Abstract
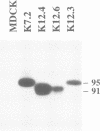
Wild-type human transferrin receptor (hTfR), like endogenous canine receptor, is expressed almost exclusively (97%) at the basolateral membrane of transfected Madin-Darbey canine kidney (MDCK) cells. We investigated the role of two distinct features of the hTfR cytoplasmic domain, namely the endocytic signal and the unique phosphorylation site, in polarized cell surface delivery. Basolateral location was not altered by point mutation of Ser24-->Ala24, indicating that phosphorylation is not involved in vectorial sorting of hTfR. The steady state distribution of hTfR was partially affected by a deletion of 36 cytoplasmic residues encompassing the internalization sequence. However, 80% of the receptors were still basolateral. As assessed by pulse-chase experiments in combination with biotinylation, newly synthesized wild-type and deletion mutant receptors were directly sorted to the domain of their steady state residency. Although both receptors could bind human transferrin, endocytosis of the deletion mutant was strongly impaired at either surface. These data indicate that the predominant basolateral targeting signal of hTfR is independent of the internalization sequence.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartles J. R., Feracci H. M., Stieger B., Hubbard A. L. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol. 1987 Sep;105(3):1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer C. B., Roth M. G. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991 Aug;114(3):413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Crise B., Rose J. K. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989 Sep 29;245(4925):1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Brändli A. W., Parton R. G., Simons K. Transcytosis in MDCK cells: identification of glycoproteins transported bidirectionally between both plasma membrane domains. J Cell Biol. 1990 Dec;111(6 Pt 2):2909–2921. doi: 10.1083/jcb.111.6.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan M. J., Anderson H. C., Palade G. E., Jamieson J. D. Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell. 1986 Aug 15;46(4):623–631. doi: 10.1016/0092-8674(86)90888-3. [DOI] [PubMed] [Google Scholar]
- Casanova J. E., Apodaca G., Mostov K. E. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991 Jul 12;66(1):65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Casanova J. E., Breitfeld P. P., Ross S. A., Mostov K. E. Phosphorylation of the polymeric immunoglobulin receptor required for its efficient transcytosis. Science. 1990 May 11;248(4956):742–745. doi: 10.1126/science.2110383. [DOI] [PubMed] [Google Scholar]
- Collawn J. F., Stangel M., Kuhn L. A., Esekogwu V., Jing S. Q., Trowbridge I. S., Tainer J. A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990 Nov 30;63(5):1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- Compton T., Ivanov I. E., Gottlieb T., Rindler M., Adesnik M., Sabatini D. D. A sorting signal for the basolateral delivery of the vesicular stomatitis virus (VSV) G protein lies in its luminal domain: analysis of the targeting of VSV G-influenza hemagglutinin chimeras. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4112–4116. doi: 10.1073/pnas.86.11.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., Johnson G. L., Kelleher D. J., Anderson J. K., Mole J. E., Czech M. P. Identification of serine 24 as the unique site on the transferrin receptor phosphorylated by protein kinase C. J Biol Chem. 1986 Jul 5;261(19):9034–9041. [PubMed] [Google Scholar]
- Fuller S. D., Simons K. Transferrin receptor polarity and recycling accuracy in "tight" and "leaky" strains of Madin-Darby canine kidney cells. J Cell Biol. 1986 Nov;103(5):1767–1779. doi: 10.1083/jcb.103.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S., von Bonsdorff C. H., Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984 Aug;38(1):65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- Gironès N., Alverez E., Seth A., Lin I. M., Latour D. A., Davis R. J. Mutational analysis of the cytoplasmic tail of the human transferrin receptor. Identification of a sub-domain that is required for rapid endocytosis. J Biol Chem. 1991 Oct 5;266(28):19006–19012. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M., Cotner T., Mann D. L., Eisenbarth G. S., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J Immunol. 1981 Jul;127(1):347–351. [PubMed] [Google Scholar]
- Hopkins C. R. Polarity signals. Cell. 1991 Sep 6;66(5):827–829. doi: 10.1016/0092-8674(91)90427-z. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Harter C., Matter K., Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991 Sep 6;66(5):907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- Iacopetta B. J., Morgan E. H., Yeoh G. C. Receptor-mediated endocytosis of transferrin by developing erythroid cells from the fetal rat liver. J Histochem Cytochem. 1983 Feb;31(2):336–344. doi: 10.1177/31.2.6300220. [DOI] [PubMed] [Google Scholar]
