Summary
Our previous study demonstrated that pregnancy increased large-conductance Ca2+-activated potassium (BKCa) channel β1 subunit (BKβ1) expression and BKCa channel activity in uterine arteries, which was abrogated by chronic hypoxia. The present study tested the hypothesis that promoter methylation/demethylation is a key mechanism in epigenetic reprogramming of BKβ1 expression patterns in uterine arteries. Ovine BKβ1 promoter of 2315 bp spanning from −2211 to +104 of the transcription start site was cloned and a Sp1-380 binding site that contains CpG dinucleotide in its core binding sequences was identified. Site-directed deletion of the Sp1 site significantly decreased the BKβ1 promoter activity. Estrogen receptor-α bound to the Sp1 site through “tethering” to Sp1, and up-regulated the expression of BKβ1. The Sp1 binding site at BKβ1 promoter was highly methylated in uterine arteries of nonpregnant sheep, and methylation inhibited transcription factor binding and BKβ1 promoter activity. Pregnancy caused a significant decrease in CpG methylation at the Sp1 binding site and increased Sp1 binding to the BKβ1 promoter and BKβ1 mRNA abundance. Chronic hypoxia during gestation abrogated this pregnancy-induced demethylation and up-regulation of BKβ1 expression. The results provide evidence of a novel mechanism of promoter demethylation in pregnancy-induced reprogramming of BKCa channel expression and function in uterine arteries, and suggest new insights of epigenetic mechanisms linking gestational hypoxia to aberrant uteroplacental circulation and increased risk of preeclampsia.
Keywords: BKCa channel, hypoxia, pregnancy, demethylation, epigenetic
Introduction
The striking increase of uterine blood flow in pregnancy is essential both for optimal growth and survival of the fetus and for cardiovascular well-being of the mother. Maladaptation of the uteroplacental circulation during pregnancy is associated with high incidence of clinical complications including preeclampsia and fetal intrauterine growth restriction.1–4 Thus, a comprehensive understanding of regulatory mechanisms of uterine vascular adaptation in pregnancy has long been sought, but has not been achieved. The large-conductance Ca2+-activated potassium (BKCa) channel plays an essential role in determining the membrane potential of vascular smooth muscle cells and vascular tone. Previous studies demonstrated a critical role of BKCa channels in the adaptation of uterine circulation and increased uterine blood flow in pregnancy.5–7
The BKca channel is abundantly expressed in vascular smooth muscle cells and is a tetramer formed by a pore-forming α subunit (KCNMA1) along with up to four accessory β subunits. The predominant β subunit in vascular smooth muscle cells is the β1 subunit (KCNMB1) and other β subunits are either undetectable or negligible.6, 8–13 BKca channels in vascular smooth muscle are primarily activated by elevation of intracellular Ca2+ concentrations, and the β1 subunit enhances Ca2+ sensitivity of BKCa channels.10, 14–17 The β1:α subunit stoichiometry is dynamic under various physiological and pathophysiological conditions and plays a vital role in regulating BKCa channel activity in vascular smooth muscle.9, 10, 14–17 Recently, we and others have discovered that pregnancy and steroid hormones cause a significant increase in the β1, but not α, subunit, resulting in the increased β1:α subunit stoichiometry and heightened BKca channel activity in uterine arteries in sheep. 6, 18–20 In addition, chronic hypoxia during gestation abrogated these changes.21 In ovine uterine arteries, both α and β1 subunits are detected exclusively in vascular smooth muscle cells with no evidence of their existence in the endothelium.6, 18, 19
These previous electrophysiological and functional studies demonstrated an exciting and novel regulatory target of BKca β1 subunit in the adaptation of uterine vascular BKca channel activity in pregnancy.5–7, 20, 21 However, the molecular mechanisms in regulating BKca β1 subunit expression remain unknown. Epigenetic mechanisms play an important role in regulating gene expression patterns and maintaining the homeostasis. DNA methylation is a chief mechanism in epigenetic modification of gene expression patterns and occurs at cytosines of the dinucleotide sequence CpG. Methylation in promoter regions is generally associated with transcription repression of the associated genes.22–27 A recent study demonstrated that promoter methylation played a key role in regulating estrogen receptor-α (ERα) expression patterns in uterine vascular adaptation to pregnancy and chronic hypoxia.28 Little is known about the epigenetic regulation of KCNMB1 gene expression patterns in vascular smooth muscle and its adaptation to pregnancy. Herein, we present evidence of a novel mechanism of promoter demethylation in pregnancy-induced reprogramming of BKCa channel expression and function in uterine arteries, and suggest new insights of epigenetic mechanisms linking gestational hypoxia to aberrant uteroplacental circulation and increased risk of preeclampsia.
Materials and Methods
Tissue Preparation and Treatment
Control nonpregnant and pregnant sheep were obtained from the Nebeker Ranch in Lancaster, CA (altitude: ~300 m; arterial Pao2: 102 ± 2 mmHg). For chronic hypoxic treatment, nonpregnant and pregnant (30 days of gestation) animals were transported to the Barcroft Laboratory, White Mountain Research Station, in Bishop, CA (altitude: 3,801 m, maternal arterial Pao2: 60 ± 2 mmHg) and maintained there for ~110 days.16, 23 Uterine arteries were isolated from nonpregnant and near-term (~140 days of gestation) pregnant sheep. Animals were anesthetized with thiamylal (10 mg/kg, i.v) followed by inhalation of 1.5% to 2.0% halothane. An incision was made in the abdomen and the uterus exposed. The resistance-sized, fourth generation branches of main uterine arteries were isolated and removed without stretching and placed into a modified Krebs solution. These arteries were used in all studies including vascular smooth muscle cell dissociation. For hormonal treatment, arteries from nonpregnant sheep were incubated in phenol red-free DMEM with 1% charcoal-stripped FBS for 48 hours at 37 °C in a humidified CO2 incubator with 21% O2 for tissues from normoxic animals and 10.5% O2 for tissues from hypoxic animals, in the absence or presence of 17β-estradiol (0.3 nmol/L; Sigma) and progesterone (100.0 nmol/L; Sigma), as reported previously.20, 21 The concentrations of 17β-estradiol and progesterone chosen are physiologically relevant and have been shown to exhibit direct genomic effects on BKCa channel function and pressure-dependent myogenic tone in the uterine artery.20, 21 All procedures and protocols were approved by the Institutional Animal Care and Use Committee and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Real-time RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, USA), and subjected to reverse transcription with iScript™ cDNA Synthesis system (Bio-Rad, Hercules, CA). The abundance of KCNMB1 mRNA was measured with real-time PCR using iQ SYBR Green Supermix (Bio-Rad), as described previously.29 Primers used were: 5′-CTGTACCACACGGAGGACACT-3′ (forward) and 5′-GTAGAGGCGCTGGAATAGGAC-3′ (reverse). Real-time PCR was performed in a final volume of 25 μl and each PCR reaction mixture consisted of 500 nM of primers and iQ SYBR Green Supermix containing 0.625 unit hot-start Taq polymerase, 400 μM each of dATP, dCTP, dGTP, and dTTP, 100 mM KCl, 16.6 mM ammonium sulfate, 40 mM Tris-HCl, 6 mM MgSO4, SYBR Green I, 20 nM fluorescing and stabilizers. The following protocol was used for real-time PCR: 95 °C for 5 minutes, followed by 45 cycles of 95 °C for 30 seconds, annealing for 30 seconds at 52 °C, and 72 °C for 45 seconds. GAPDH was used as an internal reference and serial dilutions of the positive control were done on each plate to create a standard curve for the quantification. PCR was done in triplicate and threshold cycle numbers were averaged for each sample.
