Abstract
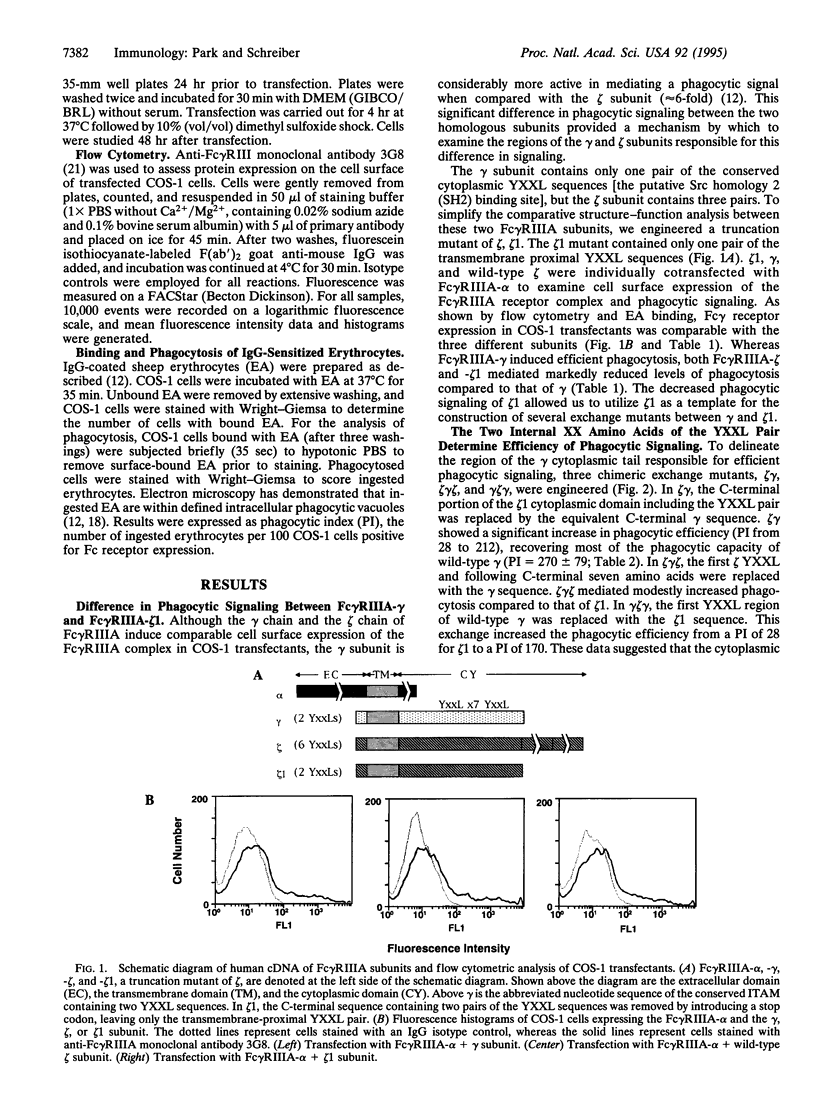
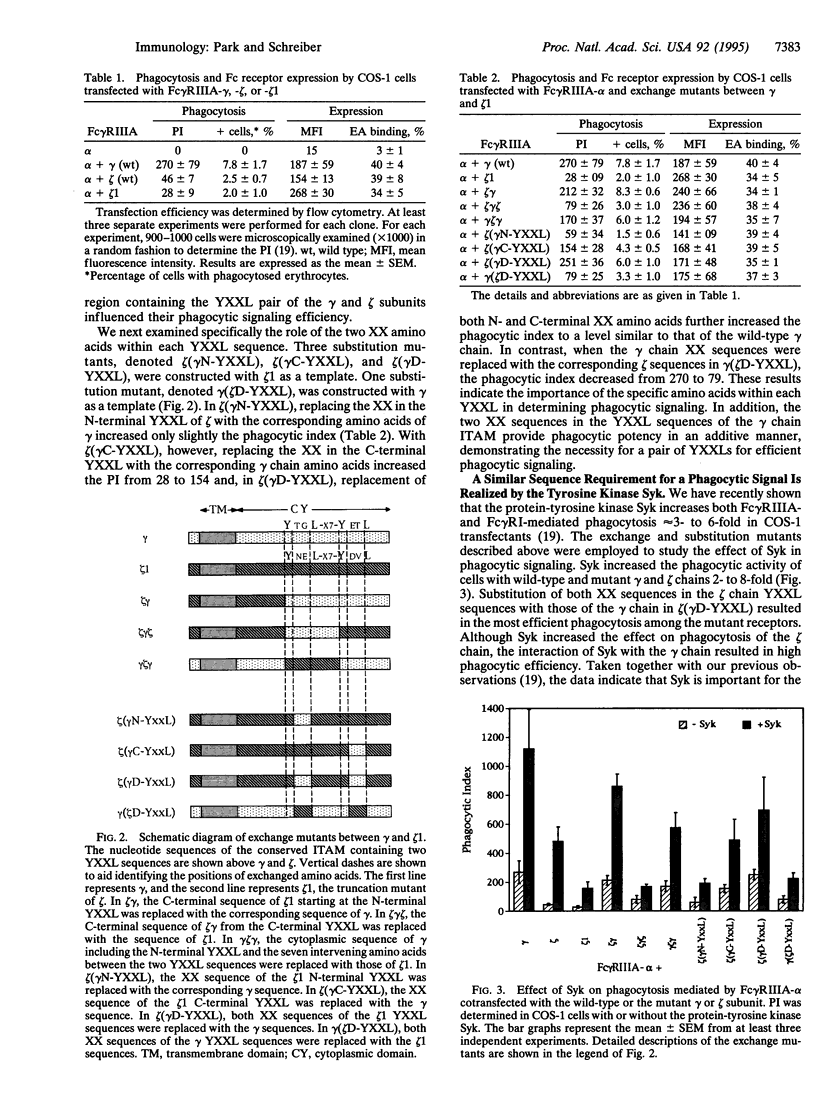
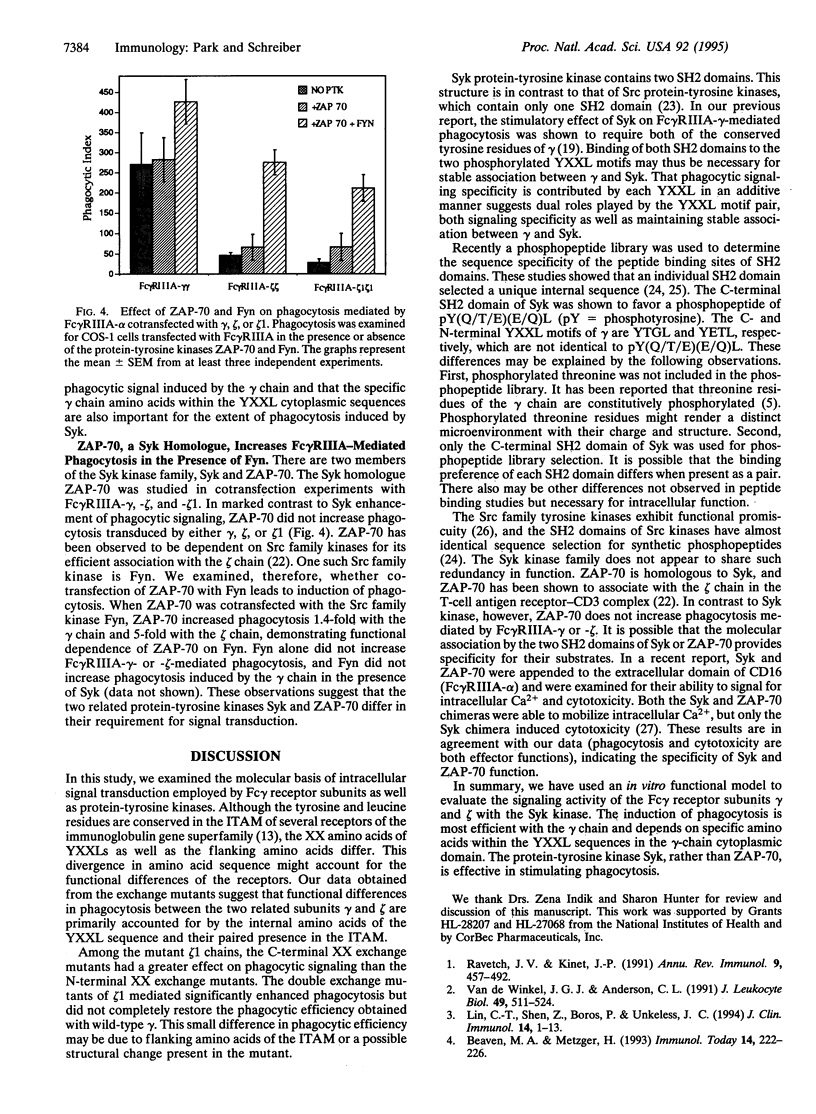
The Fc gamma receptor-associated gamma and zeta subunits contain a conserved cytoplasmic motif, termed the immunoglobulin gene tyrosine activation motif, which contains a pair of YXXL sequences. The tyrosine residues within these YXXL sequences have been shown to be required for transduction of a phagocytic signal. We have previously reported that the gamma subunit of the type IIIA Fc gamma receptor (Fc gamma RIIIA) is approximately 6 times more efficient in mediating phagocytosis than the zeta subunit of Fc gamma RIIIA. By exchanging regions of the cytoplasmic domains of the homologous gamma and zeta chains, we observed that the cytoplasmic area of the gamma chain bearing a pair of the conserved YXXL sequences is important in phagocytic signaling. Further specificity of phagocytic signaling is largely determined by the two internal XX amino acids in the YXXL sequences. In contrast, the flanking amino acids of the YXXL sequences including the seven intervening amino acids between the two YXXL sequences do not significantly affect the phagocytic signal. Furthermore, the protein-tyrosine kinase Syk, but not the related kinase ZAP-70, stimulated Fc gamma RIIIA-mediated phagocytosis. ZAP-70, however, increased phagocytosis when coexpressed with the Src family kinase Fyn. These data demonstrate the importance of the two specific amino acids within the gamma subunit YXXL cytoplasmic sequences in phagocytic signaling and explain the difference in phagocytic efficiency of the gamma and zeta chains. These results indicate the importance of Syk in Fc gamma RIIIA-mediated phagocytosis and demonstrate that ZAP-70 and syk differ in their requirement for a Src-related kinase in signal transduction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amigorena S., Salamero J., Davoust J., Fridman W. H., Bonnerot C. Tyrosine-containing motif that transduces cell activation signals also determines internalization and antigen presentation via type III receptors for IgG. Nature. 