Abstract
As ATP released from astrocytes can modulate many neural signaling systems, the triggers of and pathways for this ATP release are important. Here, the ability of mechanical strain to trigger ATP release through pannexin channels, and the effects of sustained strain on pannexin expression, were examined in rat optic nerve head astrocytes. Astrocytes released ATP when subjected to 5% equibiaxial strain or to hypotonic swelling. While astrocytes expressed mRNA for pannexins 1–3, connexin 43 and VNUT, pharmacological analysis suggested a predominant role for pannexins in mechanosensitive ATP release, with Rho kinases contributing. Astrocytes from panx1−/− mice had reduced baseline and stimulated levels of extracellular ATP, confirming the role for pannexins. Swelling astrocytes triggered a regulatory volume decrease that was inhibited by apyrase or probenecid. The swelling–induced rise in calcium was inhibited by P2X7 receptor antagonists A438079 and AZ10606120, in addition to apyrase and carbenoxelone. Extended stretch of astrocytes in vitro upregulated expression of panx1 and panx2 mRNA. A similar upregulation was observed in vivo in optic nerve head tissue from the Tg-MYOCY437H mouse model of chronic glaucoma; genes for panx1, panx2 and panx3 were increased while immunohistochemistry confirmed increased expression of pannexin 1 protein. In summary, astrocytes released ATP in response to mechanical strain, with pannexin 1 the predominant efflux pathway. Sustained strain upregulated pannexins in vitro and in vivo. Together these findings provide a mechanism by which extracellular ATP remains elevated under chronic mechanical strain, as found in the optic nerve head of patients with glaucoma.
Keywords: mechanosensitive signaling, ATP release, pannexin, P2X7 receptor, glaucoma, cell swelling
Introduction
Identifying the ways in which mechanical strain is translated into physiological signals is of considerable importance as over-simulation of these pathways can lead to pathophysiologic events in disease. Throughout the body, mechanical strain rapidly triggers the release of the transmitter ATP from both neural and non-neural cell types (Burnstock 1999; Grygorczyk et al. 2012). Astrocytes are emerging as central mediators of mechanical strain, and the ability of astrocytes to release ATP upon stretch or swelling has implications for neuronal signaling in various regions (Bennett et al. 2012; Darby et al. 2003; Halassa et al. 2009; Ostrow and Sachs 2005; Perez-Ortiz et al. 2008). However, the pathways involved in this release, the physiological effects of this release, and the consequences of chronic strain are only partially understood.
While astrocytes in many locations are subject to mechanical strain, optic nerve head astrocytes are particularly well-situated to examine the signals induced by mechanical strain. Elevated pressure in a closed system leads to a stretch of cells and their membranes (Landsman et al. 1995). The lamina cribrosa, where the astrocytes reside, is the focal center of mechanical strain produced in the posterior eye upon elevation of intraocular pressure (IOP) in glaucoma (Burgoyne 2011; Sigal and Ethier 2009). As glaucomatous eyes have both an increase in baseline IOP and a increased magnitude in the daily IOP fluctuations (Gao et al. 2012), optic nerve head astrocytes are subject to considerable stretch. These astrocytes have been implicated in the damage to retinal ganglion cell axons in chronic glaucoma (Hernandez et al. 2008; Morgan 2000), although the precise signaling mechanisms linking elevated strain to cellular injury remain unresolved. As moderate neuronal damage may occur over many years, the mechanisms that link the short term responses to mechanical strain with more chronic damage are key to understanding chronic neural degenerative diseases like glaucoma.
Several studies suggest a role for aberrant purinergic signaling in glaucoma. Human patients with either chronic or acute glaucoma have elevated levels of ATP in the anterior chamber relative to non-glaucomatous patients (Li et al. 2011a; Zhang et al. 2007). Mechanical perturbation of retinal Müller cells can evoke ATP release (Newman 2001), while increased ocular pressure triggers a release of ATP from the retina (Resta (Resta et al. 2007; Reigada et al. 2008). In vivo and in vitro studies demonstrate that excessive stimulation of the P2X7 receptor for ATP on retinal ganglion cells can lead to rapid cell death (Hu et al. 2010; Resta et al. 2007; Zhang et al. 2005).
While rapid neuronal death is clearly important, the slow time course of ganglion cell death in the chronic disease suggests sudden neuronal death may not by the primary pathological mechanism. Instead, impaired axonal transport and compression in the unmyelinated axons passing through the lamina cribrosa implicates aberrant axonal signaling in the chronic neuronal degeneration (Howell et al., 2007; Calkins 2012). Within the body of the retina, the ATP release from Müller cells is likely to stimulate receptors on the retina ganglion cell soma, and purinergic signaling in Müller cells has been well described (Bringmann et al. 2002; Keirstead and Miller 1997; Metea and Newman 2006; Newman 2004; Pannicke et al. 2001; Reifel Saltzberg et al. 2003; Wurm et al. 2009). However, the ganglion cell axons would be closer to astrocytes in the optic nerve head, and mechanosensitive ATP release from these astrocytes would be more likely to modulate axonal signaling in glaucoma. As such, identification of the conduit for this ATP release may pinpoint potential targets for intervention.
Both vesicular and channel mediated ATP release from astrocytes have been documented in other tissues (Coco et al. 2003; Pascual et al. 2005). Of the non-vesicular release pathways, connexin hemi-channels, pannexin hemi-channels, maxi-anion channels and CALMH1 channels have all been implicated (Cotrina et al. 1998; Iglesias et al. 2009; Liu et al. 2008; Taruno et al. 2013). As the pannexin hemi-channel has been identified with mechanosensitive ATP release most consistently (Bao et al. 2004; Bruzzone et al. 2005; Dubyak 2009), we asked whether mechanical perturbations could trigger a pannexin-mediated release of ATP from optic nerve head astrocytes, whether this release had functional implications, and tested if sustained mechanical strain led to changes in pannexin expression.
Materials and Methods
Animal care and use
All procedures were performed in strict accordance with the National Research Council’s “Guide for the Care and Use of Laboratory Animals” and were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC). All animals were housed in temperature-controlled rooms on a 12-h light, 12-h dark cycle with food and water ad libitum. Long-Evans rat pups of both sexes, aged postnatal day 2–5, were used as a source of optic nerve head astrocytes. Panx1−/− mice were obtained from Dr. Shestopalov (Dvoriantchikova et al. 2012), and the presence and absence of relevant constructs was confirmed with PCR. Tg-MYOCY437H mice were obtained from Drs. Sheffield and Zode (Zode et al. 2011). IOP was measured from conscious litter mate C57BL/6N and Tg-MYOCY437H mice monthly using a Tonolab rebound tonometer; each value was the mean of 9 measurements from the left and 9 from the right eye, performed between 2 and 5 pm.
Solutions and reagents
The composition of isotonic solution used in our experiments was as follows: (in mM) 105NaCl, 5KCl, 4NaHEPES, 6HEPES acid, 1.3CaCl2, 5glucose, 5NaHCO3, 60mannitol, pH7.4, 298mOsm. The composition of hypotonic solution was almost identical except that the mannitol was omitted. This yielded a solution that measured approximately 227mOsm, using a VAPRO vapor pressure osmometer (Westcor, Inc.). In some experiments, hypotonic solution was created by diluting isotonic solution with dH20 at a ratio of either 1:1 (50% hypotonic) or 1:2.3 (30% hypotonic). The osmolarity of isotonic solution diluted with dH2O was approximately 206mOsm for 30% hypotonic and 148mOsm for 50% hypotonic. All reagents were purchased through Sigma-Aldrich Co. (St. Louis, MO) unless otherwise noted.
Astrocyte cell culture
Primary rat optic nerve head astrocyte (RONHA) cultures were grown according to a protocol modified from Mandal et. al. (2009). Rat pups were sacrificed by PD5, with the optic nerve proximal to the sclera defined as the optic nerve head. This optic nerve head tissue was digested for 1 hr using 0.25% trypsin, with periodic trituration to break up the clump and create a cell suspension. Cells were washed once with Dulbecco's Modified Eagle's Medium (DMEM)/F12 containing 10% fetal bovine serum (FBS) to inactivate the trypsin and re-suspended in medium comprised of DMEM/F12, 10% FBS, 1% penicillin/streptomycin and 25ng/ml epidermal growth factor (EGF) before being plated on T25 cell culture flasks. Cultures were found to contain greater than 97% astrocytes, as defined by GFAP immunofluorescence staining (not shown). Of note, optic nerve astrocytes express more GFAP at baseline than cortical astrocytes, suggesting the cells were not necessarily reactive (Dowling 2011). Cells were trypsinized after 7 days and passaged; while no difference between cells of different passage number was observed, cells were generally used in passage 2–3. Mouse optic nerve head astrocytes were isolated from C57Bl/6J and panx−/− mice at postnatal day 15–20. The isolation procedure to obtain mouse astrocytes was the same protocol as for the rat isolation procedure, except that the optic nerve head tissue was incubated in 0.25% trypsin for only 20–30min.
Hypotonic ATP release experiments
Rat optic nerve head astrocytes were sub-cultured onto plastic 96-well plates and grown until 80% confluent. Before the challenge, cells were pre-incubated in either 50µl of isotonic solution (for control or hypotonic conditions) or 50µl isotonic solution containing an antagonist (hypotonic + drug) for 30 min. After the incubation, 50µl of either isotonic (control), dH2O (to produce a 50% hypotonic solution), or dH2O with antagonist (hypotonic + drug) was added to each well. To prevent ATP degradation, drug solutions contained 100µM of ATPase inhibitors β,γ-methylene ATP (β,γ-mATP) and ARL67156; 20μl of luciferin/luciferase working solution was then added to each well (Stock solution = 450 µl Isotonic + 50 µl water to 1 vial of ATP assay mix; Sigma, Catalog #FLAAM, working solution 100 µl of stock + 2.4 mL of isotonic solution). Luminescence was read 10x on a Luminoskan Ascent luminometer plate reader (ThermoFisher Scientific, MA) with 100 ms integration time per well. The values used here were obtained 1.5–2.5 min after addition of hypotonic solution, as trials indicate ATP levels are at their peak at 2 min and fluctuations from the solution change have subsided. Luminescence values were converted to ATP concentration by means of a standard curve performed separately for each solution used, and corrections were made for the effect of hypotonicity and the various antagonists on the curve as done previously (Reigada et al. 2006).