Reporter Gene Assay
Genomic DNA isolated from uterine arteries was used as a PCR template. Using primers designed from the bovine KCNMB1 gene promoter sequence (Gene ID, 407176), a 2315-bp ovine genomic fragment spanning −2211 bp to +104 bp relative to the KCNMB1 gene transcription start site was cloned into pCR4-TOPO vector and subsequently cloned in NheI-HindIII orientation in pGL3 basic vector (Promega, Madison, WI) to drive the transcription of the luciferase reporter gene. Site-specific deletion mutations were constructed at two putative transcription factor binding sites, AP1-652 and Sp1-380, as described previously.30 All promoter construct sequences were confirmed with DNA sequencing analyses. Smooth muscle cells were isolated from uterine arteries of control nonpregnant sheep and cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in 95% air/5% CO2, as described previously.28 Cells were grown and sub-cultured in 24-well plates with experiments performed at 70–80% confluent. Cells were transiently co-transfected with 1 μg of promoter/reporter vector along with 0.1 μg of internal control pRL-SV40 vector using endotoxin free plasmid DNA plus X-tremeGENE HP DNA Transfection Reagent (Roche) following the manufacturer’s instructions. After 48 hours, firefly and Renilla reniformis luciferase activities in cell extracts were measured in a luminometer using a dual-luciferase reporter assay system (Promega), as described previously.28, 30 The activities of the wild-type or site specific deletion constructs were then calculated by normalizing the firefly luciferase activities to R. reniformis luciferase activity, and expressed as relative to wild-type reporter activity (% WT).
Western Blot Analysis
Nuclear extracts were prepared from uterine arteries using NXTRACT CelLytic Nuclear Extraction Kit (Sigma, St Louis, MO). Protein concentrations were measured using a protein assay kit (Bio-Rad, Hercules, CA). Samples with equal amounts of protein were loaded onto 7.5% polyacrylamide gel with 0.1% SDS and separated by electrophoresis at 100 V for 90 minutes. Proteins were then transferred onto nitrocellulose membranes. Nonspecific binding sites was blocked for 1 hour at room temperature in a Tris-buffered saline solution containing 5% dry-milk. The membranes were then probed with primary antibody against Sp1 (Active motif; Carlsbad, CA). After washing, membranes were incubated with secondary horseradish peroxidase-conjugated antibodies. Proteins were visualized with enhanced chemiluminescence reagents, and blots were exposed to Hyperfilm. Results were analyzed with the Kodak ID image analysis software.
Quantitative Methylation-Specific PCR
Genomic DNA was isolated from uterine arteries using a GenElute Mammalian Genomic DNA Mini-Prep kit (Sigma), denatured with 2 N NaOH at 42 °C for 15 min, and treated with sodium bisulfite at 55 °C for 16 h, as previously described.28, 31 DNA was purified with a Wizard DNA clean up system (Promega) and dissolved in 120 μl of H2O. Bisulfite-treated DNA was used as a template for real-time fluorogenic methylation-specific PCR (MSP) using specific primers designed to amplify the regions of interest with unmethylated CpG dinucleotides or methylated CpG dinucleotides (CmG), respectively (Table S1, available in the online data supplement). GAPDH was used as an internal reference gene. Real-time MSP was performed using the iQ SYBR Green Supermix with iCycler real-time PCR system (Bio-Rad). Data are presented as the percent of methylation at the region of interest (methylated CpG/methylated CpG + unmethylated CpG x100), as described previously.28, 31
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts were collected from uterine arteries using NXTRACT CelLytic Nuclear Extraction Kit (Sigma). The oligonucleotide probes with unmethylated CpG (forward: 5′-TGGCTGCTGGGCGGGTTGGAAATG-3′; reverse: 5′-CATTTCCAACCCGCCCAGCAGCCA-3′) and methylated CmG (forward: 5′-TGGCTGCTGGGmCGGGTTGGAAATG-3′; reverse: 5′-CATTTCCAACCmCGCCCAGCAGCCA-3′) of the Sp1-380 binding site at ovine KCNMB1 promoter region were labeled and subjected to gel shift assays using the Biotin 3′ end labeling kit and LightShift Chemiluminescent EMSA Kit (Pierce Biotechnology, Rockford, IL), as described previously.28, 31 Briefly, single stranded oligos were incubated with Terminal Deoxynucleotidyl Transferase (TdT) and Biotin-11-dUTP in binding mixture for 30 min at 37 °C. The TdT adds a biotin labeled dUTP to the 3′-end of the oligonucleotides. The oligos were extracted using chloroform and isoamyl alcohol to remove the enzyme and unincorporated biotin-11-dUTP. Dot blots were performed to ensure the oligos were labeled equally. Combining sense and antisense oligos and exposing to 95 °C for 5 min was done to anneal complementary oligos. The labeled oligonucleotides were then incubated with or without nuclear extracts in the binding buffer (from LightShift kit). Binding reactions were performed in 20 μl containing 50 fmol oligonucleotieds probes, 1× binding buffer, 1 μg of poly (dI-dC), and 5 μg of nuclear extracts. For competitions studies, increasing concentrations of non-labeled oligonucleotides were added to binding reactions. For super-shift assays, 2 μl of affinity purified Sp1 antibody (Active Motif) or ERα antibody (Thermo Scientific, Fremont, CA) were added to the binding reaction. The samples were then run on a native 5% polyacrylamide gel. The contents of the gel were then transferred to a nylon membrane (Pierce) and crosslinked to the membrane using a UV crosslinker (125 mJoules/cm2). Membranes were blocked and then visualized using the reagents provided in the LightShift kit.