- Iacopetta B. J., Rothenberger S., Kühn L. C. A role for the cytoplasmic domain in transferrin receptor sorting and coated pit formation during endocytosis. Cell. 1988 Aug 12;54(4):485–489. doi: 10.1016/0092-8674(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Jing S. Q., Spencer T., Miller K., Hopkins C., Trowbridge I. S. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990 Feb;110(2):283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn L. C., Barbosa J. A., Kamarck M. E., Ruddle F. H. An approach to the cloning of cell surface protein genes. Selection by cell sorting of mouse L-cells that express HLA or 4F2 antigens after transformation with total human DNA. Mol Biol Med. 1983 Oct;1(3):335–352. [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Quaroni A., Nichols B., Rodriguez-Boulan E. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line. J Cell Biol. 1990 Oct;111(4):1351–1361. doi: 10.1083/jcb.111.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A., Sambuy Y., Mostov K., Rodriguez-Boulan E. Vectorial targeting of an endogenous apical membrane sialoglycoprotein and uvomorulin in MDCK cells. J Cell Biol. 1990 May;110(5):1533–1539. doi: 10.1083/jcb.110.5.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A., Sambuy Y., Patzak A., Patil N., Chao M., Rodriguez-Boulan E. An internal deletion in the cytoplasmic tail reverses the apical localization of human NGF receptor in transfected MDCK cells. J Cell Biol. 1991 Nov;115(3):607–618. doi: 10.1083/jcb.115.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Caras I. W., Davitz M. A., Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989 Nov;109(5):2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey D., Feracci H., Gorvel J. P., Rigal A., Soulié J. M., Maroux S. Evidence for the transit of aminopeptidase N through the basolateral membrane before it reaches the brush border of enterocytes. J Membr Biol. 1987;96(1):19–25. doi: 10.1007/BF01869331. [DOI] [PubMed] [Google Scholar]
- Matter K., Brauchbar M., Bucher K., Hauri H. P. Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2). Cell. 1990 Feb 9;60(3):429–437. doi: 10.1016/0092-8674(90)90594-5. [DOI] [PubMed] [Google Scholar]
- McClelland A., Kühn L. C., Ruddle F. H. The human transferrin receptor gene: genomic organization, and the complete primary structure of the receptor deduced from a cDNA sequence. Cell. 1984 Dec;39(2 Pt 1):267–274. doi: 10.1016/0092-8674(84)90004-7. [DOI] [PubMed] [Google Scholar]
- McQueen N. L., Nayak D. P., Stephens E. B., Compans R. W. Basolateral expression of a chimeric protein in which the transmembrane and cytoplasmic domains of vesicular stomatitis virus G protein have been replaced by those of the influenza virus hemagglutinin. J Biol Chem. 1987 Nov 25;262(33):16233–16240. [PubMed] [Google Scholar]
- McQueen N., Nayak D. P., Stephens E. B., Compans R. W. Polarized expression of a chimeric protein in which the transmembrane and cytoplasmic domains of the influenza virus hemagglutinin have been replaced by those of the vesicular stomatitis virus G protein. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9318–9322. doi: 10.1073/pnas.83.24.9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., de Bruyn Kops A., Deitcher D. L. Deletion of the cytoplasmic domain of the polymeric immunoglobulin receptor prevents basolateral localization and endocytosis. Cell. 1986 Nov 7;47(3):359–364. doi: 10.1016/0092-8674(86)90592-1. [DOI] [PubMed] [Google Scholar]
- Mostov K., Apodaca G., Aroeti B., Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J Cell Biol. 1992 Feb;116(3):577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Okamoto C. T., Shia S. P., Bird C., Mostov K. E., Roth M. G. The cytoplasmic domain of the polymeric immunoglobulin receptor contains two internalization signals that are distinct from its basolateral sorting signal. J Biol Chem. 1992 May 15;267(14):9925–9932. [PubMed] [Google Scholar]
- Owen D., Kühn L. C. Noncoding 3' sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987 May;6(5):1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. C., Scalera V., Simmons N. L. Identification of two strains of MDCK cells which resemble separate nephron tubule segments. Biochim Biophys Acta. 1981 Feb 18;673(1):26–36. [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Roman L. M., Garoff H. Alteration of the cytoplasmic domain of the membrane-spanning glycoprotein p62 of Semliki Forest virus does not affect its polar distribution in established lines of Madin-Darby canine kidney cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2607–2618. doi: 10.1083/jcb.103.6.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Gundersen D., Patil N., Rodriguez-Boulan E. The large external domain is sufficient for the correct sorting of secreted or chimeric influenza virus hemagglutinins in polarized monkey kidney cells. J Cell Biol. 1987 Mar;104(3):769–782. doi: 10.1083/jcb.104.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberger S., Iacopetta B. J., Kühn L. C. Endocytosis of the transferrin receptor requires the cytoplasmic domain but not its phosphorylation site. Cell. 1987 May 8;49(3):423–431. doi: 10.1016/0092-8674(87)90295-9. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M., Lisanti M., Graeve L., Le Bivic A., Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989 Mar;107(3):277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Schneider C., Owen M. J., Banville D., Williams J. G. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984 Oct 18;311(5987):675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokode M., Pathak R. K., Hammer R. E., Brown M. S., Goldstein J. L., Anderson R. G. Cytoplasmic sequence required for basolateral targeting of LDL receptor in livers of transgenic mice. J Cell Biol. 1992 Apr;117(1):39–46. doi: 10.1083/jcb.117.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- Zurzolo C., Le Bivic A., Quaroni A., Nitsch L., Rodriguez-Boulan E. Modulation of transcytotic and direct targeting pathways in a polarized thyroid cell line. EMBO J. 1992 Jun;11(6):2337–2344. doi: 10.1002/j.1460-2075.1992.tb05293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]