ATP release from mouse cells followed a similar protocol with the following modifications. C57Bl6J and panx−/− optic nerve head astrocytes (passage 2) were plated in adjacent wells in a 96-well plate and grown to confluence. After washing, 50µl isotonic + 10µl luciferin-luciferase working solution was added to each well. In some wells, 100µM β,γ-mATP was added to inhibit degradation of extracellular ATP by ecto-ATPases. After taking 50 measurements of baseline ATP levels, 50µl water + 10µl luciferase working solution was added using the internal dispensing system. One hundred measurements were recorded of the hypotonic induced ATP release with a 100 ms integration time. As in rat, measurements were made 1.5–2.5 min after addition of hypotonic solution to quantify ATP levels.
Mechanical stretch ATP release experiments
Rat optic nerve head astrocytes were sub-cultured onto sterilized silicone sheets and allowed to grow for 24 hours (Silastic, Speciality Manufacturing, Saginaw, MI). In some cases, media maintaining the cells was removed prior to the experiment and replaced with isotonic buffer solution containing 100µM β,γ-mATP and ARL67156 to prevent ATP degradation. Using a custom-designed apparatus (Winston et al. 1989), astrocytes were subjected to a 5% equibiaxial strain at approximately 0.3 Hz for 2 min. This mild degree of strain was below that calculated as the mean strain present in the lamina cribrosa with a rise in IOP to 50 mm Hg (Sigal and Ethier 2009; Sigal et al. 2005; Sigal et al. 2007); our imposition of cyclical strain is consistent with short term changes in pressure (ocular pulse) observed in primate eyes using remote telemetry (Downs et al. 2011). While the magnitude of this cyclic strain in the living ONH is currently unknown, the imposed values are not inconsistent with computations, accounting for the strain amplification that has been predicted by lamina microarchitecture. After stretch, the silicone sheet was examined to confirm cell attachment, and a 100µl aliquot of the extracellular solution was removed and stored at −80°C until ATP quantification. The ATP concentrations of the samples were determined via the luciferin/luciferase reaction in 96-well plates, as described above.
Lactose dehydrogenase (LDH) cytotoxicity assay
In order to rule out the possibility that increased extracellular ATP was due to cell death and lysis, we utilized a commercially available, colorimetric assay for quantifying cytolysis through LDH activity (Cytotoxicity Detection Kit PLUS LDH, Roche Applied Science, Germany). 100µl samples of the extracellular solution were taken before and after stimulation of cells by hypotonic solution or mechanical stretch as outlined above and measured for LDH activity following the manufacturer’s instructions as described (Xia et al. 2012). LDH activity in each test sample was normalized to ATP levels in lysed cells as a maximum and isotonic solution as a minimal response to calculate percentile as 5=100x(max-test)/(max-min).
PCR/qPCR
For cultured astrocytes, media was aspirated from cultured cells and 1 ml of TRIzol reagent (Invitrogen/Life technologies, NY) was added for stretched cells on silicone supports. For cells in 96-well plates, 50µl Trizol was added per well. Total RNA was extracted using the protocol recommended by the manufacturer. For 96-well plates, 20 samples were combined. RNA concentration and purity was assessed using a Nanodrop spectrophotometer (ThermoFisher Scientific). To create cDNA for PCR, 1 µg of total RNA was reverse transcribed using the First Strand Synthesis Kit (Applied Biosystems) using the manufacturer’s instructions. PCR was performed using the Taq PCR Core Kit (Qiagen) and a thermocycler (Applied Biosystems). Primer sequences and expected product sizes are listed in Table 1. Primers were designed in house using Primer 3 software (http://primer3.wi.mit.edu/). PCR products were visualized on a 2.0% agarose gel in TAE buffer using ethidium bromide to stain DNA.
Table 1.
List of PCR primers used in the study.
| Gene Name (NCBI Accession #) |
Species | Sequence (5’ → 3’) | Expected Product Size (bp) |
|---|---|---|---|
| NTPdase1 (NM_022587.1) |
Rat | F: AGGAGCCTGAAGAGCTACCC R: GTCTGATTTAGGGGCACGAA |
224 |
| P2X7 (NM_019256.1) |
Rat | F: GGAAGATCCGGAAGGAGTTC R: GACATGGACGGGAGAGAAAA |
392 |
| Pannexin1 (NM_199397.2) |
Rat | F: GCTGTGGGCCATTATGTCTT R: GCAGCCAGAGAATGGACTTC |
234 |
| Pannexin2 (NM_199409.2) |
Rat | F: CAAGGGGAGTGGAGGTGATA R: GTGGGGTATGGGATTTCCTT |
264 |
| Pannexin3 (NM_199398.1) |
Rat | F: TTTCCCTTGCTAGAGCGGTA R: GGGGCTCTAGAAGGCTCTGT |
104 |
| Connexin43 (NM_012567.2) |
Rat | F: TCCTTGGTGTCTCTCGCTTT R: GAGCAGCCATTGAAGTAGGC |
167 |
| β-actin (NM_031144.3) |
Rat | F: AGCCATGTACGTAGCCATCC R: ACCCTCATAGATGGGCACAG |
115 |
| GFAP (NM_017009.2) |
Rat | F: GCAGGAGTACCAGGATCTAC R: ATCTGGAGGTTGGAGAAAGT |
129 |
| VNUT (NM_001108613.1) |
Rat | F: GGAACTCCACCTCGGATGTA R: GTTTTGAACTGTGGGCCTGT |
396 |
| β-actin (NM_007393.3) |
Mouse | F: TTGCTGACAGGATGCAGAAG R: ACATCTGCTGGAAGGTGGAC |
141 |
| Pannexin 1 (NM_019482.2) |
Mouse | F: GGCCACGGAGTATGTGTTCT R: TACAGCAGCCCAGCAGTATG |
247 |
| Pannexin 2 (NM_001002005.2) |
Mouse | F: TGCCCATCTCCTACCTATGC R: CAAGACGATGTCCACACCTG |
176 |
| Pannexin 3 (NM_172454.2) |
Mouse | F: TTTCCCTTGCTAGAGCGGTA R: TTGCTGCACTGGAGACACTC |
271 |
To quantify gene expression changes in cultured astrocytes following stretch or swelling, cells were grown to 80% confluence and subjected to either mechanical stretch (5% strain, 0.3 Hz for 4 hours then rested for 20 hours) or hypotonicity. Appropriate untreated controls were also grown simultaneously. RNA was isolated and cDNA created as described above. qPCR was performed using SYBR Green master mix and a real-time thermocycler (Applied Biosystems). Changes in mRNA expression were calculated using the ΔΔCT method as described (Guha et al. 2013), using β-actin as a housekeeping gene. Analogous approaches were used for optic nerve head material from 8 month old C57Bl6N and Tg-MYOCY437H mice, using mouse specific primers in Table 1.
Regulatory volume decrease (RVD)
Regulatory volume decrease was determined based on an approach used previously (Mitchell et al. 2002; Xia et al. 2012). Briefly, astrocytes were sub-cultured sparsely on glass coverslips and allowed to attach for 24 hrs. Cells were loaded with 4μM calcein-AM and 0.02% Pluronic at room temperature for 40 min, followed by a gentle wash. Cells were imaged with an inverted microscope equipped for fluorescence (Nikon Diaphot, Nikon USA every 20 sec, with the fluorescence excited at 488nM and emitted >520nm recorded with a camera and processed (all Photon Technologies International, Inc., Lawrenceville, NJ). Cells were first perfused at room temperature with isotonic control solution followed by hypotonic solution (addition of 30% H20). To determine cell area, images were thresholded to remove background fluorescence and cell area was defined as the number of non-black pixels after thresholding. The area values were converted to volume using standard equations, assuming the image represented the maximal cross sectional area. Regulatory volume decrease was defined as 100x(H-H30)/(H–I), where H = peak cell volume in hypotonic solution, H30 = volume after 30 min in hypotonic solution and I= volume in isotonic solution.
Intracellular calcium measurement
Pharmacological manipulation of intracellular Ca2+ levels was monitored with a plate reader as previously described (Reigada et al. 2005); this approach provides the most reproducible levels for comparisons. In brief, rat optic nerve head astrocytes from were grown to 80% confluence in black 96-well plates; the clear bottoms enabled inspection of cell density. Cells were loaded with 5µM Fura-2 AM for 30 min at room temperature in the dark. After washing, cells were pre-incubated with isotonic solution + drug for 10–30min. Pre-incubation solutions were removed and replaced with the drug in either isotonic or hypotonic solution. Experimental solutions and drugs included isotonic solution (Mg2+-free), hypotonic solution (same composition as isotonic, but mannitol-free), and hypotonic plus 1U/mL apyrase, 10µM A438079, 10µM AZ10606120, or 10µM carbenoxelone. Fluorescence was measured with a Fluoroskan Ascent Fluorometer (Thermo Scientific). Parameters were 340nm/380nm excitation and 527nm emission, 60ms integration time, 30s interval time, 11 measurements. The 340nm/380nm ratios in isotonic and hypotonic were normalized to the mean level in isotonic over the 5.5 minutes for each drug.
