Abstract
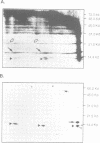
A previously unreported B cell specific gene, which we have named 8HS-20, was isolated from the cDNA library of a pre-B cell clone by subtraction and differential hybridization. This gene is selectively expressed as a 0.75 kb transcript in pre-B and bone marrow-derived B cell lines; a transcript of the same size is also found in bone marrow and, albeit at low levels, in spleen. The deduced amino acid sequence of the 8HS-20 cDNA displayed homology to a B cell specific gene, VpreB-1, and to members of the immunoglobulin supergene family including V lambda, V kappa, VH, TCRV alpha, V beta and CD8. Biochemical analysis using purified antiserum against 8HS-20 oligopeptides indicates that the gene encodes proteins with mol. wts of 13.5, 14, 15.5 and 16 kDa, which associate with mu chains in pre-B cell lines, and that these molecules are expressed concomitantly with VpreB-1 and lambda 5 gene products in the same cell lines.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer S. R., Kudo A., Melchers F. Structure and pre-B lymphocyte restricted expression of the VpreB in humans and conservation of its structure in other mammalian species. EMBO J. 1988 Jan;7(1):111–116. doi: 10.1002/j.1460-2075.1988.tb02789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Hozumi N., Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978 Dec;15(4):1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Bossy D., Milili M., Zucman J., Thomas G., Fougereau M., Schiff C. Organization and expression of the lambda-like genes that contribute to the mu-psi light chain complex in human pre-B cells. Int Immunol. 1991 Nov;3(11):1081–1090. doi: 10.1093/intimm/3.11.1081. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Schrader J. W. The regulation of growth and differentiation of a murine B cell lymphoma. II. The inhibition of WEHI 231 by anti-immunoglobulin antibodies. J Immunol. 1981 Jun;126(6):2466–2469. [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Campbell K. S., Cambier J. C. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990 Feb;9(2):441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H., Dmitrovsky E., Hieter P. A., Mitchell K., Leder P., Turoczi L., Kirsch I. R., Hollis G. F. Identification of three new Ig lambda-like genes in man. J Exp Med. 1986 Feb 1;163(2):425–435. doi: 10.1084/jem.163.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherayil B. J., Pillai S. The omega/lambda 5 surrogate immunoglobulin light chain is expressed on the surface of transitional B lymphocytes in murine bone marrow. J Exp Med. 1991 Jan 1;173(1):111–116. doi: 10.1084/jem.173.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chou H. S., Behlke M. A., Godambe S. A., Russell J. H., Brooks C. G., Loh D. Y. T cell receptor genes in an alloreactive CTL clone: implications for rearrangement and germline diversity of variable gene segments. EMBO J. 1986 Sep;5(9):2149–2155. doi: 10.1002/j.1460-2075.1986.tb04478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Era T., Ogawa M., Nishikawa S., Okamoto M., Honjo T., Akagi K., Miyazaki J., Yamamura K. Differentiation of growth signal requirement of B lymphocyte precursor is directed by expression of immunoglobulin. EMBO J. 1991 Feb;10(2):337–342. doi: 10.1002/j.1460-2075.1991.tb07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Zachau H. G. Correct transcription of an immunoglobulin kappa gene requires an upstream fragment containing conserved sequence elements. Nature. 1984 Jul 5;310(5972):71–74. doi: 10.1038/310071a0. [DOI] [PubMed] [Google Scholar]
- Goding J. W., Harris A. W. Subunit structure of cell surface proteins: disulfide bonding in antigen receptors, Ly-2/3 antigens, and transferrin receptors of murine T and B lymphocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4530–4534. doi: 10.1073/pnas.78.7.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hermanson G. G., Eisenberg D., Kincade P. W., Wall R. B29: a member of the immunoglobulin gene superfamily exclusively expressed on beta-lineage cells. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6890–6894. doi: 10.1073/pnas.85.18.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S., Hirabayashi T. Developmental change of protein constituents in chicken gizzards. Dev Biol. 1983 Jun;97(2):483–493. doi: 10.1016/0012-1606(83)90105-7. [DOI] [PubMed] [Google Scholar]
