPS vaccinees | Controls | P value | 23vPPS | Control | P value | 23vPPS | Control | P value |
| 4 | 0.67 | 1.76 | 0.0003 | 86.4 | 30.0 | <0.0001 | 88.3 | 55.0 | <0.0001 |
| 6B | 0.80 | 2.16 | <0.0001 | 91.5 | 61.7 | 0.0001 | 95.0 | 90.0 | 0.2985 |
| 9V | 0.77 | 1.80 | <0.0001 | 96.6 | 71.7 | 0.0002 | 96.7 | 93.3 | 0.4022 |
| 14 | 0.72 | 1.74 | <0.0001 | 93.2 | 66.7 | 0.0003 | 90.0 | 85.0 | 0.4076 |
| 19F | 0.58 | 1.44 | 0.0001 | 91.7 | 40.0 | <0.0001 | 85.0 | 53.3 | 0.0002 |
| 18C | 0.93 | 2.18 | <0.0001 | 88.3 | 51.7 | <0.0001 | 86.7 | 73.3 | 0.0679 |
| 23F | 0.82 | 2.28 | <0.0001 | 90.0 | 65.0 | 0.001 | 95.0 | 78.3 | 0.0072 |
| 1 | 1.32 | 2.25 | 0.0162 | 100.0 | 96.7 | 0.1538 | 100.0 | 100.0 | N/A |
| 5 | 1.10 | 2.47 | <0.0001 | 100.0 | 85.0 | 0.0018 | 98.3 | 95.0 | 0.3091 |
| Mean GMR | 0.83 | 1.98 | <0.0001 | ||||||
Abbreviations: 23vPPS, 23-valent pneumococcal polysaccharide; GMR, geometric mean ratio.
3.2. Seroprotective titers
The proportion of subjects at one year with seroprotective titers >0.35 mcg/mL for individual serotypes ranged from 85 to 100% for the vaccinated mothers and from 53 to 100% for the control mothers (Table 1). The percentage of mothers in the control group with antibody levels above the 0.35 mcg/mL threshold increased during the 12 month postpartum period. Percentage increase from delivery to 12 months post-delivery ranged from 3.4% to 83.3% for individual serotypes.
4. Discussion
Our study examined maternal pneumococcal antibody concentrations during the postpartum year after immunization of pregnant Asian women with either 23vPPS vaccine or influenza vaccine. To our knowledge, this is the first report of pneumococcal immunization in pregnant women with follow-up of vaccine recipients more than 12 months after immunization. Antibody concentrations in vaccinees did not decline substantially during the 12 months after antenatal immunization, in contrast to previous studies that demonstrated a significant decline in post-immunization anti-pneumococcal antibodies in adults over one to two years [11,14]. Musher et al. demonstrated a post-immunization decline of anti-pneumococcal antibody responses in adults greater than 50 years of age [14]. Primary immunization or re-immunization of the adult subjects with 23vPPS resulted in significant increases in IgG to all serotypes peaking at 30–60 days after immunization and declining over the next one to two years. However, the durability of antibody responses in later years of life may not apply to young adult women.
Santosham, et al. vaccinated non-pregnant, healthy women 18–40 years of age from the Gila River Indian Community in Arizona in a study of antibody persistence following pneumococcal polysaccharide or Haemophilus influenzae type b (Hib) immunization [11]. Women were vaccinated prior to pregnancy and followed through pregnancy for a total observation period of 37 months from the time of immunization. Post-immunization pneumococcal antibodies declined 30–60% in pregnant women and their infants. Infants born to women immunized pre-partum with pneumococcal vaccine had pneumococcal antibody concentrations similar to those of infants born to unimmunized mothers.
Interestingly, in the current study, women who did not receive 23vPPS during pregnancy showed a substantial increase of GMC for most serotypes in the first year after immunization. This suggests that natural exposure to their infants colonized with pneumococcus stimulates maternal antibody production. However, infants in the Mother's Gift study were randomized to receive either pneumococcal conjugate vaccine or Haemophilus influenzae type b (Hib) conjugate vaccine, potentially altering the acquisition of vaccine serotypes in mothers. Similar maternal antibody findings were demonstrated by Goldblatt et al. in the United Kingdom [15]; these family studies showed substantial increases in maternal antibody levels in association with infant colonization. In 121 families participating in the study, 3767 nasopharyngeal swabs were collected, revealing 932 (25%) culture-positive for S. pneumoniae in all family members. Mean carriage was highest (52%) in children 0–2 years of age, and documented carriage of a particular serotype in any one family member resulted in a significant increase in the antibody concentration for that serotype in adults living in the same household.
Our study had several potential limitations. Data from this South Asian setting may have limited generalizability, as S.pneumoniae appears to colonize infants at much earlier ages in low-resource environments [2,3]. The high prevalence of maternal and infant maternal nasal colonization may affect serum antibody concentrations. We are currently awaiting further analysis of maternal and infant nasal colonization data from the Mother's Gift study to evaluate nasal pneumococcal colonization rates in this tropical setting.