Immunohistochemical staining
Rat optic nerve head astrocytes cells were grown to ∼80% confluence on 12mm glass coverslips and then fixed with cold methanol/acetone (80%/20%) solution for 20 minutes at 4°C. Cells were blocked with 10% goat serum in a phosphate buffered saline solution (PBS) for 90 min. A polyclonal rabbit anti-glial fibrillary acidic protein (GFAP, 1:250 dilution, EMD Millipore, MA) antibody was applied overnight at 4°C, followed by a goat anti-rabbit IgG Alexa-Fluor 488 secondary antibody (1:500 dilution, Abcam Inc, MA) for 60 min. For pannexin 1 staining of cells, the cells were fixed with 4% paraformaldehyde in PBS for 20 min. at room temperature, washed 3x with PBS and permeablized for 1hr. with PBS containing 5% donkey serum and 0.3% TritonX-100. Coverslips were incubated with goat polyclonal antibody against pannexin 1 (1:200, Abcam, Inc.) diluted in PBS containing 1% donkey serum and 0.3% TritonX-100 overnight at 4°C. A secondary antibody (donkey anti-goat FITC, 1:500 dilution in blocking solution, Abcam Inc.) was added for 2 hrs. Cells were incubated with DAPI and mounted using SlowFade Gold Anti-fading Media (Invitrogen). Images were acquired using a Nikon Diaphot microscope and ImagePro software (MediaCybernetics). Incubation with only the secondary antibody showed no non-specific binding (results not shown).
For immunostaining C57Bl6N and of Tg-MYOCY437H mouse optic nerve heads, mice were perfused with 4% paraformaldehyde and the eye enucleated. Eyes were fixed overnight and incubated in 30% sucrose for 24 h. Tissues were then embedded in O.C.T compound, and cryosectioned at 8μm. Sections were incubated with a blocking solution containing 0.1% Triton-X, and immunostained with a pannexin 1 primary antibody (goat polyclonal antibody #SC-49695; Santa Cruz Biotechnology). Secondary donkey anti-goat Alexa555 conjugated antibody was applied (1:500). Images were acquired using a Nikon A1 confocal microscope at the University of Pennsylvania Live Cell Imaging Center.
Data Analysis
Data are reported as mean ± SEM. Statistical analysis used a one-way ANOVA with appropriate post-hoc test, or tests on ranks where data were not normally distributed. Results with p<0.05 were considered significant. In data from experiments with plate-readers, n=the number of wells with 3–7 plates typically used.
Results
Astrocytes release ATP in response to mechanical strain or osmotic stress
Initial experiments measured the unstimulated baseline levels of ATP in the bath surrounding optic nerve head astrocytes and examined the presence of ecto-ATPase enzymes on the cells. Baseline levels of ATP in the bath were low, although significantly distinguishable from background. However addition of the ecto-ATPase inhibitors β,γ-methylene ATP (100µM) and ARL-67156 (100µM) to the extracellular media increased ATP levels over 9 fold to 1.9 nM (Fig. 1A). While these absolute levels are low, they represent the mean bath ATP levels sampled at a distance from the cell membrane and have thus been diluted many fold; concentrations at the membrane surface are expected to be several orders of magnitude greater (Beigi et al. 1999). These observations suggested that ATP is released basally at low levels from the astrocytes and that ectoATPase enzymes are present on the astrocyte membrane to rapidly control levels of released ATP.
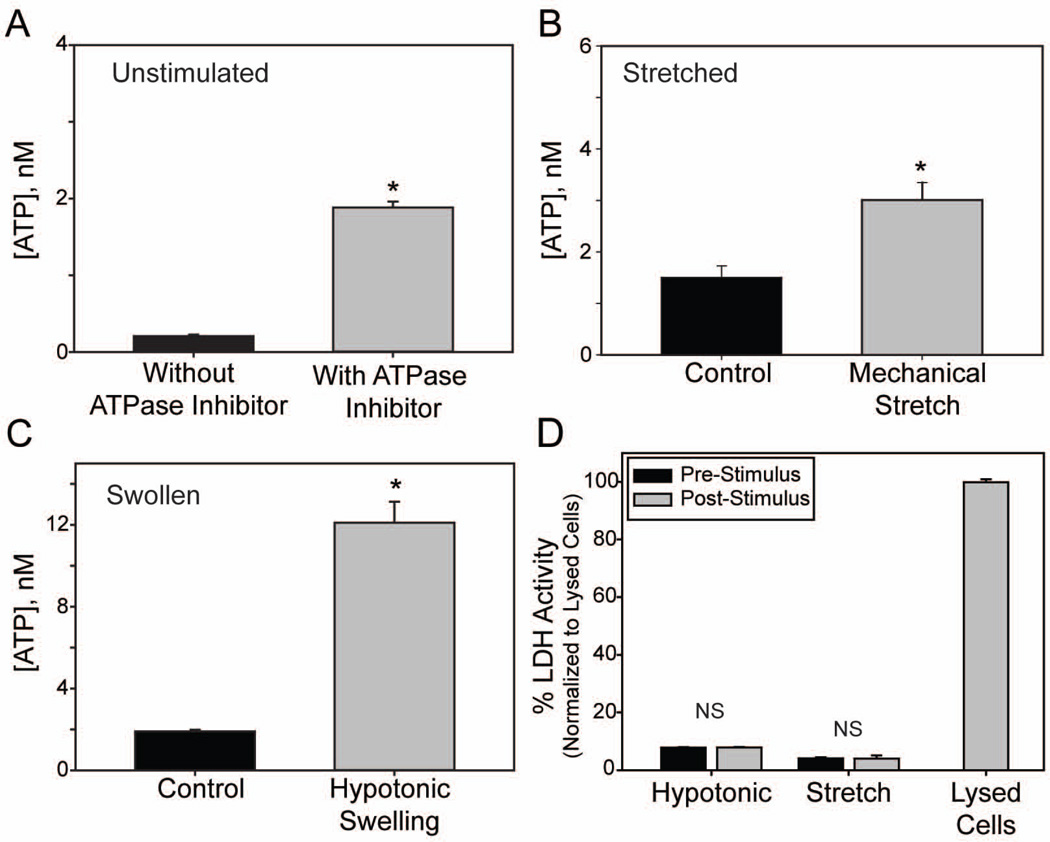
Figure 1.
Mechanical stretch and hypotonic swelling of astrocytes releases ATP. (A): Comparison of basal extracellular ATP concentrations from non-stimulated (control) cells in the absence or presence of the ecto-ATPase inhibitors β,γ-methylene ATP and ARL67156 (100µM each; n=385, 147 respectively) (B): Effects of mechanical stretch (5% equibiaxial strain, 0.3 Hz for 2 min) on extracellular ATP concentrations. ATP levels were determined from 100µl samples taken before and after cells were stretched, n=4 each. (C): Effects of hypotonic swelling (50% dH2O) on extracellular ATP concentrations measured between 1.5–2.5min after addition of hypotonicity, n=140. All solutions in (B) and (C) had 100µM β,γ-methylene ATP and ARL67156. (D): Measurement of extracellular lactose dehydrogenase (LDH) as an index of cell lysis. LDH activity was measured before and after stimuli (hypotonic swelling or mechanical stretch). NS = no significant. Levels expressed as % of that measured from lysed cells; n=20 for hypotonic, 4 for stretch and 21 for lysed cells. *p<0.05, unpaired Students’ t-test in A,C; paired in B,D.
To determine whether optic nerve head astrocytes released ATP in response to mechanical perturbation, cells grown on a silicone substrates were subjected to a mild 5% equibiaxial strain at 0.3 Hz for 2 min and concentrations of ATP in the bath were determined before and after the stretch. EctoATPase inhibitors were present in the bath to ensure focus on ATP release over degradation. Mild stretch doubled the extracellular ATP concentration (Fig. 1B). To determine whether this ATP release represented a general response to mechanical strain, astrocytes were swollen in a hypotonic solution. Swelling the astrocytes led to a 6-fold rise in the concentration of extracellular ATP when measured 2 min after initial exposure to hypotonic solution (Fig. 1C). In the absence of ectoATPase inhibitors, hypotonic swelling still increased extracellular ATP levels 8 fold, although levels were scaled down.
To confirm that the increased extracellular ATP observed was due to physiological release and not from a stress-induced rupture of the cellular membrane, we performed a lactate dehydrogenase (LDH) assay on aliquots of the extracellular solution of cells subjected to osmotic stress or mechanical stretch. Samples taken from either stretched or swelled cells exhibited low LDH activity, not significantly different from LDH activity in non-stimulated cells (Fig. 1D). In contrast, lysing cells led to a large increase in LDH activity. Together, these observations imply that mechanical strain leads to a physiological release of ATP from the astrocyte that is distinct from cell lysis.