Chromatin Immunoprecipitation (ChIP) and Re-ChIP
Chromatin extracts were prepared from uterine arteries. ChIP assays were performed using the ChIP-IT Express kit (Active Motif), as previously described.28,29 Briefly, tissues were exposed to 1% formaldehyde for 10 min to crosslink and maintain DNA/protein interactions. The reactions were stopped with glycine, tissues were washed, and chromatin were isolated and sheared into medium fragments (100 – 1000 bp) using a sonicator. ChIP reactions were performed using an ERα or Sp1 antibodies to precipitate the transcription factor/DNA complex. Re-ChIP reactions were performed with re-ChIP kit (Active Motif) using a Sp1 antibody and then ERα antibody to precipitate the transcription factor/DNA complex. Crosslinking was then reversed and proteins were digested with proteinase K. Primers flanking the SP1-380 binding site were: 5′-GTCAAAGGCTGAGGGTTTTG-3′ (forward) and 5′-GGAGGAGGAGTGGAAGCTCT-3′ (reverse). PCR amplification products were visualized on 2% agarose gel stained with ethidium bromide. For quantitative real-time PCR amplification, 45 cycles of real-time PCR were carried out with 3 min initial denaturation followed by 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s, using the iQ SYBR Green Supermix with iCycler real-time PCR system (Bio-Rad).
Site-Directed CpG Methylation Mutagenesis and Reporter Gene Assay
The effect of site-directed CpG methylation on KCNMB1 promoter activity was determined as described previously.23 Briefly, to determine the effect of methylation at the Sp1-380 on KCNMB1 promoter activity, two unique cutting sites were engineered at the Sp1-380 site, with SacII at 5′ upstream and SmaI at 3′ downstream of the Sp1-380 site. A customized 39 bp SacII/SmaI oligonucleotide fragment with methylation at the CpG-383 was synthesized by IDT, and ligated to the KCNMB1 promoter-reporter plasmid with pGL3. An identical 39 bp SacII/SmaI fragment with unmethylated CpG-383 at the Sp1-380 site was served as a control. Amount of formed ligation product was quantified by real-time PCR (forward primer: 5′-GTGACCTGCCTGGGGTCACA; reverse primer: 5′-GGGCTCATCAGCAGCTGGAG) and equal amount of plasmid was used in a transfection assay. Activities of each promoter-reporter constructs were determined as described above. The Sp1-380 methylated or unmethylated oligo pairs used for in vitro methylation assay at the KCNMB1 promoter are listed as following: Sp1-M-sense 5′P-GGGTTTTGGCTGCTGGGCmGGGTTGGAAATGCCAGCCC-3′; Sp1-M-antisense 5′P-GGGCTGGCATTTCCAACCCmGCCCAGCAGCCAAAACCCGC-3′; Sp1-UM-sense 5′P-GGGTTTTGGCTGCTGGGCGGGTTGGAAATGCCAGCCC-3′; Sp1-UM-antisense 5′P-GGGCTGGCATTTCCAACCCGCCCAGCAGCCAAAACCCGC-3′.
Data analysis
Data are expressed as mean ± SEM. Statistical significance (P < 0.05) was determined by analysis of variance (ANOVA) followed by Neuman-Keuls post hoc testing or Student’s t test, where appropriate.
Results
Pregnancy Up-regulated KCNMB1 mRNA Expression in Uterine Arteries
Our previous studies demonstrated that pregnancy or steroid hormone treatment significantly increased KCNMB1 protein abundance in uterine arteries, which was inhibited by chronic hypoxia during gestation.20, 21 Consistent with this finding, pregnancy and steroid hormone treatment significantly increased KCNMB1 mRNA abundance in uterine arteries (Figure 1). In sheep acclimatized to long-term high altitude hypoxia, however, this pregnancy or steroid hormone-induced increase in KCNMB1 mRNA expression was abrogated (Figure 1).
Figure 1. Effect of pregnancy and chronic hypoxia on KCNMB1 mRNA expression.
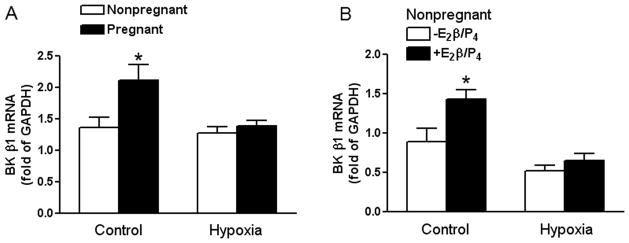
Panel A: Uterine arteries were isolated from nonpregnant and pregnant sheep maintained at sea level (Control) and long-term high-altitude hypoxia (Hypoxia). Panel B: Uterine arteries were isolated from nonpregnant sheep maintained at sea level (Control) and long-term high-altitude hypoxia (Hypoxia), and were treated ex vivo with 17β-estradiol (E2β; 0.3 nmol/L) plus progesterone (P4; 100.0 nmol/L) under 21% O2 (Control) or 10.5% O2 (Hypoxia), respectively for 48 hours. KCNMB1 mRNA abundance was determined by real-time RT-PCR. Data are mean ± SEM. * P < 0.05. n=5
Sequencing of Ovine KCNMB1 Gene Proximal Regulatory Region
To investigate the mechanisms underlying this pregnancy-induced increase in KCNMB1 gene transcription, the proximal regulatory region upstream of the ovine KCNMB1 gene was cloned based on the bovine KCNMB1 proximal promoter sequences obtained from GenBank (Gene ID, 407176). As shown in Figure S1 (available in the online data supplement), the sequence of this regulatory region consists of a 2315-bp ovine genomic fragment spanning from −2211 bp to +104 bp relative to the transcription start site (TSS). This regulatory region shows over 90% of homology to the bovine KCNMB1 promoter.