1992 Jul 23;358(6384):337–341. doi: 10.1038/358337a0. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Metzger H. Signal transduction by Fc receptors: the Fc epsilon RI case. Immunol Today. 1993 May;14(5):222–226. doi: 10.1016/0167-5699(93)90167-j. [DOI] [PubMed] [Google Scholar]
- Chan A. C., Iwashima M., Turck C. W., Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992 Nov 13;71(4):649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Campbell K. S., Kazlauskas A., Johnson S. A., Hertz M., Potter T. A., Pleiman C., Cambier J. C. The B cell antigen receptor complex: association of Ig-alpha and Ig-beta with distinct cytoplasmic effectors. Science. 1992 Oct 2;258(5079):123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992 Jan 2;355(6355):78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Chang P., Silverstein S. C. Tyrosine phosphorylation of the gamma subunit of Fc gamma receptors, p72syk, and paxillin during Fc receptor-mediated phagocytosis in macrophages. J Biol Chem. 1994 Feb 4;269(5):3897–3902. [PubMed] [Google Scholar]
- Horton R. M., Cai Z. L., Ho S. N., Pease L. R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990 May;8(5):528–535. [PubMed] [Google Scholar]
- Huang M. M., Indik Z., Brass L. F., Hoxie J. A., Schreiber A. D., Brugge J. S. Activation of Fc gamma RII induces tyrosine phosphorylation of multiple proteins including Fc gamma RII. J Biol Chem. 1992 Mar 15;267(8):5467–5473. [PubMed] [Google Scholar]
- Indik Z. K., Park J. G., Pan X. Q., Schreiber A. D. Induction of phagocytosis by a protein tyrosine kinase. Blood. 1995 Mar 1;85(5):1175–1180. [PubMed] [Google Scholar]