Mechanisms of ATP release in astrocytes
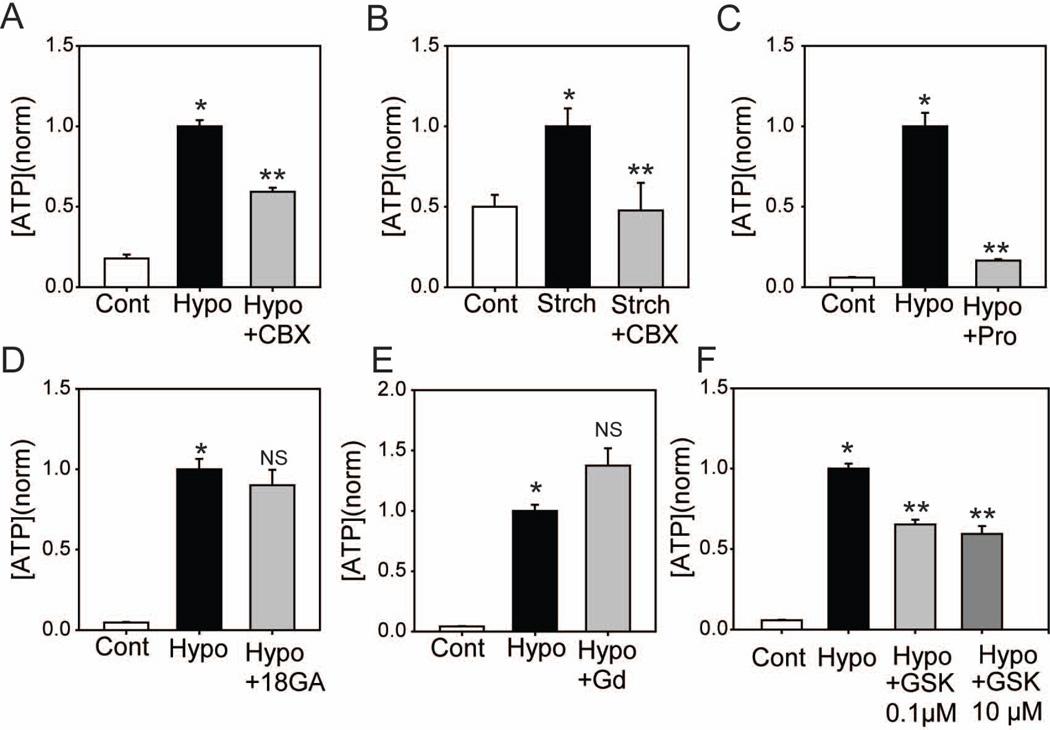
Several approaches were used to try to determine which putative mechanisms were responsible for the mechanosensitive ATP release from optic nerve head astrocytes. The ability of various pharmacological agents to block release was tested first. Carbenoxolone reduced ATP release from cells exposed either to swelling (Fig 2A) or stretch (Fig. 2B). The ability of probenecid to prevent release was also examined; while probenecid has numerous targets, it has been reported to inhibit pannexins but not connexins (Silverman et al. 2009). Probenecid blocked the swelling-induced ATP release (Fig. 2C). In contrast to carbenoxelone and probenecid, neither 18α-glycyrrhetinic acid (Fig. 2D) nor GdCl3 (Fig. 2E) significantly altered ATP release from swollen astrocytes.
Figure 2.
Pharmacological characterization of mechanosensitive ATP release (A): Hypotonic swelling (Hypo) increased extracellular ATP levels over control (Cont) and this rise was reduced by 10µM carbenoxolone (CBX n=190). (B): Mechanical stretch (Strch, as in Fig. 1B) increased extracellular ATP levels over unstretched controls (Cont). Carbenoxolone (CBX, 10µM) inhibited this increase (n=4).(C) Probenecid also inhibited swelling-induced ATP release (2mM, n=60). (D): ATP release was not blocked by 18α-Glycyrrhetinic acid (18α-GA, 50μM, n=70), or (E) gadolinium chloride (GdCl3, 50μM, n=66). (F): Increased extracellular ATP in response to hypotonic solution is partially reduced by the Rho kinase inhibitor GSK269962 at 0.1µM and 10µM (n=60). Throughout the Figure, ATP values were normalized to the mean levels attained upon swelling or stretching for each trial. * p<0.05 vs. Control, ** p<0.05 vs. Hypotonic alone.
The mechanosensitive opening of the pannexin channel can be mediated by the small GTPase RhoA pathway. Specifically, swelling-induced pannexin channel opening appears to be blocked by inhibitors of the Rho family of kinases in airway epithelial cells (Seminario-Vidal et al. 2011). In optic nerve head astrocytes, pretreatment with the non-specific Rho-kinase inhibitor GSK269962 partially decreased ATP release in response to hypotonic swelling. Release was blocked 35% by 0.1µM and 41% by 10µM GSK269962 (Fig. 3F).
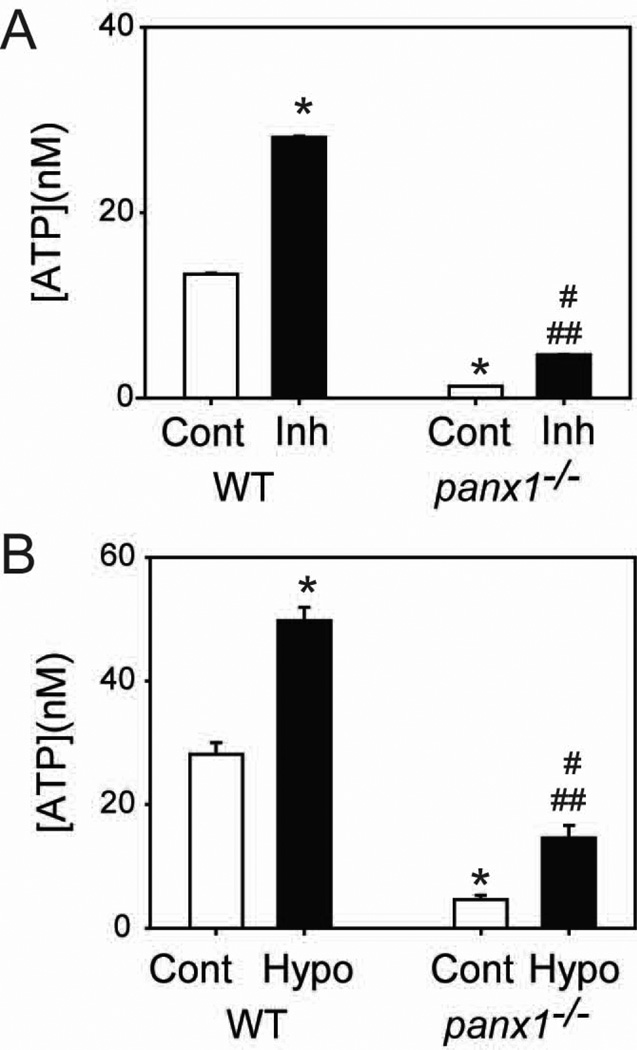
Figure 3.
ATP release from astrocytes of panx1−/− mice. (A) Baseline levels of ATP surrounding astrocytes were much lower with cells from panx1−/− mice than wild type although both were raised by 100µM β,γ-mATP (Inh). Baseline levels were much lower in astrocytes from panx1−/− mice (n=5). (B). While hypotonicity raised the ATP surrounding astrocytes from both wild type and panx1−/− mice, the magnitude of ATP release from the panx1−/− cells was reduced (n=5). Experiments in (B) were performed in the presence of β,γ-mATP. In both panels, * = p<0.05 vs. WT control, # =p<0.05 vs. panx1−/− control, and ## = p<0.05 vs. WT hypo.
While the observed pharmacological profile was consistent with pannexin channels being responsible for at least some of the mechanosensitive ATP release from optic nerve head astrocytes, none of the available drugs are specific for a particular class of channel. As such, we repeated the experiments in pannexin 1 knockout (panx1−/−) mice. Optic nerve head astrocytes obtained from wild type mice showed a baseline ATP release that was increased by the inhibition of ectoATPases similar to that in rats (Fig. 3A). Baseline levels of ATP were decreased 10-fold in astrocytes from panx1−/− mice although ectoATPase inhibition elevated these levels too; ectoATPase inhibitors were thus included in all measures of ATP release. The amount of ATP released upon exposure to hypotonicity was significantly reduced in the panx1−/− mice (Fig. 3B). This supports the pharmacological characterization described above and implies that pannexin 1 makes a substantial contribution to the mechanosensitive ATP release from optic nerve head astrocytes, but that other conduits are also present.
Extracellular ATP released from optic nerve head astrocytes acts in an autocrine manner to regulate cell volume
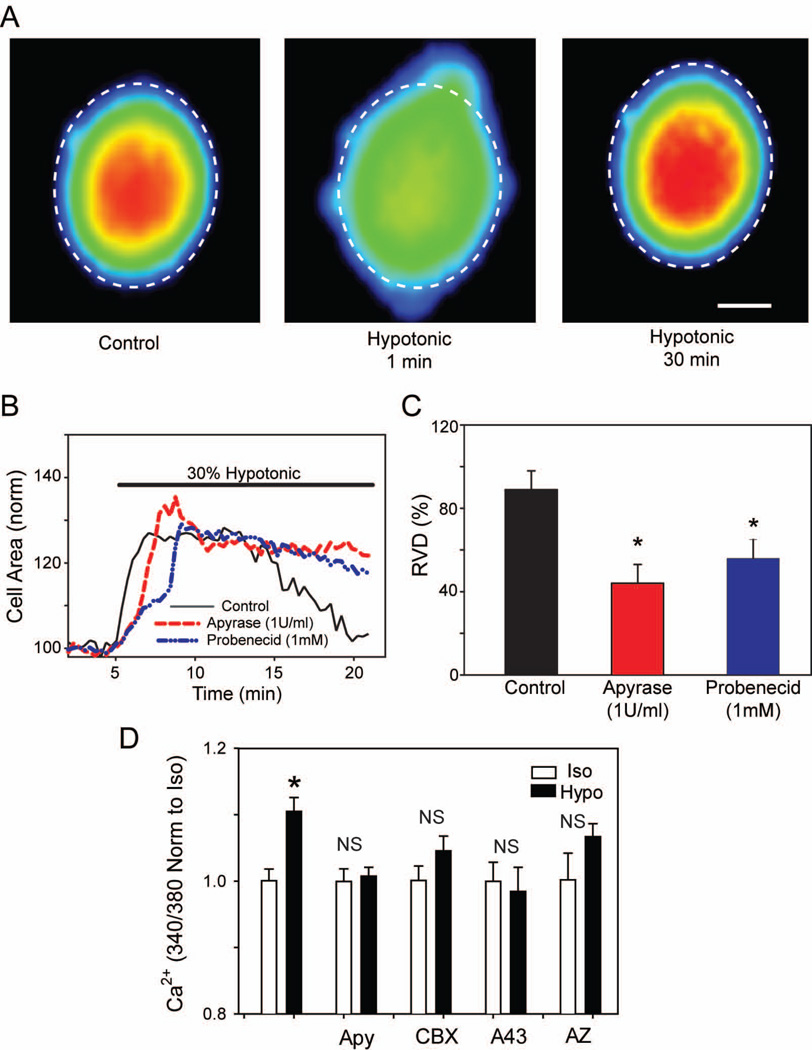
ATP released via mechanical strain often autostimulates the cells from which it came, with the feedback regulating physiological processes influenced by mechanical strain (Wang et al. 1996). For example, the initial stretch from the influx of water that accompanies exposure to hypotonic solution is followed by ATP release and autostimulation of purinergic receptors leading to efflux of ions and a regulatory volume decrease (RVD, (Okada et al. 2001)). To determine if ATP also plays a role in RVD in optic nerve astrocytes, we examined the rate at which the astrocytes recovered their original size following stimulation with a hypotonic solution. Hypotonic solution initially increased cell area by approximately 30%, but area returned to normal within 15 min (Fig. 4A). However, in the presence of the ATP hydrolase apyrase, RVD was significantly slowed, with cells still approximately 12–15% larger than control 30 min after hypotonic stimulation (Fig. 4B–C). Similar results were observed in the presence of the pannexin channel inhibitor probenecid (Fig. 4B–C), consistent with a requirement for pannexin-mediated ATP release for RVD.