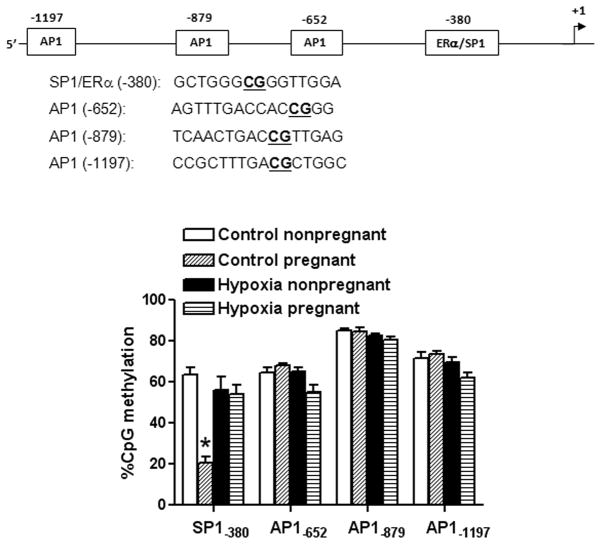
Pregnancy Decreased CpG Methylation at the Sp1-380 Binding Site
Bioinformatics analysis of the ovine KCNMB1 promoter sequence identified several putative transcription factor binding sites that contained CpG dinucleotides in or near their core binding sequences, including Sp1 at −380, AP1 at −652, −879 and −1202 (Figure S1). CpG methylation patterns at these binding sites were determined by quantitative methylation-specific PCR. As shown in Figure 2, CpG dinucleotides of these binding sites at KCNMB1 gene promoter were highly methylated in uterine arteries of nonpregnant sheep. Pregnancy selectively decreased CpG methylation at the Sp1-380 binding site (Figure 2). This pregnancy-mediated demethylation was not seen in uterine arteries from animals acclimatized to long-term high altitude hypoxia (Figure 2).
Figure 2. Effect of pregnancy and chronic hypoxia on KCNMB1 promoter methylation.
Uterine arteries were isolated from nonpregnant and pregnant sheep maintained at sea level (Control) and long-term high-altitude hypoxia (Hypoxia). CpG methylation at Sp1-380, Ap1-652, Ap1-879 and Ap1-652 binding sites was determined. Data are mean ± SEM. * P < 0.05, versus nonpregnant. n=5
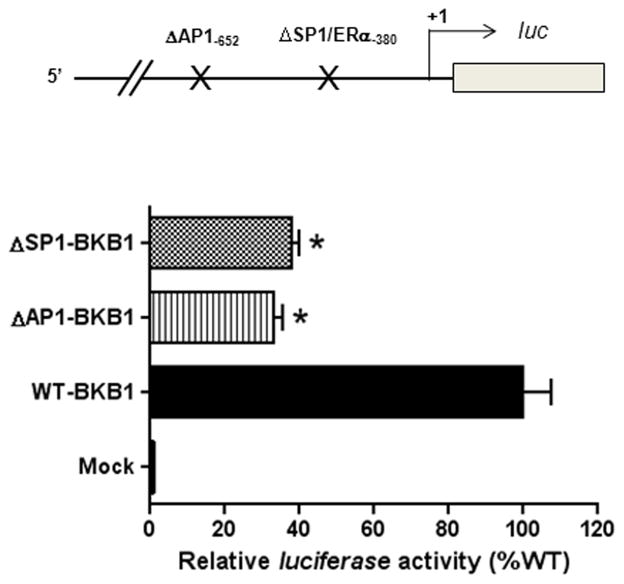
Deletion of the Sp1-380 Binding Site Inhibited KCNMB1 Promoter Activity
To determine the functional significance of Sp1-380 binding site in regulating KCNMB1 promoter activity, reporter gene assay was performed with KCNMB1 promoter constructs containing site-directed deletion at the Sp1-380, as we as AP1-652 sites in uterine arterial smooth muscle cells. As shown in Figure 3, deletion of Sp1-380 or AP1-652 sites resulted in a significant decrease in KCNMB1 promoter activity, indicating a strong stimulatory role of these promoter elements in driving the transcription of ovine KCNMB1 promoter.
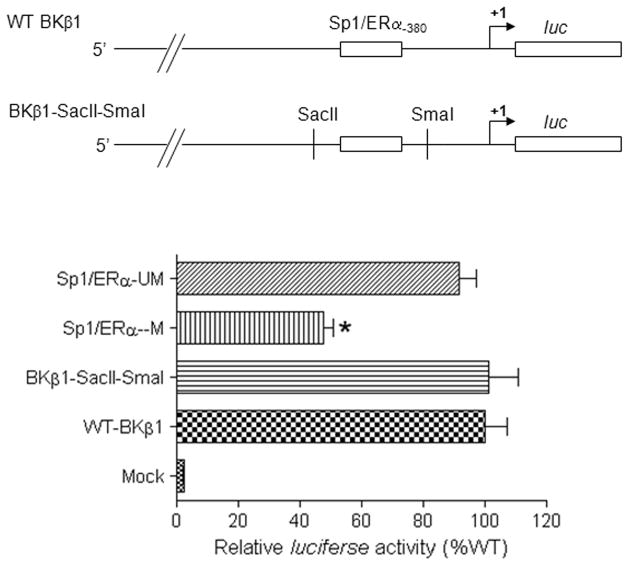
Figure 3. Effect of Sp1-380 and Ap1-652 deletion on KCNMB1 promoter activity.
