Abstract
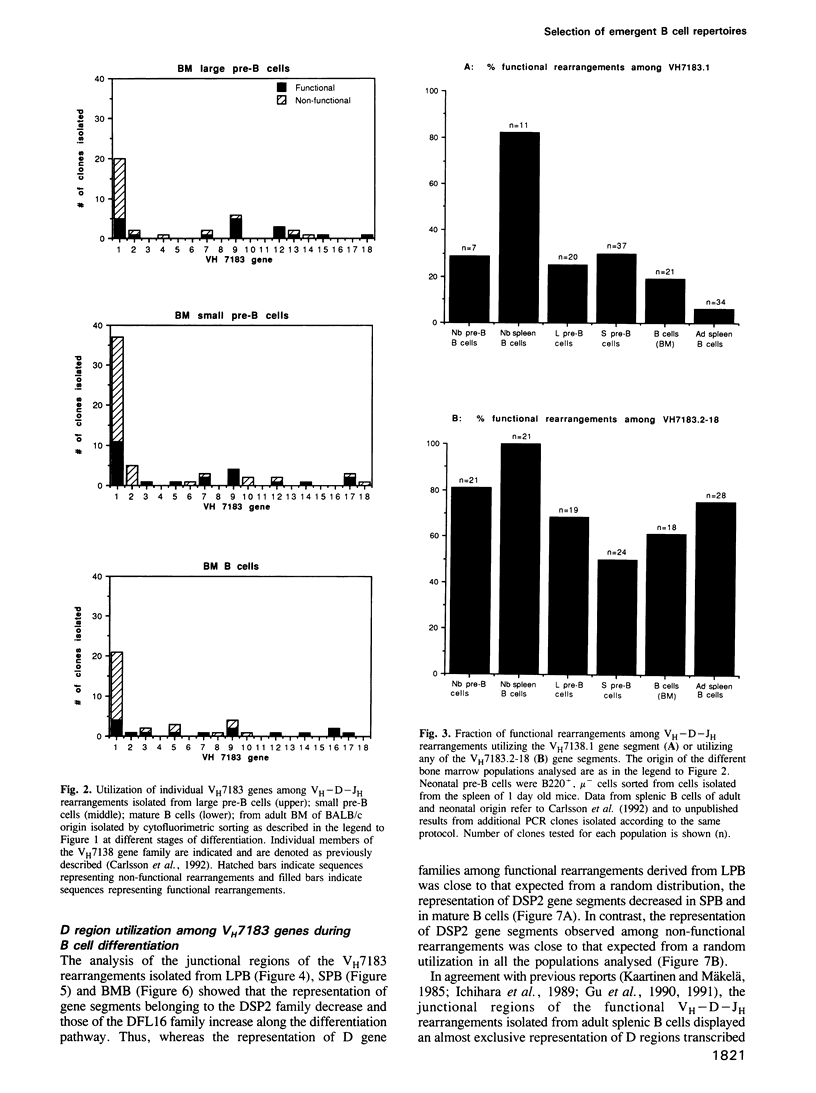
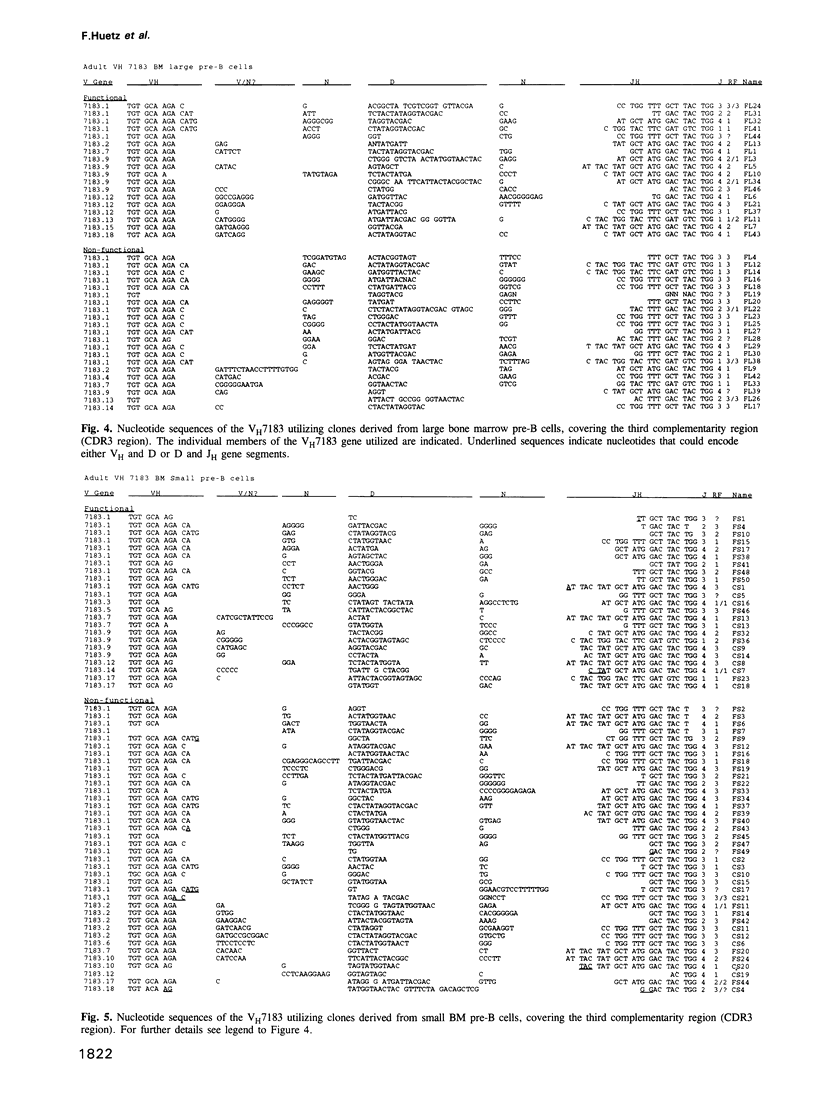
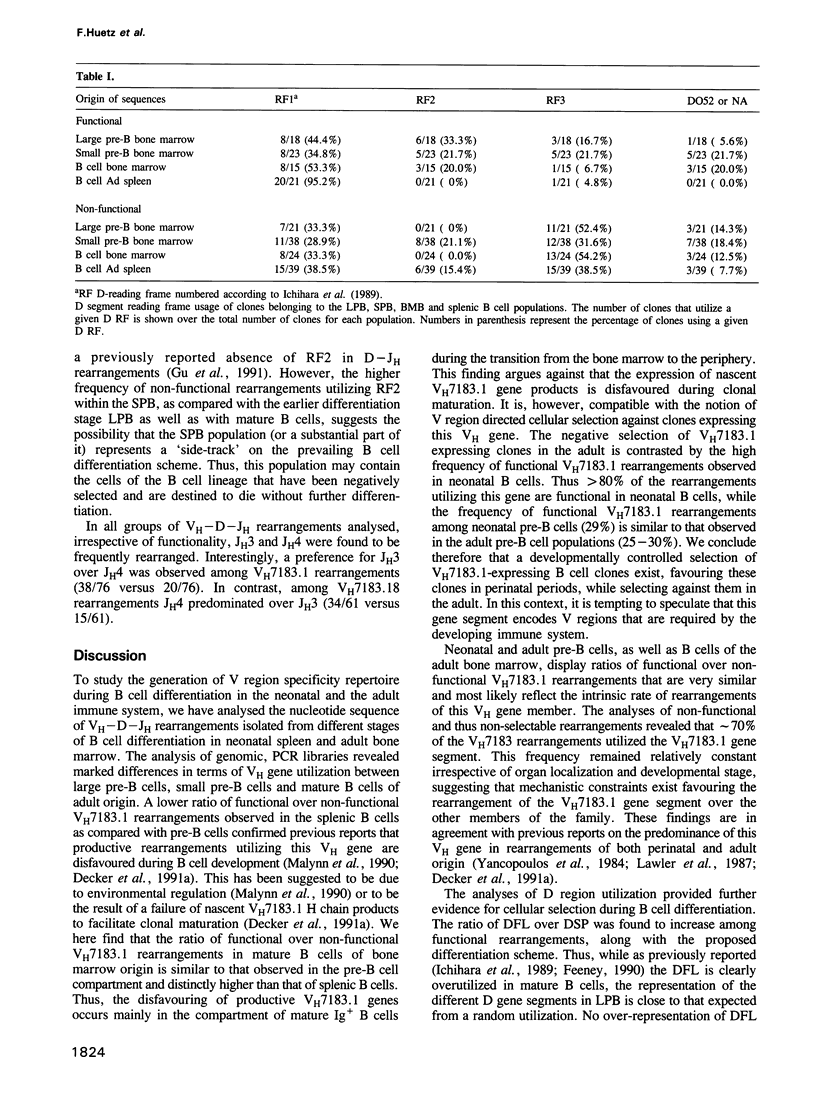
We here analyse the repertoire of VH7183 rearrangements isolated from different stages of B cell differentiation in adult mice. The nucleotide sequence analyses of VH7183-D-JH rearrangements derived from large pre-B cells (B220+, mu-), small pre-B cells (B220+, mu-) and mature B cells (B220+, mu+) isolated from adult bone marrow revealed a sequential accumulation, among functional rearrangements, of D segments of the FL16 family and a depletion of D segments using the second and the third reading frame (RF). One member (VH7183.1) of the VH7183 gene family was utilized in 60-80% of the rearrangements of all populations analysed. In neonates the majority of the rearrangements utilizing this gene was found to be functional. In contrast, > 96% of the VH7183 rearrangements isolated from adult spleen were non-functional. These data provide evidence for cellular selection of VH regions acting at different points of the B cell differentiation pathway and at the transition of B cells from the bone marrow to the periphery.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlsson L., Overmo C., Holmberg D. Developmentally controlled selection of antibody genes: characterization of individual VH7183 genes and evidence for stage-specific somatic diversification. Eur J Immunol. 1992 Jan;22(1):71–78. doi: 10.1002/eji.1830220112. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. Immunoglobulin gene rearrangement during pre-B cell differentiation. J Mol Cell Immunol. 1983;1(1):31–41. [PubMed] [Google Scholar]
- Costa T. E., Suh H., Nussenzweig M. C. Chromosomal position of rearranging gene segments influences allelic exclusion in transgenic mice. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2205–2208. doi: 10.1073/pnas.89.6.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker D. J., Boyle N. E., Klinman N. R. Predominance of nonproductive rearrangements of VH81X gene segments evidences a dependence of B cell clonal maturation on the structure of nascent H chains. J Immunol. 1991 Aug 15;147(4):1406–1411. [PubMed] [Google Scholar]