Figure 4.
Extracellular ATP plays a role in regulatory volume decrease and calcium signaling. (A): Example of increase in cell area with hypotonic solution and subsequent RVD. All three images are of the same control cell at different time points in the experiment. Dashed line indicates the circumference of the cell in isotonic conditions. Color indicates strength of calcein staining, with blue representing low intensity and red indicating high intensity, bar = 5µm. (B) Representative time courses depicting the changes in cell area of rat optic nerve head astrocytes following perfusion with a 30% hypotonic solution with or without treatment with compounds that decrease extracellular ATP concentrations. Control cells are represented as a solid black line; cells treated with apyrase (1U/ml) are represented as a dashed red line and cells treated with the pannexin antagonist probenecid (1mM) are represented as a dashed blue line. (C): Summary of the extent of the regulatory volume decrease 30 minutes after start of perfusion of hypotonic solution (defined in Methods); n=16, 11 & 14, respectively. *p<0.05. (D). The calcium rise induced by exposure of astrocytes to hypotonic solution is reduced by apyrase (Apy, 1U/ml), carbenoxelone (CBX, 10µM), A438079 (A43, 10 µM) and AZ10606120 (AZ, 10µM). The ratio of light excited at 340 and 380 nm in cells loaded with fura-2 is used as an index of Ca2+, with ratios normalized to the mean value in isotonic solution for each drug. *=p<0.05, NS = no significant difference, n=7–23.
To provide further functional evidence for autocrine actions of released ATP, we examined the effect of swelling on intracellular calcium levels. When the astrocytes were subjected to hypotonic solution, intracellular Ca2+ levels increased significantly (Fig. 4D). Preincubation with apyrase inhibited the rise in Ca2+, consistent with the autocrine stimulation above. Likewise, carbenoxelone prevented the rise in Ca2+ initiated by hypotonicity. As ATP released through pannexin channels is reported to autostimulate certain splice variants of the P2X7 receptor (Xu et al. 2012), the ability of P2X7 antagonists A438079 and AZ10606120 to prevent the rise in Ca2+ was examined. Both A438079 and AZ10606120 inhibited the Ca2+ response to hypotonicity. Taken together these findings suggest that mechanical strain leads both to ATP release and to autocrine activation of P2X7 receptors in optic nerve head astrocytes.
Pannexin channel proteins are expressed in optic nerve head astrocytes
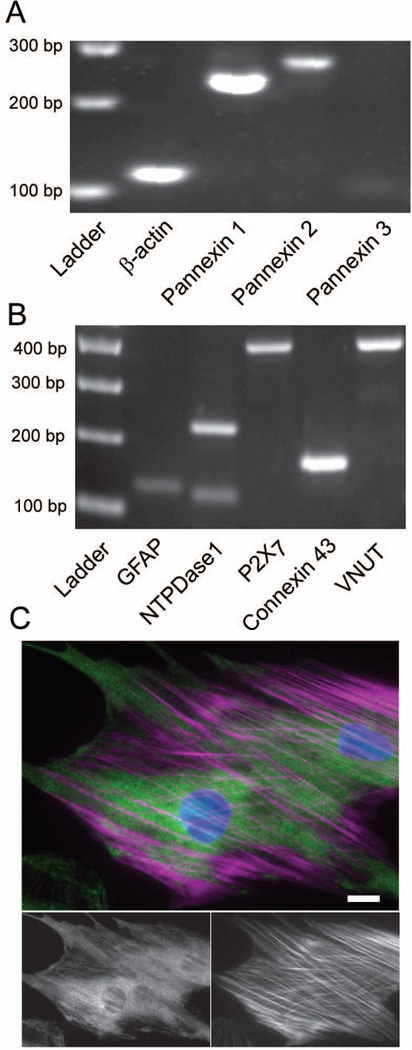
Given that pannexin 1 was implicated in the mechanosensitive ATP release from optic nerve astrocytes, release mechanisms were characterized on a molecular and protein level. ATP release is a primitive response, and numerous pathways for release are present in mammalian cells (Corriden and Insel 2010). PCR analysis confirmed that the pannexin hemichannel was present in unstimulated astrocytes, with pannexin 1, and to a smaller extent pannexin 2, expressed (Fig. 5A). Levels of pannexin 3 were faint. PCR also indicated the presence of the vesicular nucleotide transporter (VNUT, also referred to as SLC17A9) and confirmed the presence of gap junction protein connexin 43 (Fig 5B). The ionotropic purinergic receptor P2X7 and the ectonucleotidase nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) were also present. The cellular location of pannexin channels was determined using immunohistochemistry. Pannexin 1 was localized in discrete regions throughout the astrocytes (Fig. 5C). Some, but not all, staining for pannexin was associated with actin.
Figure 5.
Rat optic nerve head astrocytes express a number of possible mechanisms for releasing ATP. (A): Micrograph of PCR products for pannexin hemichannel isoforms as well as the positive control β-actin. (B): PCR products for other targets that have been shown to play a role in ATP release and signaling, plus the positive control glial fibrillary acidic protein (GFAP). NTPDase 1: Ectonucleoside triphosphate diphosphohydrolase 1. VNUT: Vesicular Nucleotide Transporter. Expected product sizes are listed in Table 1. (C). Immunofluorescence of cultured rat optic nerve head astrocytes stained for pannexin 1 (green), F-actin (magenta) and nuclear DNA (blue), bar: 20µm. Pannexin 1 staining alone is shown on the bottom left while F-actin alone is shown on the bottom right.
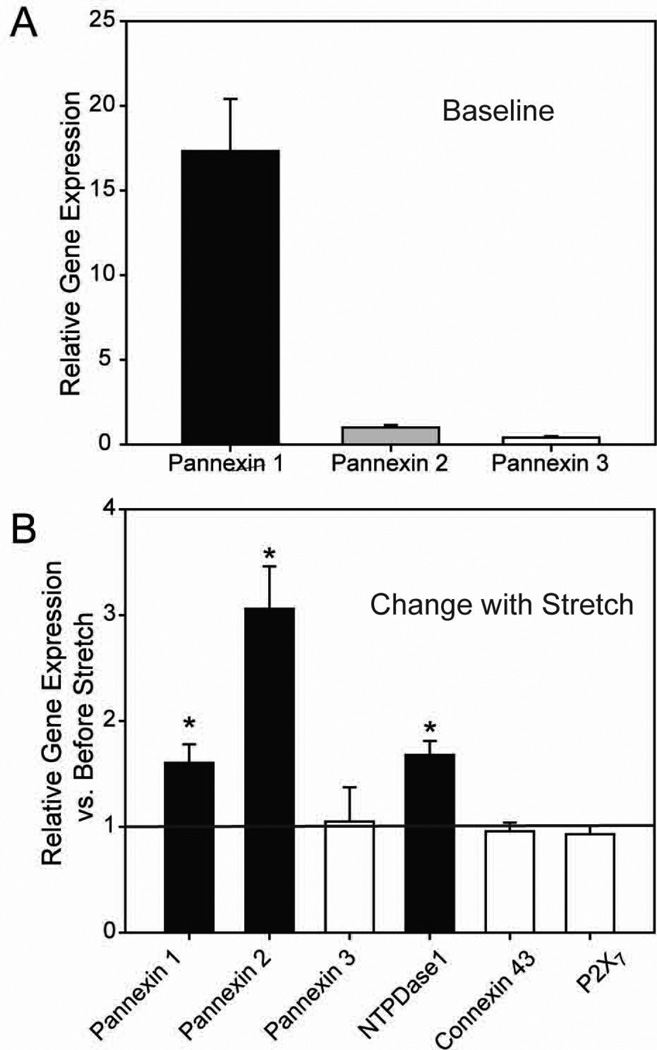
Pannexin isoforms are up-regulated following stretch in isolated astrocytes
Exposure of optic nerve head astrocytes to mechanical strain can alter expression of numerous genes (Johnson et al. 2007). Given that chronic strain can make the cells more sensitive, we asked whether expression of pannexin genes was affected by mechanical strain using quantitative PCR (qPCR). Analysis of baseline levels indicated that pannexin 1 was the predominant isoform present in astrocytes (Fig. 6A). mRNA message for both pannexin 1 and 2 is up-regulated following an extended stretch (4 hours of 5% strain at 0.3 Hz and RNA extracted 20 hours later, Fig. 6B). mRNA for the ectonucleotidase NTPDase1 was also increased 1.6-fold; as NTPDase1 is a marker for chronic changes in extracellular ATP levels (Lu et al. 2007) this finding is consistent with a sustained elevation in ATP in response to extended stretch. Message for pannexin 3, the gap-junction protein connexin 43 and the P2X7 receptor remained unchanged after stretch, however. It is worth noting that although expression of pannexin 2 rose twice as much as pannexin 1 upon stretch, initial expression of pannexin 1 was over 15 fold greater than pannexin 2 (Fig. 6B). As such, levels of pannexin 1 are expected to be greater after stretch too.