A 2315 bp fragment of ovine KCNMB1 promoter region spanning from −2211 bp to +104 bp relative to the transcription start site was amplified by PCR and inserted into pGL3 to yield the full-length promoter reporter plasmid (WT-KCNMB1). Two site-specific deletion mutations at Ap1-652 (ΔAP1-KCNMB1) and Sp1-380 (ΔSp1-KCNMB1) were constructed. Constructs were transfected into uterine arterial smooth muscle cells. Firefly and Renilla reniformis luciferase activities were measured in a luminometer using a dual-luciferase reporter assay system. Data are mean ± SEM. *P < 0.05, versus WT-BKβ1. n=5
CpG Methylation Blocked Sp1 Binding to KCNMB1 Promoter
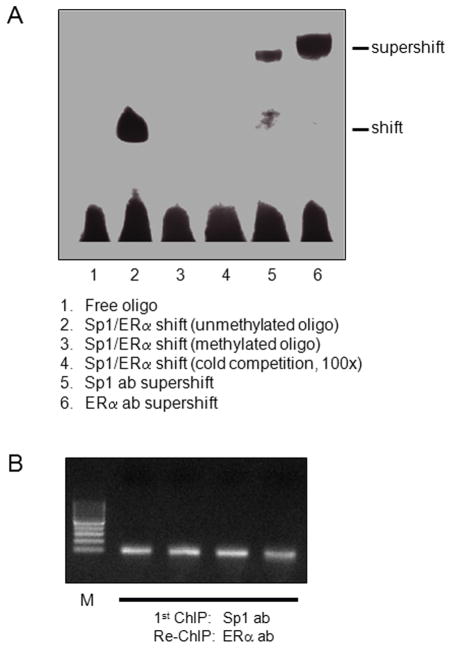
Given that the Sp1-380 binding site has a strong stimulatory effect on KCNMB1 promoter activity, and that pregnancy selectively altered the methylation status at this binding site, our further investigation was focused on the Sp1 element. To evaluate Sp1 binding to the Sp1-380 site at KCNMB1 promoter, we took the approach of electrophoretic mobility shift assay with oligonucleotide probes encompassing the putative Sp1-380 binding site. As shown in Figure 4A, incubation of nuclear extracts from uterine arteries with double-stranded oligonucleotide probes encompassing the putative Sp1-380 binding site resulted in the appearance of a DNA-protein complex (lane 2), the formation of which was inhibited in the presence of 100-fold excess of unlabeled Sp1 probes (lane 4), demonstrating a specific binding of nuclear proteins to the Sp1-380 oligonucleotide probes. Super-shift analyses showed that both Sp1 (lane 5) and ERα (lane 6) antibodies caused super-shifting of the DNA-protein complex, indicating that both Sp1 and ERα were capable of binding to this Sp1-380 binding site. The crosstalk of ERα and Sp1 in binding to the Sp1-380 site at KCNMB1 promoter was further determined by Re-ChIP assay, performed with an ERα antibody using chromatins that were immunoprecipitated by a Sp1 antibody in uterine arteries. As shown in Figure 4B, ERα and Sp1 interacted and bound at the same time to Sp1-380 binding site, indicating their cooperation in the regulation of KCNMB1 gene expression. Of importance, oligonucleotide probes with methylated CpG at the core binding sequence of the Sp1-380 site inhibited the binding of nuclear proteins (Figure 4A, lane 3).
Figure 4. Effect of CpG methylation on Sp1/ERα binding to Sp1-380 element.
Panel A: Nuclear extracts were prepared from uterine arteries and incubated with double-stranded oligonucleotide probes containing unmethylated or methylated CpG at the Sp1-380 binding element in the absence (lane 1 to 3) or presence (lane 4) of unlabeled cold competitor oligonucleotides at a 100-fold molar excess. Lanes 5 and 6, nuclear extracts were incubated with oligonucleotide probes in the presence of antibodies against Sp1 or ERα, respectively, showing supershift of the Sp1/ERα-DNA complex. Panel B: Chromatins were prepared from uterine arteries and immunoprecipitated by a Sp1 antibody followed by re-immunoprecipitation with an ERα antibody in a re-ChIP assay. PCR products of primers flanking the SP1-380 binding site were visualized by ethidium bromide on an agarose gel, showing the crosstalk of ERα and Sp1 in binding to the Sp1-380 site at KCNMB1 promoter.
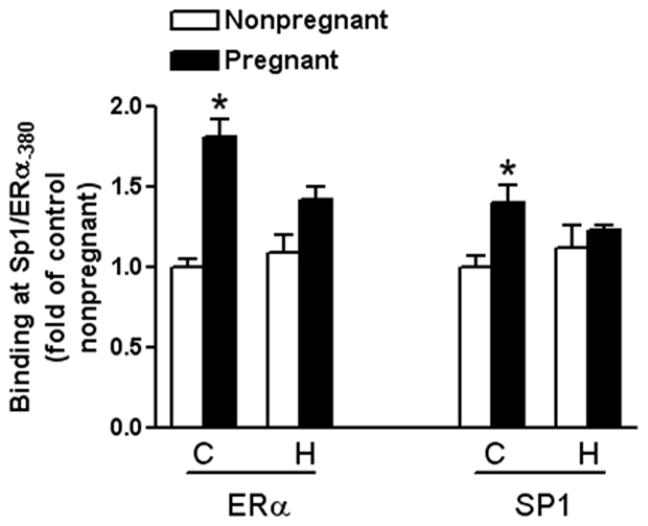
Pregnancy Increased Sp1 and ERα Binding to the Sp1-380 Binding Site
Because pregnancy caused demethylation of the Sp1-380 binding site in uterine arteries, and gestational hypoxia abrogated this adaptation (Figure 2), we then determined the effect of pregnancy and chronic hypoxia on Sp1 and ERα binding to the Sp1 element at KCNMB1 promoter in vivo in the context of intact chromatin via chromatin immunoprecipitation (ChIP) assays. As shown in Figure 5, pregnancy significantly increased both ERα and Sp1 binding at the Sp1-380 site in uterine arteries. In contrast, in animals acclimatized to long-term high-altitude hypoxia, this pregnancy-induced increase of ERα and Sp1 binding was abolished (Figure 5).
Figure 5. Effect of pregnancy and chronic hypoxia on Sp1 and ERα binding to KCNMB1 promoter.
ERα and Sp1 binding to KCNMB1 promoter in vivo in the context of intact chromatin was determined with chromatin immunoprecipitation (ChIP) assays in uterine arteries from nonpregnant or pregnant sheep of normoxic control (C) and long-term high-altitude hypoxia (H). Data are mean ± SEM. * P < 0.05, versus nonpregnant. n=5
Methylation of Sp1-380 Site Reduced KCNMB1 Promoter Activity
The functional significance of CpG methylation rendering the inhibition of Sp1/ERα binding to the promoter was further investigated by determining the effect of site-specific CpG methylation of the Sp1-380 site on KCNMB1 promoter activity. To create a constitutive site-directed CmG at the Sp1-380 site, two restriction enzyme sites, with SacII site at the 5′ upstream and SmaI site at the 3′ downstream of the Sp1-380 site, were engineered in KCNMB1 promoter-luciferase reporter gene constructs. As shown in Figure 6, the insertion of SacII and SmaI sites had no significant effect on the promoter activity compared with the wild-type KCNMB1 promoter. However, the CmG at the Sp1-380 site significantly reduced the KCNMB1 promoter activity (Figure 6).