- Decker D. J., Boyle N. E., Koziol J. A., Klinman N. R. The expression of the Ig H chain repertoire in developing bone marrow B lineage cells. J Immunol. 1991 Jan 1;146(1):350–361. [PubMed] [Google Scholar]
- Dildrop R., Krawinkel U., Winter E., Rajewsky K. VH-gene expression in murine lipopolysaccharide blasts distributes over the nine known VH-gene groups and may be random. Eur J Immunol. 1985 Nov;15(11):1154–1156. doi: 10.1002/eji.1830151117. [DOI] [PubMed] [Google Scholar]
- Feeney A. J. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990 Nov 1;172(5):1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas A. A., Andrade L., Lembezat M. P., Coutinho A. Selection of VH gene repertoires: differentiating B cells of adult bone marrow mimic fetal development. Int Immunol. 1990;2(1):15–23. doi: 10.1093/intimm/2.1.15. [DOI] [PubMed] [Google Scholar]
- Gu H., Förster I., Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990 Jul;9(7):2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Kitamura D., Rajewsky K. B cell development regulated by gene rearrangement: arrest of maturation by membrane-bound D mu protein and selection of DH element reading frames. Cell. 1991 Apr 5;65(1):47–54. doi: 10.1016/0092-8674(91)90406-o. [DOI] [PubMed] [Google Scholar]
- Hozumi N., Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara Y., Hayashida H., Miyazawa S., Kurosawa Y. Only DFL16, DSP2, and DQ52 gene families exist in mouse immunoglobulin heavy chain diversity gene loci, of which DFL16 and DSP2 originate from the same primordial DH gene. Eur J Immunol. 1989 Oct;19(10):1849–1854. doi: 10.1002/eji.1830191014. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Kudo A., Melchers F. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J Exp Med. 1990 Sep 1;172(3):969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Sun L., Watanabe T. Monoclonal rat antibodies to murine IgM determinants. J Immunol Methods. 1981;42(1):17–26. doi: 10.1016/0022-1759(81)90220-9. [DOI] [PubMed] [Google Scholar]