Figure 6.
Mechanical stretch alters gene expression in cultured rat optic nerve head astrocytes. (A): Relative expression of pannexin isoforms in unstretched astrocytes. Gene expression is normalized to pannexin 2 levels. Base pair (bp) sizes are indicated at left (B): Mechanical stretch of cultured rat optic nerve head astrocytes (5% equibiaxial strain, 0.3Hz for 4 hours followed by rest for 20 hours) significantly increased expression of pannexin 1, pannexin 2 and NTPDase1 mRNA, while pannexin 3, connexin 43 and P2X7 mRNA remained unchanged. *p<0.05 as compared by paired Students’ t-tests to non-stretched controls (indicated by the dashed line). n=3 for each column.
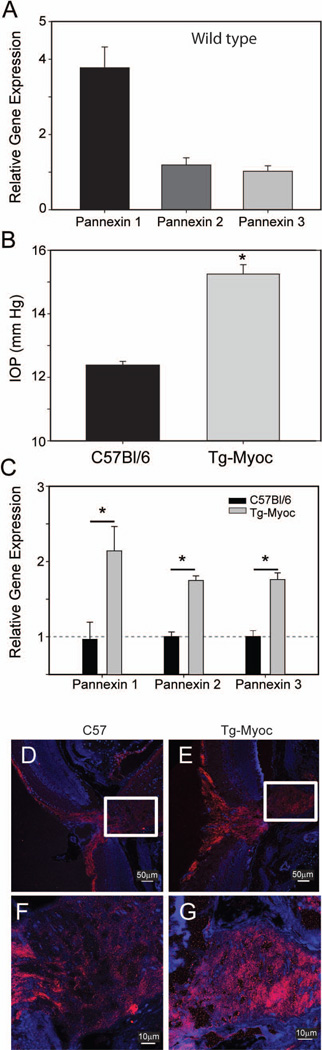
Pannexin isoforms are up-regulated in the Tg-MYOCY437H mouse model of chronic glaucoma
While the use of isolated astrocytes enables the increase in gene expression to be examined in a cell-specific manner, we also examined pannexin mRNA expression in a genetic mouse model of chronic glaucoma in order to determine if pannexin expression is altered in this clinically-relevant model of sustained stretch. In the Tg-MYOCY437H model of chronic glaucoma, transgenic mice expressing the Y437H mutation of the human myocilin gene exhibit increased IOP, increased retinal ganglion cell death and axonal degeneration as they age (Zode et al. 2011). For our experiments, the optic nerve heads from 8 month old wild-type mice and their transgenic Tg-MYOCY437H litter mates were removed and the material tested for differences in pannexin gene expression. The optic nerve head of wild type mice expressed all three isoforms of pannexin, with pannexin 1 being the most abundant form (Fig. 7A). At 8 months of age, the IOP showed a modest but significant increase in Tg-MYOCY437H mice as compared to age-matched controls (Fig. 7B). Relative gene expression for all three isoforms of pannexin was significantly increased in the optic nerve head of Tg-MYOCY437H mice (Fig. 7C). This suggests that mechanosensitive ATP release via pannexin channels may be enhanced by stretch in chronic glaucoma.
Figure 7.
Increased pannexin expression in the optic nerve head on Tg-MYOCY437H mice with elevated IOP. (A): Relative gene expression for pannexin isoforms in the optic nerve head of wild type mice. Results are an average of optic nerve head tissue taken from 3 separate mice and are normalized to the lowest expressed isoform, pannexin 3. (B): IOP in wild-type C57Bl/6N mice and Tg-MYOCY437H (Tg-Myoc) mice at 8 months of age. n=7,8 mice, respectively. (C): Increases in expression of pannexin isoforms1-3 in Tg-MYOCY437H (grey), as compared to wild-type mice (black). n=3. * = p<0.05 vs. wild type. (D–G). Immunohistochemistry confirming localization of pannexin 1 in optic nerve head astrocytes. Staining for pannexin 1 (red) and nuclei (blue) in a cross section of the optic nerve head demonstrating staining in the nerve fiber layer and along the optic nerve head of wild type (D) and Tg-MYOCY437H mice (E). The optic nerve head region inside the box is magnified below, showing that both wild type (F) and Tg-MYOCY437H (G) mice have staining surrounding nuclei, consistent with astrocytes. The staining is greater in the Tg-MYOCY437H mice.
The presence of pannexin 1 in optic nerve astrocytes was confirmed with immunohistochemistry (Fig. 7D, F). Globular staining in the optic nerve head surrounding nuclear staining for DAPI was consistent with astrocyte staining. This staining was greater in the optic nerve head of Tg-MyocY437H mice (Fig. 7E,G), suggesting the increased mRNA for pannexin in this model was also reflected at the protein level. Together, this increase in panx expression, combined with the identification of pannexin as a pathway for stretch-dependent ATP release, provides a mechanism for the upregulation of ATP release with sustained mechanical strain.
Discussion
This study indicates that pannexins contribute to the mechanosensitive release of ATP from optic nerve head astrocytes and that the expression of pannexins is likely increased by chronic stretch. In particular, the study has demonstrated that astrocytes release ATP upon stretch and swelling (Fig. 1). The preferential block by antagonists carbenoxelone and probenecid suggests that pannexins are the predominant pathway to the ATP release that accompanies mechanical strain, although other mechanisms may also contribute (Fig. 2). Both baseline and swelling-induced ATP release are reduced in optic nerve head astrocytes from panx1−/− mice, confirming a role for pannexin 1 in ATP release (Fig. 3). The mechanosensitive release of ATP makes a functional contribution to cell physiology, as the regulatory volume decrease was dependent on permeant pannexins and the presence of extracellular ATP while the rise in Ca2+ accompanying cell swelling was blocked by pannexin and P2X7 receptor antagonists (Fig. 4). Pannexin 1 was present on both a molecular and protein level in the astrocytes (Fig. 5). Both pannexin 1 and pannexin 2 were increased after stretch in vitro. (Fig. 6). All three pannexin isoforms were increased in the optic nerve head of Tg-MyocY437H mice with a chronic elevation of IOP (Fig. 7). Together, these findings imply a role for pannexins in the response to acute stretch, and suggest a mechanism for enhanced involvement under conditions of prolonged mechanical strain.
Contribution of pannexins to the mechanosensitive release of ATP from optic nerve head astrocytes
The ability of probenecid and low levels of carbenoxelone to prevent the mechanosensitive release of ATP from the astrocytes implicates pannexins in this release (Ma et al. 2009). The 10µM level of carbenoxelone used in this study has been reported to preferentially inhibit pannexin channels (Bruzzone et al. 2005). However, carbenoxelone can also inhibit amino acid release from cortical astrocytes from P2X7−/− mice at this concentration (Ye et al. 2009), indicating the specificity may be complex even at this level. While the block by probenecid (Silverman et al. 2009), combined with the lack of block by Gd3+ or 18α-GA is consistent with pannexin channels as a conduit for the mechanosensitive ATP release, all these compounds have multiple sites of action and thus the pharmacological characterization is just suggestive. However, the reduction in both baseline and stimulated ATP levels in the panx1−/− mice provides a more definitive identification of pannexins in this release. This is consistent with the altered ATP release in cortical astrocytes from panx1 knock-out mice (Suadicani et al. 2012) and in astrocytes in which pannexin1 has been knocked down (Iglesias et al. 2009). Whether the residual ATP release in astrocytes from panx1−/− mice in the present study reflects a contribution from pannexin 2 or from other conduits is unknown. The recent identification of leucine-rich repeat-containing 8 (LRRC8) channels as swelling-activated anion channels may be of relevance in this regard (Qiu et al. 2014; Voss et al. 2014). The permeability of LRRC8 to taurine (Voss et al. 2014) and the similarities with pannexin channels (Abascal and Zardoya 2012) suggest LRRC8 may also contribute to the mechanosensitive efflux of ATP, although this remains to be demonstrated directly.
Although pannexins are clearly involved in connecting mechanical strain to ATP release in astrocytes, the present data do not indicate whether they act as a mechanosensor or as a “middle man”. Pannexins were shown to have intrinsic mechanosensitive properties in isolated patch experiments (Bao et al. 2004). Whether the association between pannexins and actin in Figure 5 indicates a more direct role in mechanosensitivity or is merely a reflection of the cultured condition, as found in cortical astrocytes (Oberheim et al. 2009), remains to be determined. An upstream role for TRPV4 has been implicated in the swelling-activated ATP release from pannexins in lens (Shahidullah et al. 2012) and airway epithelial cells (Seminario-Vidal et al. 2011). However, experiments to determine a role of TRPV4 in the present study were inconclusive due to the substantial effect of inhibitor HC067047 on the luciferase standard curve (not shown). Rho kinase was implicated in at least some of the mechanosensitive ATP release in the airway epithelial cells, consistent with the ability of Rho kinase inhibitor GSK269962 to prevent the mechanosensitive release in the present study. Interestingly, GSK269962 never blocked more than 42% of the ATP release even with a 100-fold rise in concentration; if the Rho kinase is involved in trafficking of pannexin it may not have impacted channels already in the membrane. However, it is also possible that several pathways for ATP release exist and not all of the release involves Rho kinase.
ATP release is a primitive response, and numerous pathways for release are present in mammalian cells (Corriden and Insel 2010). Astrocytes may use various mechanisms for ATP release depending upon the stimuli. Astrocytoma cells employ Ca2+ dependent vesicular mechanisms for ATP release triggered by receptors while the same cells use a non-vesicular pathway for ATP release when swollen (Joseph et al. 2003). The presence of a similar dual-response system in optic nerve head astrocytes would explain why VNUT was expressed but mechanosensitive release was largely blocked by pannexin-channel inhibitors. G-protein coupled receptors act synergistically with osmotic stress to modulate ATP release from 1321N1 astrocytes (Blum et al. 2010), and while such a synergistic mechanism is also possible from optic nerve head astrocytes, the above data indicate that it isn’t necessary. Non-vesicular ATP release from cortical astrocytes can be regulated by circadian genes (Marpegan et al. 2011); as IOP also changes on a circadian basis, the temporal coordination between IOP levels and ATP release from optic nerve head astrocytes may have important implications.