Figure 6. Effect of CpG methylation at Sp1-380 site on KCNMB1 promoter activity.
Full length wild-type (WT-KCNMB1), methylated CpG at Sp1-380 (Sp1/ERα-M), unmethylated CpG at Sp1-380 (Sp1/ERα-UM), and a dual insertion of SacII and SmaI sites (KCNMB1-SacII-SmaI) constructs were transfected into uterine arterial smooth muscle cells. Firefly and Renilla reniformis luciferase activities were measured in a luminometer using a dual-luciferase reporter assay system. Data are mean ± SEM. * P < 0.05, versus KCNMB1-SacII-SmaI. n=5
Pregnancy Increased Sp1/ERα Binding Affinity to the Sp1-380 Site
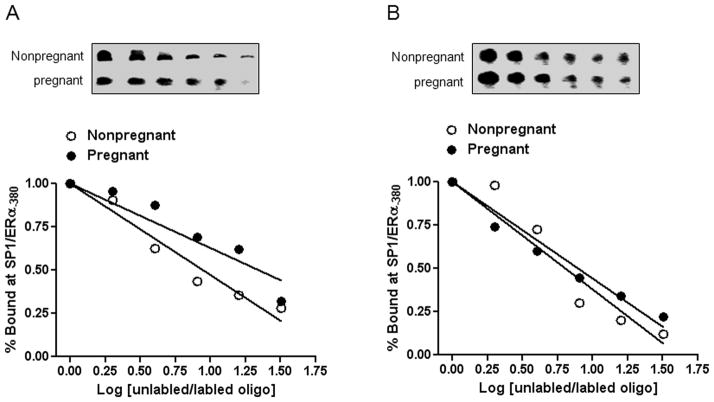
In addition to regulating promoter methylation, we determined whether pregnancy affected Sp1/ERα binding affinity to the Sp1-380 site. In both normoxia control and hypoxic animals, no significant difference in nuclear Sp1 protein abundance was observed in uterine arteries between pregnant and nonpregnant sheep (Figure S2, available in the online data supplement). To determine the binding affinity of Sp1/ERα to the Sp1-380 site, competition EMSA assay was performed in pooled nuclear extracts from uterine arteries in each group of animals with increasing ratios of unlabelled/labelled oligonucleotides encompassing the Sp1-380 site. In normoxia control sheep, pregnancy significantly increased the binding affinity of nuclear extracts to the Sp1-380 site (Figure 7). In sheep acclimatized to long-term high-altitude hypoxia, however, no significant difference was observed in Sp1/ERα binding affinity between pregnant and nonpregnant animals (Figure 7).
Figure 7. Effect of pregnancy and chronic hypoxia on Sp1/ERα binding affinity.
Pooled nuclear extracts of uterine arteries were incubated with labeled oligonucleotide probes containing the Sp1-380 binding site in the presence of 1, 2, 4, 8, 16, and 32 folds of the unlabeled oligonucleotides. A: Normoxic control; B: Long-term high-altitude hypoxia.
Discussion
The previous electrophysiological and functional studies demonstrated that pregnancy and steroid hormones induced a significant increase in the BKca β1 subunit expression, resulting in the increased BKca channel activity in uterine arteries in sheep.5–7, 20, 21 The present study provides evidence of a novel mechanism of promoter demethylation in pregnancy-induced reprogramming of increased BKCa channel expression and function in uterine arteries. In addition, this pregnancy-induced adaptation is inhibited by chronic hypoxia during gestation. We had cloned the proximal regulatory region upstream of the ovine KCNMB1 gene based on the bovine KCNMB1 proximal promoter sequences before the ovine genome sequence was available at NCBI. The cloned ovine KCNMB1 promoter sequence is nearly identical to that in the ovine genome sequence and has over 90% homology to the bovine KCNMB1 promoter. Both of them lack the canonical TATA-like element. Multiple Ap1 and Sp1 response elements were identified by bioinformatics analysis at both bovine and ovine KCNMB1 promoters, and these elements are located almost in the same region. Unlike the bovine Ap1 sites which lack the CpGs, the ovine Ap1 sites contain CpG dinucleotides within or close to their core binding sequences. In contrast, the Sp1 binding site identified is highly homologous among ovine (Sp1-380), bovine (Sp1-417) and human (Sp1-387) KCNMB1 gene promoters, thus showing translational applicability.
The finding that both Sp1 and ERα antibodies caused super-shifting of the DNA-protein complex formed from binding of uterine artery nuclear extracts with the double-stranded oligonucleotide probes containing the Sp1 element is intriguing and indicates a true ERα/Sp1 binding site at ovine KCNMB1 promoter. Similar findings were obtained in the Sp1 binding elements at ERα promoter in uterine arteries28 as well as protein kinase C-ε (PKCε) promoter in the heart,29 in which ERα bound to the Sp1 sites. Our previous study demonstrated that estrogen increased KCNMB1 protein abundance in uterine arteries.20 In agreement with this finding, the present study demonstrated steroid hormone-induced increase in KCNMB1 mRNA expression. Given that ovine KCNMB1 promoter region contains no estrogen response element (ERE), the finding of ERα binding to the Sp1 site is of importance, and provides a novel mechanism that estrogen may regulate KCNMB1 gene expression through binding of ERα to the Sp1 site through “tethering” to Sp1 at the KCNMB1 promoter. Several studies have demonstrated an alternative mode of activation by estrogen via interactions with other transcription factors in addition to binding to ERE.32, 33 ER can interact with both Ap1 and Sp1 transcription factors and bind to their binding sites in regulating the expression of genes including human creatine kinase B, c-myc, heat shock protein 27 and ovine estrogen receptor in the uterine arteries.28, 34 In the present study, the crosstalk of ERα and Sp1 in binding to the Sp1-380 response element at KCNMB1 promoter was further determined in vivo in the context of intact chromatin by chromatin reimmunoprecipitation assay in uterine arteries.