Maternal immunization with 23vPPS increases anti-pneumococcal antibody concentrations, and while the maternal antibody concentrations decline slightly, they tend to be maintained above putative protective antibody thresholds for at least one year. The overall concentration of antibodies in the pneumococcal immunization group was higher than that in controls throughout the study period. These data of sustained presence of maternal serum anti-pneumococcal antibodies for at least one year in both vaccination groups suggest that further studies are needed to determine the need for repeat doses of 23vPPS vaccine in subsequent pregnancies more than a year later. Because hyporesponsiveness to previous polysaccharide vaccine has been reported after repeat immunization of adults, additional data are needed to determine the optimal interval for maternal antenatal immunization. We have undertaken an evaluation of an additional 23vPPS dose several years after the antenatal dose.
Acknowledgements
We thank all of the women and infants who participated in the trial; the Ethical Review Committee at the International Centre for Diarrheal Disease Research, Bangladesh; and the staff of the International Centre for Diarrheal Disease Research, Bangladesh.
References
- [1].WHO Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly Epidemiol Rec. 2007;82:93–104. [PubMed] [Google Scholar]
- [2].Enwere G, Biney E, Cheung YB, Zaman SM, Okoko B, Oluwalana C, et al. Epidemiologic and clinical characteristics of community-acquired invasive bacterial infections in children aged 2–29 months in The Gambia. Pediatr Infect Dis J. 2006;25:700–5. doi: 10.1097/01.inf.0000226839.30925.a5. [DOI] [PubMed] [Google Scholar]
- [3].Traore Y, Tameklo TA, Njanpop-Lafourcade BM, Lourd M, Yaro S, Niamba D, et al. Incidence, seasonality, age distribution, and mortality of pneumococcal meningitis in Burkina Faso and Togo. Clin Infect Dis. 2009;48(Suppl 2):S181–9. doi: 10.1086/596498. [DOI] [PubMed] [Google Scholar]
- [4].Mudhune S, Wamae M. Report on invasive disease and meningitis due to Haemophilus influenzae and Streptococcus pneumonia from the Network for Surveillance of Pneumococcal Disease in the East African Region. Clin Infect Dis. 2009;48(Suppl 2):S147–52. doi: 10.1086/596494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shahid NS, Steinhoff MC, Hoque SS, Begum T, Thompson C, Siber GR. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet. 1995;346:1252–7. doi: 10.1016/s0140-6736(95)91861-2. [DOI] [PubMed] [Google Scholar]
- [6].Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- [7].Francis JP, Richmond PC, Pomat WS, Michael A, Keno H, Phuanukoonnon S, et al. Maternal antibodies to pneumolysin but not to pneumococcal surface protein A delay early pneumococcal carriage in high-risk Papua New Guinean infants. Clin Vaccine Immunol. 2009;16:1633–8. doi: 10.1128/CVI.00247-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Quiambao BP, Nohynek HM, Kayhty H, Ollgren JP, Gozum LS, Gepanayao CP, et al. Immunogenicity and reactogenicity of 23-valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine. 2007;25:4470–7. doi: 10.1016/j.vaccine.2007.03.021. [DOI] [PubMed] [Google Scholar]
- [9].Lopes CR, Berezin EN, Ching TH, Canuto Jde S, Costa VO, Klering EM. Ineffectiveness for infants of immunization of mothers with pneumococcal capsular polysaccharide vaccine during pregnancy. Braz J Infect Dis. 2009;13:104–6. [PubMed] [Google Scholar]
- [10].Munoz FM, Englund JA, Cheesman CC, Maccato ML, Pinell PM, Nahm MH, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine. 2001;20:826–37. doi: 10.1016/s0264-410x(01)00397-8. [DOI] [PubMed] [Google Scholar]
- [11].Santosham M, Englund JA, McInnes P, Croll J, Thompson CM, Croll L, et al. Safety and antibody persistence following Haemophilus influenzae type b conjugate or pneumococcal polysaccharide vaccines given before pregnancy in women of childbearing age and their infants. Pediatr Infect Dis J. 2001;20:931–40. doi: 10.1097/00006454-200110000-00005. [DOI] [PubMed] [Google Scholar]
- [12].Whaley MJ, Rose C, Martinez J, Laher G, Sammons DL, Smith JP, et al. Inter-laboratory comparison of three multiplexed bead-based immunoassays for measuring serum antibodies to pneumococcal polysaccharides. Clin Vaccine Immunol. 2010;17:862–9. doi: 10.1128/CVI.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Siber GR, Chang I, Baker S, Fernsten P, O'Brien KL, Santosham M, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007;25:3816–26. doi: 10.1016/j.vaccine.2007.01.119. [DOI] [PubMed] [Google Scholar]
- [14].Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, et al. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201:516–24. doi: 10.1086/649839. [DOI] [PubMed] [Google Scholar]
- [15].Goldblatt D, Hussain M, Andrews N, Ashton L, Virta C, Melegaro A, et al. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J Infect Dis. 2005;192:387–93. doi: 10.1086/431524. [DOI] [PubMed] [Google Scholar]