- Lawler A. M., Lin P. S., Gearhart P. J. Adult B-cell repertoire is biased toward two heavy-chain variable-region genes that rearrange frequently in fetal pre-B cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2454–2458. doi: 10.1073/pnas.84.8.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malynn B. A., Yancopoulos G. D., Barth J. E., Bona C. A., Alt F. W. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J Exp Med. 1990 Mar 1;171(3):843–859. doi: 10.1084/jem.171.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek K. Analysis of junctional diversity during B lymphocyte development. Science. 1990 Nov 9;250(4982):820–823. doi: 10.1126/science.2237433. [DOI] [PubMed] [Google Scholar]
- Osmond D. G., Nossal G. J. Differentiation of lymphocytes in mouse bone marrow. II. Kinetics of maturation and renewal of antiglobulin-binding cells studied by double labeling. Cell Immunol. 1974 Jul;13(1):132–145. doi: 10.1016/0008-8749(74)90233-0. [DOI] [PubMed] [Google Scholar]
- Osmond D. G. Population dynamics of bone marrow B lymphocytes. Immunol Rev. 1986 Oct;93:103–124. doi: 10.1111/j.1600-065x.1986.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Kearney J. F., Chang S. P., Hood L. E. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985 Mar 29;227(4694):1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. Formation of disulphide-linked mu 2 omega 2 tetramers in pre-B cells by the 18K omega-immunoglobulin light chain. Nature. 1987 Sep 10;329(6135):172–174. doi: 10.1038/329172a0. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986 Dec 11;324(6097):579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Structure and evolution of mammalian VH families. Int Immunol. 1990;2(1):41–50. doi: 10.1093/intimm/2.1.41. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Tsubata T., Reth M. The products of pre-B cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J Exp Med. 1990 Sep 1;172(3):973–976. doi: 10.1084/jem.172.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]