The functional significance of the Sp1-380 binding element in the regulation of the ovine KCNMB1 gene expression was demonstrated by the finding that deletion of this site significantly decreased the KCNMB1 promoter activity. The importance of promoter Sp1 elements in mediating estrogen response in regulating gene expression has been demonstrated in several genes including estrogen-mediated regulation of ERα transcription in uterine arteries. This was demonstrated by the finding that the estrogen-induced increase in ovine ERα promoter activity in uterine arterial smooth muscle cells was abrogated by the deletion of the Sp1 binding site.28
In the present study, we have shown that the CpG dinucleotides transcription factor binding sites at KCNMB1 gene promoter were highly methylated in uterine arteries of nonpregnant sheep, indicating a reduced expression of KCNMB1 and BKCa channel activity in nonpregnant uterine arteries as compared with uterine arteries from pregnant animals.20 Given that ovine, similar to bovine, KCNMB1 gene promoter lacks a TATA-like element, the enrichment of CpGs found at the promoter suggests a rigorous regulation of KCNMB1 gene expression by methylation. In contrast to KCNMB1 gene, BKCa α subunit gene promoter lacks GC box or Sp1 binding site and its expression is not regulated by pregnancy or chronic hypoxia.20, 21 Although the transcriptional regulation by DNA methylation is commonly observed in CpG islands located around the promoter region and is mediated by the methylation-specific binding of methylated CpG-binding proteins,35, 36 CpG methylation of specific transcription factor binding sites can alter gene expression through changes in the binding affinity of transcription factors to promoters.37–39 The present study provides several lines of evidence showing that CpG methylation at Sp1-380 binding site suppresses transcription factor binding and KCNMB1 promoter activity. Thus, the finding that nuclear extracts from uterine arteries shifted oligonucleotides encompassing the Sp1 binding site with unmethylated CpG but failed to cause a shift with methylated CpG, indicates that CpG methylation in a sequence-specific binding site may directly inhibit the transcription factor binding. Similar findings were obtained with Sp1 elements at ERα and PKCε promoters.28, 29 Additionally, we demonstrated that the binding of Sp1 and ERα to the Sp1-380 site was reduced in vivo in the context of intact chromatin in uterine arteries of nonpregnant animals that had higher levels of CpG methylation. Of importance, the functional significance of CpG methylation at the Sp1-380 binding site in regulating KCNMB1 gene expression was demonstrated by site-specific methylation of KCNMB1 promoter selectively at Sp1-380 site, showing that the mutation of CmG at the Sp1 binding site significantly decreased the promoter activity. In agreement to this finding, our previous studies have shown that site-specific methylation of Sp1 elements inhibits ERα and PKCε promoter activities.28, 40 These findings suggest a common mechanism of CpG methylation at Sp1 binding elements in regulating gene transcription.
The finding that pregnancy selectively decreased CpG methylation in the SP1-380 binding site at KCNMB1 promoter is intriguing and suggests a highly novel mechanism of pregnancy-induced DNA demethylation in a sequence-specific manner at transcription factor binding elements in a promoter region. Thus, this pregnancy-mediated promoter demethylation increased Sp1/ERα binding to the Sp1 element and up-regulated KCNMB1 gene expression in uterine arteries. While the mechanisms of pregnancy-induced demethylation in uterine arteries remain to be determined, several recent studies have suggested a robust mechanism of ten-eleven translocation 1-3 (TET1-3) proteins in active DNA demethylation, resulting in increased expression of associated genes in adult tissues both in vitro and in vivo.41–48 Indeed, estrogen was recently reported to induce active DNA demethylation on promoters of estrogen-response genes. For example, the pS2 gene underwent a switch from repression to expression under the stimulation of estrogen, which was caused by cyclic rounds of active methylation and demethylation at its promoter.49, 50 In the present study, we have shown that chronic hypoxia during gestation inhibits this pregnancy-induced promoter demethylation and abrogates pregnancy-mediated increase in Sp1/ERα binding and KCNMB1 gene expression in uterine arteries. Given the complexity of potential DNA demethylation mechanisms, separate studies are warranted and are currently undergoing to investigate how pregnancy induces KCNMB1 gene demethylation and how hypoxia inhibits this demethylation in uterine arteries. The finding that pregnancy increased the binding affinity of nuclear extracts to the Sp1-380 site suggests that, in addition to inducing promoter demethylation, pregnancy also enhances Sp1/ERα binding to unmethylated Sp1-380 site at KCNMB1 promoter. Gestational hypoxia did not alter maternal plasma estrogen levels but significantly suppressed ERα expression in uterine arteries,28, 51 suggesting a possible cause in hypoxic abrogation of the pregnancy-induced adaptation. Of importance functionally, our previous studies demonstrated that pregnancy- and steroid hormone-induced up-regulation of β1:α subunit stoichiometry significantly increased the BKca channel activity and decreased myogenic tone in uterine arteries, which was abolished by chronic hypoxia during gestation.20, 21
Perspectives
During gestation, uterine arteries undergo profound physiological adaptation to increase uterine blood flow both for optimal growth and survival of the fetus and for cardiovascular well-being of the mother. Yet, the molecular mechanisms for this adaptation remain largely elusive. The BKca channel plays a critical role in the adaptation of uterine circulation and increased uterine blood flow in pregnancy. The present investigation provides evidence of a highly novel mechanism of promoter demethylation at sequence-specific transcription factor binding sites in epigenetic upregulation of BKCa channel expression and activity in uterine vascular adaptation to pregnancy. Of importance, the finding that chronic hypoxia inhibits this pregnancy-induced adaptation suggests a novel epigenetic mechanism linking gestational hypoxia to aberrant uteroplacental circulation. Given that impaired uteroplacental circulation in both humans and several animal models has been implicated in preeclampsia,52, 53 the present findings may help improve the understanding of preeclampsia, albeit it is a human specific disorder. In addition, because of the vital importance of BKca channel function in determining vascular tone in virtually all vascular beds, and the extremely limited current knowledge in epigenetic regulation of BKca channel expression and activity in vascular smooth muscle in general, the present finding indeed has a broad impact in comprehensive understanding of molecular mechanisms in regulating BKca channel activity and the homeostasis of vascular tone.