Upregulation of pannexins upon chronic stretch
The ability of sustained stretch to upregulate expression of pannexin genes in isolated optic nerve head astrocytes, combined with the increase in pannexin expression in the optic nerve head of the Tg-MYOCY437H mice has important implications for the contribution of the channel to disease. In both cultured rat astrocytes and material from mouse optic nerve head, baseline expression of mRNA for panx1 was greatest (assuming analogous primer efficiency). However, the increase in panx1, and particularly of panx2 in cultured astrocytes, signals the presence of an important positive feedback loop that may lead to increased ATP release with sustained stretch. This is strongly supported by the increased expression of panx1, panx2 and panx3 in material from the optic nerve head of the Tg-MYOCY437H mice, in which the intraocular pressure was moderately elevated for several months. Of relevance in this regard is the age-related increase in panx1 expression in rats (Mawhinney et al. 2011), further supporting potential relevance of this channel activity to age-mediated progression of glaucoma.
It is interesting that panx3 was elevated in the animal model but not in vitro. The relative contributions of the three isoforms to the optic nerve head response to stretch remains to be determined. While pannexin 1 is the most widely distributed isoform, all three have been associated with ATP release (Li et al. 2011b; Penuela et al. 2013). Both pannexin 2 and 3 have been associated with development (Vogt et al. 2005) and differentiation (Ishikawa et al. 2011); this role may be consistent with the stretch-dependent upregulation, although the signals connecting cell stretch to elevated transcription may differ from those involved in the developmentally-triggered changes.
Physiological implications
These data implicating pannexins in ATP release from optic nerve head astrocytes, combined with the observation that pannexin expression increases with sustained stretch, suggest a mechanism for maintaining an elevated extracellular ATP upon sustained mechanical strain. The increase in both pannexin and NTPDase1 message in swollen astrocytes above supports this connection as NTPDase1 can act as a marker for the maintained elevation of extracellular ATP in other ocular cells (Lu et al. 2007). The elevation of extracellular ATP in humans with chronic angle closure glaucoma also confirms this pattern has clinical relevance (Li et al. 2011a).
While the upregulation of pannexins provides a mechanism for sustained ATP release under chronic strain, the consequences of this release may be either beneficial or detrimental. ATP released from retinal glia can be converted to adenosine and protect retinal ganglion cells by stimulation A1 and A3 adenosine receptors (Hartwick et al. 2004; Newman 2001; Newman 2003; Zhang et al. 2006). In contrast, stimulation of the P2X7 receptor for ATP can kill retinal ganglion cells both in vitro and in vivo (Hu et al. 2010; Zhang et al. 2005). As such, the net effect of increased extracellular ATP resulting from upregulation of pannexins may depend on the relative levels of adenosine and P2X7 receptors. The ability of retinal ganglion cells to survive exposure to decades of mechanical strain suggests that the enhanced ATP release, mediated by pannexin upregulation, may in fact be beneficial.
In the brain, the increased expression of pannexin channels upon chronic stretch, combined with the identification of pannexins as conduits for ATP release from glial cells, may have important implications as the release of ATP from glial cells has recently been implicated in several key events. For example, an increased release of ATP from astrocytes led to an enhanced excitability of hippocampal neurons (Lee et al. 2013), while long-term depression of synaptic transmission in the hippocampus was also attributed to astrocytic ATP release (Chen et al. 2013). The pannexin-mediated release of ATP from microglial cells influenced inflammatory responses (Orellana et al. 2013), while ATP released from astrocytes is implicated in sleep dynamics (Blutstein and Haydon 2013). An elevation in pannexin expression in response to mechanical strain, such as following traumatic brain injury or edema, could lead to chronic modification in any of these systems.
Main Points.
Mechanical strain triggers ATP release from astrocytes via pannexin 1
Released ATP autostimulates P2X7 receptors
Sustained stretch increased pannexin expression
Mechanism for sustained elevation of extracellular ATP under chronic stretch
Acknowledgements
This work is supported by grants from the NIH EY015537 (CHM), EY013434 (CHM), EY017045 (AML), EY001583 (CHM and AML), DK069898 (EJM), EY10564 (VCS), EY06915 (NAD), EY09532 (NAD), EY021517 (VIS), GM075770 (JMB), Research to Prevent Blindness (AML), the Paul and Evanina Bell Mackall Foundation Trust (AML), and the Jody Sack Fund (WL). The authors would like to thank Mortimer Civan for the use of the osmometer, C. Ross Ethier for comments on the strain protocol and the University of Pennsylvania Live Cell Imaging Core. V.C. Sheffield is investigator of the Howard Hughes Medical Institute. CHM would like to thank the Lasker/IRRF Glaucoma initiative for inspiration.
Preliminary versions of this work have appeared in abstract form (Argall et al. 2011; Beckel et al. 2011; Beckel et al. 2012)
References
- Abascal F, Zardoya R. LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell-cell communication. Bioessays. 2012;34:551–560. doi: 10.1002/bies.201100173. [DOI] [PubMed] [Google Scholar]
- Argall AJ, Beckel JM, Lim JC, Shahidullah M, Macarak EJ, Laties AM, Delamere NA, Mitchell CH. Stretch-induced ATP release from optic nerve astrocytes. Faseb J. 2011;25:650. 18. [Google Scholar]
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004a;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Argall AJ, Lim JC, Shahidullah M, Macarak EJ, Laties AM, Delamere NA, Mitchell CH. Optic Nerve Astrocytes Release ATP upon Mechanical Strain. Invest Ophthalmol Vis Sci. 2011;52:1819. [Google Scholar]
- Beckel JM, Xia J, Lim JC, Macarak EJ, Mitchell CH. Reduced GFAP Expression in Optic Nerve Head Astrocytes Linked To Pannexin-mediated Release Of ATP. Invest Ophthalmol Vis Sci. 2012;53:2011. [Google Scholar]
- Beigi R, Kobatake E, Aizawa M, Dubyak GR. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol. 1999;276:C267–C278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Garre JM, Orellana JA, Bukauskas FF, Nedergaard M, Saez JC. Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 2012;1487:3–15. doi: 10.1016/j.brainres.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum AE, Walsh BC, Dubyak GR. Extracellular osmolarity modulates G protein-coupled receptor-dependent ATP release from 1321N1 astrocytoma cells. Am J Physiol Cell Physiol. 2010;298:C386–C396. doi: 10.1152/ajpcell.00430.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blutstein T, Haydon PG. The Importance of astrocyte-derived purines in the modulation of sleep. Glia. 2013;61:129–139. doi: 10.1002/glia.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Weick M, Biedermann B, Uhlmann S, Kohen L, Wiedemann P, Reichenbach A. Activation of P2Y receptors stimulates potassium and cation currents in acutely isolated human Muller (glial) cells. Glia. 2002;37:139–152. doi: 10.1002/glia.10025. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005a;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011;93:120–132. doi: 10.1016/j.exer.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Progress in Retinal and Eye Research. 2012;31:702–701. doi: 10.1016/j.preteyeres.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Tan Z, Zeng L, Zhang X, He Y, Gao W, Wu X, Li Y, Bu B, Wang W, Duan S. Heterosynaptic long-term depression mediated by ATP released from astrocytes. Glia. 2013;61:178–191. doi: 10.1002/glia.22425. [DOI] [PubMed] [Google Scholar]
- Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal. 2010;3 doi: 10.1126/scisignal.3104re1. pre1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby M, Kuzmiski JB, Panenka W, Feighan D, MacVicar BA. ATP released from astrocytes during swelling activates chloride channels. J Neurophysiol. 2003;89:1870–1877. doi: 10.1152/jn.00510.2002. [DOI] [PubMed] [Google Scholar]
- Dowling JE. Astrocytes and glaucomatous neurodegeneration. Lasker/IRRF Initiative for Innovation in Vision Science. 2011 [Google Scholar]
- Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011;52:7365–7375. doi: 10.1167/iovs.11-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak GR. Both sides now: multiple interactions of ATP with pannexin-1 hemichannels. Am J Physiol Cell Physiol. 2009;296:C235–C241. doi: 10.1152/ajpcell.00639.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoriantchikova G, Ivanov D, Barakat D, Grinberg A, Wen R, Slepak VZ, Shestopalov VI. Genetic ablation of Pannexin1 protects retinal neurons from ischemic injury. PLoS One. 2012;7:e31991. doi: 10.1371/journal.pone.0031991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Miller JP, Miglior S, Beiser JA, Torri V, Kass MA, Gordon MO. The effect of changes in intraocular pressure on the risk of primary open-angle glaucoma in patients with ocular hypertension: an application of latent class analysis. BMC Med Res Methodol. 2012;12:151. doi: 10.1186/1471-2288-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygorczyk R, Furuya K, Sokabe M. Imaging and characterization of stretch-induced ATP release from alveolar A549 cells. J Physiol. 2012;591:1195–1215. doi: 10.1113/jphysiol.2012.244145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha S, Baltazar GC, Coffey EE, Tu L-A, Lim JC, Beckel JM, Eysteinsson T, Lu W, O’Brien-Jenkins A, Patel S, Laties AM, Mitchell CH. Lysosomal alkalinization, lipid oxidation, impaired autophagy and reduced phagosome clearance triggered by P2X7 receptor activation in retinal pigmented epithelial cells. Faseb J. 2013;27:4500–4509. doi: 10.1096/fj.13-236166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57:343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwick ATE, Lalonde MR, Barnes S, Baldridge WH. Adenosine A(1)-receptor modulation of glutamate-induced calcium influx in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:3740–3748. doi: 10.1167/iovs.04-0214. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Miao H, Lukas T. Astrocytes in glaucomatous optic neuropathy. Prog Brain Res. 2008;173:353–373. doi: 10.1016/S0079-6123(08)01125-4. [DOI] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, Whitmore AV, Masland RH, John SW. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Lu W, Zhang M, Zhang X, Argall AJ, Patel S, Lee GE, Kim YC, Jacobson KA, Laties AM, Mitchell CH. Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp Eye Res. 2010;91:425–432. doi: 10.1016/j.exer.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, Yamada Y. Pannexin 3 functions as an ER Ca(2+) channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol. 2011;193:1257–1274. doi: 10.1083/jcb.201101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Jia L, Cepurna WO, Doser TA, Morrison JC. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2007;48:3161–3177. doi: 10.1167/iovs.06-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem. 2003;278:23331–23342. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- Keirstead SA, Miller RF. Metabotropic glutamate receptor agonists evoke calcium waves in isolated Muller cells. Glia. 1997;21:194–203. [PubMed] [Google Scholar]