- Hollis G. F., Evans R. J., Stafford-Hollis J. M., Korsmeyer S. J., McKearn J. P. Immunoglobulin lambda light-chain-related genes 14.1 and 16.1 are expressed in pre-B cells and may encode the human immunoglobulin omega light-chain protein. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5552–5556. doi: 10.1073/pnas.86.14.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach J., Leclercq L., Radbruch A., Rajewsky K., Reth M. A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J. 1988 Nov;7(11):3451–3456. doi: 10.1002/j.1460-2075.1988.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach J., Lottspeich F., Reth M. Identification of the genes encoding the IgM-alpha and Ig-beta components of the IgM antigen receptor complex by amino-terminal sequencing. Eur J Immunol. 1990 Dec;20(12):2795–2799. doi: 10.1002/eji.1830201239. [DOI] [PubMed] [Google Scholar]
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990 Feb 22;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- Hughes-Jones N. C., Bye J. M., Beale D., Coadwell J. Nucleotide sequences and three-dimensional modelling of the VH and VL domains of two human monoclonal antibodies specific for the D antigen of the human Rh-blood-group system. Biochem J. 1990 May 15;268(1):135–140. doi: 10.1042/bj2680135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias A., Lamers M., Köhler G. Expression of immunoglobulin delta chain causes allelic exclusion in transgenic mice. Nature. 1987 Dec 3;330(6147):482–484. doi: 10.1038/330482a0. [DOI] [PubMed] [Google Scholar]
- Ikawa Y., Aida M., Inoue Y. Isolation and characterization of high and low differentiation-inducible Friend leukemia lines. Gan. 1976 Oct;67(5):767–770. [PubMed] [Google Scholar]
- Karasuyama H., Kudo A., Melchers F. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J Exp Med. 1990 Sep 1;172(3):969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr W. G., Cooper M. D., Feng L., Burrows P. D., Hendershot L. M. Mu heavy chains can associate with a pseudo-light chain complex (psi L) in human pre-B cell lines. Int Immunol. 1989;1(4):355–361. doi: 10.1093/intimm/1.4.355. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Watanabe T., Sun L., Scheid M. P. Antigens displayed on murine B lymphocyte precursors. J Immunol. 1981 Dec;127(6):2262–2268. [PubMed] [Google Scholar]
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992 May 29;69(5):823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Davis R. L., Watanabe S. M., Ponticelli A. S., Schiff-Maker L., Rosenberg N., Witte O. N. Only site-directed antibodies reactive with the highly conserved src-homologous region of the v-abl protein neutralize kinase activity. J Virol. 1984 Jul;51(1):223–232. doi: 10.1128/jvi.51.1.223-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A., Melchers F. A second gene, VpreB in the lambda 5 locus of the mouse, which appears to be selectively expressed in pre-B lymphocytes. EMBO J. 1987 Aug;6(8):2267–2272. doi: 10.1002/j.1460-2075.1987.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakauchi H., Nolan G. P., Hsu C., Huang H. S., Kavathas P., Herzenberg L. A. Molecular cloning of Lyt-2, a membrane glycoprotein marking a subset of mouse T lymphocytes: molecular homology to its human counterpart, Leu-2/T8, and to immunoglobulin variable regions. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5126–5130. doi: 10.1073/pnas.82.15.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S., Ogawa M., Nishikawa S., Kunisada T., Kodama H. B lymphopoiesis on stromal cell clone: stromal cell clones acting on different stages of B cell differentiation. Eur J Immunol. 1988 Nov;18(11):1767–1771. doi: 10.1002/eji.1830181117. [DOI] [PubMed] [Google Scholar]
- Nishimoto N., Kubagawa H., Ohno T., Gartland G. L., Stankovic A. K., Cooper M. D. Normal pre-B cells express a receptor complex of mu heavy chains and surrogate light-chain proteins. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6284–6288. doi: 10.1073/pnas.88.14.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Shaw A. C., Sinn E., Danner D. B., Holmes K. L., Morse H. C., 3rd, Leder P. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987 May 15;236(4803):816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Ikuta K., Katsura Y., Nishikawa S. Stepwise progression of B cell malignancy occurred in a bone marrow stromal cell-dependent pre-B cell clone. Leukemia. 1989 Apr;3(4):282–288. [PubMed] [Google Scholar]