- Kiener P. A., Rankin B. M., Burkhardt A. L., Schieven G. L., Gilliland L. K., Rowley R. B., Bolen J. B., Ledbetter J. A. Cross-linking of Fc gamma receptor I (Fc gamma RI) and receptor II (Fc gamma RII) on monocytic cells activates a signal transduction pathway common to both Fc receptors that involves the stimulation of p72 Syk protein tyrosine kinase. J Biol Chem. 1993 Nov 15;268(32):24442–24448. [PubMed] [Google Scholar]
- Kinet J. P. The gamma-zeta dimers of Fc receptors as connectors to signal transduction. Curr Opin Immunol. 1992 Feb;4(1):43–48. doi: 10.1016/0952-7915(92)90122-u. [DOI] [PubMed] [Google Scholar]
- Kolanus W., Romeo C., Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993 Jul 16;74(1):171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- Liao F., Shin H. S., Rhee S. G. Cross-linking of Fc gamma RIIIA on natural killer cells results in tyrosine phosphorylation of PLC-gamma 1 and PLC-gamma 2. J Immunol. 1993 Apr 1;150(7):2668–2674. [PubMed] [Google Scholar]
- Lin C. T., Shen Z., Boros P., Unkeless J. C. Fc receptor-mediated signal transduction. J Clin Immunol. 1994 Jan;14(1):1–13. doi: 10.1007/BF01541170. [DOI] [PubMed] [Google Scholar]
- Lowell C. A., Soriano P., Varmus H. E. Functional overlap in the src gene family: inactivation of hck and fgr impairs natural immunity. Genes Dev. 1994 Feb 15;8(4):387–398. doi: 10.1101/gad.8.4.387. [DOI] [PubMed] [Google Scholar]
- Paolini R., Jouvin M. H., Kinet J. P. Phosphorylation and dephosphorylation of the high-affinity receptor for immunoglobulin E immediately after receptor engagement and disengagement. Nature. 1991 Oct 31;353(6347):855–858. doi: 10.1038/353855a0. [DOI] [PubMed] [Google Scholar]