- Landsman AS, Meaney DF, Cargill RS, 2nd, Macarak EJ, Thibault LE. High strain rate tissue deformation. A theory on the mechanical etiology of diabetic foot ulcerations. William J. Stickel Gold Award. J Am Podiatr Med Assoc. 1995;85:519–527. doi: 10.7547/87507315-85-10-519. [DOI] [PubMed] [Google Scholar]
- Lee HU, Yamazaki Y, Tanaka KF, Furuya K, Sokabe M, Hida H, Takao K, Miyakawa T, Fujii S, Ikenaka K. Increased astrocytic ATP release results in enhanced excitability of the hippocampus. Glia. 2013;61:210–224. doi: 10.1002/glia.22427. [DOI] [PubMed] [Google Scholar]
- Li A, Zhang X, Zheng D, Ge J, Laties AM, Mitchell CH. Sustained elevation of extracellular ATP in aqueous humor from humans with primary chronic angle-closure glaucoma. Exp Eye Res. 2011a;93:528–533. doi: 10.1016/j.exer.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bjelobaba I, Yan Z, Kucka M, Tomic M, Stojilkovic SS. Expression and roles of pannexins in ATP release in the pituitary gland. Endocrinology. 2011b;152:2342–2352. doi: 10.1210/en.2010-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Toychiev AH, Takahashi N, Sabirov RZ, Okada Y. Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res. 2008;18:558–565. doi: 10.1038/cr.2008.49. [DOI] [PubMed] [Google Scholar]
- Lu W, Reigada D, Sevigny J, Mitchell CH. Stimulation of the P2Y1 receptor up-regulates nucleoside-triphosphate diphosphohydrolase-1 in human retinal pigment epithelial cells. J Pharmacol Exp Ther. 2007;323:157–164. doi: 10.1124/jpet.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegan L, Swanstrom AE, Chung K, Simon T, Haydon PG, Khan SK, Liu AC, Herzog ED, Beaule C. Circadian regulation of ATP release in astrocytes. J Neurosci. 2011;31:8342–8350. doi: 10.1523/JNEUROSCI.6537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawhinney LJ, de Rivero Vaccari JP, Dale GA, Keane RW, Bramlett HM. Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats. BMC Neurosci. 2011;12:123. doi: 10.1186/1471-2202-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Calcium signaling in specialized glial cells. Glia. 2006;54:650–655. doi: 10.1002/glia.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Fleischhauer JC, Stamer WD, Peterson-Yantorno K, Civan MM. Human trabecular meshwork cell volume regulation. Am J Physiol Cell Physiol. 2002;283:C315–C326. doi: 10.1152/ajpcell.00544.2001. [DOI] [PubMed] [Google Scholar]
- Morgan JE. Optic nerve head structure in glaucoma: astrocytes as mediators of axonal damage. Eye. 2000;14:437–444. doi: 10.1038/eye.2000.128. [DOI] [PubMed] [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial modulation of synaptic transmission in the retina. Glia. 2004;47:268–274. doi: 10.1002/glia.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD) J Physiol. 2001;532:3–16. doi: 10.1111/j.1469-7793.2001.0003g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Montero TD, von Bernhardi R. Astrocytes inhibit nitric oxide-dependent Ca2+ dynamics in activated microglia: involvement of ATP released via pannexin 1 channels. Glia. 2013;61:2023–2037. doi: 10.1002/glia.22573. [DOI] [PubMed] [Google Scholar]
- Ostrow LW, Sachs F. Mechanosensation and endothelin in astrocytes--hypothetical roles in CNS pathophysiology. Brain Res Brain Res Rev. 2005;48:488–508. doi: 10.1016/j.brainresrev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Pannicke T, Weick M, Uckermann O, Wheeler-Schilling T, Fries JE, Reichel MB, Mohr C, Stahl T, Fluess M, Kacza J, Seeger J, Richt JA, Reichenbach A. Electrophysiological alterations and upregulation of ATP receptors in retinal glial Muller cells from rats infected with the Borna disease virus. Glia. 2001;35:213–223. doi: 10.1002/glia.1086. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013;1828:15–22. doi: 10.1016/j.bbamem.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Perez-Ortiz JM, Serrano-Perez MC, Pastor MD, Martin ED, Calvo S, Rincon M, Tranque P. Mechanical lesion activates newly identified NFATc1 in primary astrocytes: implication of ATP and purinergic receptors. Eur J Neurosci. 2008;27:2453–2465. doi: 10.1111/j.1460-9568.2008.06197.x. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Dubin Adrienne E, Mathur J, Tu B, Reddy K, Miraglia Loren J, Reinhardt J, Orth Anthony P, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157:447–458. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifel Saltzberg JM, Garvey KA, Keirstead SA. Pharmacological characterization of P2Y receptor subtypes on isolated tiger salamander Muller cells. Glia. 2003;42:149–159. doi: 10.1002/glia.10198. [DOI] [PubMed] [Google Scholar]
- Reigada D, Lu W, Mitchell CH. Glutamate acts at NMDA receptors on fresh bovine and on cultured human retinal pigment epithelial cells to trigger release of ATP. J Physiol. 2006;575:707–720. doi: 10.1113/jphysiol.2006.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigada D, Lu W, Zhang M, Mitchell CH. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience. 2008;157:396–404. doi: 10.1016/j.neuroscience.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigada D, Lu W, Zhang X, Friedman C, Pendrak K, McGlinn A, Stone RA, Laties AM, Mitchell CH. Degradation of extracellular ATP by the retinal pigment epithelium. Am J Physiol Cell Physiol. 2005;289:C617–C624. doi: 10.1152/ajpcell.00542.2004. [DOI] [PubMed] [Google Scholar]
- Resta V, Novelli E, Vozzi G, Scarpa C, Caleo M, Ahluwalia A, Solini A, Santini E, Parisi V, Di Virgilio F, Galli-Resta L. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci. 2007;25:2741–2754. doi: 10.1111/j.1460-9568.2007.05528.x. [DOI] [PubMed] [Google Scholar]
- Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O’Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286:26277–26286. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA. TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. Am J Physiol Cell Physiol. 2012;302:C1751–C1761. doi: 10.1152/ajpcell.00010.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal IA, Ethier CR. Biomechanics of the optic nerve head. Exp Eye Res. 2009;88:799–807. doi: 10.1016/j.exer.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2005;46:4189–4199. doi: 10.1167/iovs.05-0541. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Predicted extension, compression and shearing of optic nerve head tissues. Exp Eye Res. 2007;85:312–322. doi: 10.1016/j.exer.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suadicani SO, Iglesias R, Wang J, Dahl G, Spray DC, Scemes E. ATP signaling is deficient in cultured Pannexin1-null mouse astrocytes. Glia. 2012;60:1106–1116. doi: 10.1002/glia.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt A, Hormuzdi SG, Monyer H. Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Brain Res Mol Brain Res. 2005;141:113–120. doi: 10.1016/j.molbrainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Voss FK, Ullrich F, Münch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014 doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci U S A. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston FK, Macarak EJ, Gorfien SF, Thibault LE. A system to reproduce and quantify the biomechanical environment of the cell. J Appl Physiol. 1989;67:397–405. doi: 10.1152/jappl.1989.67.1.397. [DOI] [PubMed] [Google Scholar]
- Wurm A, Erdmann I, Bringmann A, Reichenbach A, Pannicke T. Expression and function of P2Y receptors on Muller cells of the postnatal rat retina. Glia. 2009;57:1680–1690. doi: 10.1002/glia.20883. [DOI] [PubMed] [Google Scholar]
- Xia J, Lim JC, Lu W, Beckel JM, Macarak EJ, Laties AM, Mitchell CH. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J Physiol. 2012;10:2285–2304. doi: 10.1113/jphysiol.2012.227983. 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XJ, Boumechache M, Robinson LE, Marschall V, Gorecki DC, Masin M, Murrell-Lagnado RD. Splice variants of the P2X7 receptor reveal differential agonist dependence and functional coupling with pannexin-1. J Cell Sci. 2012;125:3776–3789. doi: 10.1242/jcs.099374. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Oberheim N, Kettenmann H, Ransom BR. Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia. 2009;57:258–269. doi: 10.1002/glia.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li A, Ge J, Reigada D, Laties AM, Mitchell CH. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp Eye Res. 2007;85:637–643. doi: 10.1016/j.exer.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang M, Laties AM, Mitchell CH. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:2183–2191. doi: 10.1167/iovs.05-0052. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang M, Laties AM, Mitchell CH. Balance of purines may determine life or death of retinal ganglion cells as A3 adenosine receptors prevent loss following P2X7 receptor stimulation. J Neurochem. 2006;98:566–575. doi: 10.1111/j.1471-4159.2006.03900.x. [DOI] [PubMed] [Google Scholar]
- Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011;121:3542–3553. doi: 10.1172/JCI58183. [DOI] [PMC free article] [PubMed] [Google Scholar]