- Okabe T., Bauer S. R., Kudo A. Pre-B lymphocyte-specific transcriptional control of the mouse VpreB gene. Eur J Immunol. 1992 Jan;22(1):31–36. doi: 10.1002/eji.1830220106. [DOI] [PubMed] [Google Scholar]
- Okabe T., Watanabe T., Kudo A. A pre-B- and B cell-specific DNA-binding protein, EBB-1, which binds to the promoter of the VpreB1 gene. Eur J Immunol. 1992 Jan;22(1):37–43. doi: 10.1002/eji.1830220107. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parslow T. G., Blair D. L., Murphy W. J., Granner D. K. Structure of the 5' ends of immunoglobulin genes: a novel conserved sequence. Proc Natl Acad Sci U S A. 1984 May;81(9):2650–2654. doi: 10.1073/pnas.81.9.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten P., Yokota T., Rothbard J., Chien Y., Arai K., Davis M. M. Structure, expression and divergence of T-cell receptor beta-chain variable regions. Nature. 1984 Nov 1;312(5989):40–46. doi: 10.1038/312040a0. [DOI] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. Formation of disulphide-linked mu 2 omega 2 tetramers in pre-B cells by the 18K omega-immunoglobulin light chain. Nature. 1987 Sep 10;329(6135):172–174. doi: 10.1038/329172a0. [DOI] [PubMed] [Google Scholar]
- Reininger L., Shibata T., Ozaki S., Shirai T., Jaton J. C., Izui S. Variable region sequences of pathogenic anti-mouse red blood cell autoantibodies from autoimmune NZB mice. Eur J Immunol. 1990 Apr;20(4):771–777. doi: 10.1002/eji.1830200410. [DOI] [PubMed] [Google Scholar]
- Reth M., Hombach J., Wienands J., Campbell K. S., Chien N., Justement L. B., Cambier J. C. The B-cell antigen receptor complex. Immunol Today. 1991 Jun;12(6):196–201. doi: 10.1016/0167-5699(91)90053-V. [DOI] [PubMed] [Google Scholar]
- Reth M., Hämmerling G. J., Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur J Immunol. 1978 Jun;8(6):393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- Reth M., Petrac E., Wiese P., Lobel L., Alt F. W. Activation of V kappa gene rearrangement in pre-B cells follows the expression of membrane-bound immunoglobulin heavy chains. EMBO J. 1987 Nov;6(11):3299–3305. doi: 10.1002/j.1460-2075.1987.tb02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Kashiwamura S., Kimoto M., Thalmann P., Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO J. 1988 Nov;7(11):3457–3464. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986 Dec 11;324(6097):579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff C., Milili M., Fougereau M. Isolation of early immunoglobulin lambda-like gene transcripts in human fetal liver. Eur J Immunol. 1989 Oct;19(10):1873–1878. doi: 10.1002/eji.1830191018. [DOI] [PubMed] [Google Scholar]
- Stavnezer J., Kekish O., Batter D., Grenier J., Balazs I., Henderson E., Zegers B. J. Aberrant recombination events in B cell lines derived from a kappa-deficient human. Nucleic Acids Res. 1985 May 24;13(10):3495–3514. doi: 10.1093/nar/13.10.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori T., Miyazoe I., Shirasawa T., Taniguchi M., Graf T. A temperature-sensitive mutant of Abelson murine leukemia virus confers inducibility of IgM expression to transformed lymphoid cells. EMBO J. 1987 Apr;6(4):951–956. doi: 10.1002/j.1460-2075.1987.tb04844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori T., Mizuguchi J., Miyazoe I., Nakanishi M., Shigemoto K., Kimoto H., Shirasawa T., Maruyama N., Taniguchi M. Two types of mu chain complexes are expressed during differentiation from pre-B to mature B cells. EMBO J. 1990 Aug;9(8):2493–2500. doi: 10.1002/j.1460-2075.1990.tb07428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubata T., Reth M. The products of pre-B cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J Exp Med. 1990 Sep 1;172(3):973–976. doi: 10.1084/jem.172.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubata T., Tsubata R., Reth M. Cell surface expression of the short immunoglobulin mu chain (D mu protein) in murine pre-B cells is differently regulated from that of the intact mu chain. Eur J Immunol. 1991 Jun;21(6):1359–1363. doi: 10.1002/eji.1830210605. [DOI] [PubMed] [Google Scholar]