- Park J. G., Isaacs R. E., Chien P., Schreiber A. D. In the absence of other Fc receptors, Fc gamma RIIIA transmits a phagocytic signal that requires the cytoplasmic domain of its gamma subunit. J Clin Invest. 1993 Oct;92(4):1967–1973. doi: 10.1172/JCI116790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. G., Murray R. K., Chien P., Darby C., Schreiber A. D. Conserved cytoplasmic tyrosine residues of the gamma subunit are required for a phagocytic signal mediated by Fc gamma RIIIA. J Clin Invest. 1993 Oct;92(4):2073–2079. doi: 10.1172/JCI116804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Kinet J. P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989 Mar 30;338(6214):383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Klausner R. D. Tyrosine kinases and tyrosine-based activation motifs. Current research on activation via the T cell antigen receptor. J Biol Chem. 1992 Dec 15;267(35):24913–24916. [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., McGlade J., Olivier P., Pawson T., Bustelo X. R., Barbacid M., Sabe H., Hanafusa H., Yi T. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994 Apr;14(4):2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Kobayashi T., Kondo J., Takahashi K., Nakamura H., Suzuki J., Nagai K., Yamada T., Nakamura S., Yamamura H. Molecular cloning of a porcine gene syk that encodes a 72-kDa protein-tyrosine kinase showing high susceptibility to proteolysis. J Biol Chem. 1991 Aug 25;266(24):15790–15796. [PubMed] [Google Scholar]
- Wirthmueller U., Kurosaki T., Murakami M. S., Ravetch J. V. Signal transduction by Fc gamma RIII (CD16) is mediated through the gamma chain. J Exp Med. 1992 May 1;175(5):1381–1390. doi: 10.1084/jem.175.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Winkel J. G., Anderson C. L. Biology of human immunoglobulin G Fc receptors. J Leukoc Biol. 1991 May;49(5):511–524. doi: 10.1002/jlb.49.5.511. [DOI] [PubMed] [Google Scholar]