Supplementary Material
Novelty and Significance.
What Is New?
KCNMB1 promoter is highly methylated in uterine arteries of nonpregnant sheep.
Pregnancy induces promoter demethylation and upregulation of KCNMB1 expression in uterine arteries.
Gestational hypoxia abrogates pregnancy-induced epigenetic reprogramming of KCNMB1 expression.
What Is Relevant?
The BKCa channel plays a critical role in regulating uterine blood flow during pregnancy, and suppression of KCNMB1 leads to reductions in BKCa channel activity.
Gestational hypoxia and reduced uteroplacental perfution are major risks for preeclampsia.
New insights of molecular mechanisms of KCNMB1 expression have impact in comprehensive understanding of BKca channel activity and vascular function in the homeostasis of blood pressure regulation.
Summary
The present study demonstrates a novel mechanism of promoter demethylation in pregnancy-induced reprogramming of BKCa channel expression and function in uterine arteries, and suggests new insights of epigenetic mechanisms linking gestational hypoxia to aberrant uteroplacental circulation and increased risk of preeclampsia.
Acknowledgments
Source of Funding
This work was supported by National Institutes of Health grants HD031226 (L.Z.), HL089012 (L.Z.) and HL110125 (L.Z.).
Footnotes
Disclosures
None.
References
- 1.Julian CG, Galan HL, Wilson MJ, Desilva W, Cioffi-Ragan D, Schwartz J, Moore LG. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol Regul Integr Comp Physiol. 2008;295:R906–915. doi: 10.1152/ajpregu.00164.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore LG, Charles SM, Julian CG. Humans at high altitude: Hypoxia and fetal growth. Respir Physiol Neurobiol. 2011;178:181–190. doi: 10.1016/j.resp.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in colorado. Am J Obstet Gynecol. 1999;180:1161–1168. doi: 10.1016/s0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- 4.Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA, Moore LG. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol (1985) 1995;79:15–22. doi: 10.1152/jappl.1995.79.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld CR, Cornfield DN, Roy T. Ca(2+)-activated k(+) channels modulate basal and e(2)beta-induced rises in uterine blood flow in ovine pregnancy. Am J Physiol Heart Circ Physiol. 2001;281:H422–431. doi: 10.1152/ajpheart.2001.281.1.H422. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld CR, Liu XT, DeSpain K. Pregnancy modifies the large conductance ca2+-activated k+ channel and cgmp-dependent signaling pathway in uterine vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296:H1878–1887. doi: 10.1152/ajpheart.01185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenfeld CR, Roy T, DeSpain K, Cox BE. Large-conductance ca2+-dependent k+ channels regulate basal uteroplacental blood flow in ovine pregnancy. J Soc Gynecol Investig. 2005;12:402–408. doi: 10.1016/j.jsgi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Ahn YT, Kim YM, Adams E, Lyu SC, Alvira CM, Cornfield DN. Hypoxia-inducible factor-1alpha regulates kcnmb1 expression in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2012;302:L352–359. doi: 10.1152/ajplung.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu XQ, Zhang L. Function and regulation of large conductance ca(2+)-activated k+ channel in vascular smooth muscle cells. Drug Discov Today. 2012;17:974–987. doi: 10.1016/j.drudis.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka Y, Koike K, Alioua A, Shigenobu K, Stefani E, Toro L. Beta1-subunit of maxik channel in smooth muscle: A key molecule which tunes muscle mechanical activity. J Pharmacol Sci. 2004;94:339–347. doi: 10.1254/jphs.94.339. [DOI] [PubMed] [Google Scholar]
- 12.Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mslo and slowpoke family of potassium channels. J Biol Chem. 1994;269:3921–3924. [PubMed] [Google Scholar]
- 13.Wulf H, Hay-Schmidt A, Poulsen AN, Klaerke DA, Olesen J, Jansen-Olesen I. Molecular investigations of bk(ca) channels and the modulatory beta-subunits in porcine basilar and middle cerebral arteries. J Mol Histol. 2009;40:87–97. doi: 10.1007/s10735-009-9216-3. [DOI] [PubMed] [Google Scholar]
- 14.Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted bk channel beta1 subunit gene feature abnormal ca(2+) spark/stoc coupling and elevated blood pressure. Circ Res. 2000;87:E53–60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 15.Cox DH, Aldrich RW. Role of the beta1 subunit in large-conductance ca(2+)-activated k(+) channel gating energetics. Mechanisms of enhanced ca(2+) sensitivity. J Gen Physiol. 2000;116:411–432. doi: 10.1085/jgp.116.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, ca(2+) activated k(+) channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao G, Zhao Y, Pan B, Liu J, Huang X, Zhang X, Cao C, Hou N, Wu C, Zhao KS, Cheng H. Hypersensitivity of bkca to ca2+ sparks underlies hyporeactivity of arterial smooth muscle in shock. Circ Res. 2007;101:493–502. doi: 10.1161/CIRCRESAHA.107.157271. [DOI] [PubMed] [Google Scholar]
- 18.Khan LH, Rosenfeld CR, Liu XT, Magness RR. Regulation of the cgmp-cpkg pathway and large-conductance ca2+-activated k+ channels in uterine arteries during the ovine ovarian cycle. Am J Physiol Endocrinol Metab. 2010;298:E222–228. doi: 10.1152/ajpendo.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagar D, Liu XT, Rosenfeld CR. Estrogen regulates {beta}1-subunit expression in ca(2+)-activated k(+) channels in arteries from reproductive tissues. Am J Physiol Heart Circ Physiol. 2005;289:H1417–1427. doi: 10.1152/ajpheart.01174.2004. [DOI] [PubMed] [Google Scholar]
- 20.Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson S, Zhang L. Pregnancy upregulates large-conductance ca(2+)-activated k(+) channel activity and attenuates myogenic tone in uterine arteries. Hypertension. 2011;58:1132–1139. doi: 10.1161/HYPERTENSIONAHA.111.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson SM, Zhang L. Chronic hypoxia suppresses pregnancy-induced upregulation of large-conductance ca2+-activated k+ channel activity in uterine arteries. Hypertension. 2012;60:214–222. doi: 10.1161/HYPERTENSIONAHA.112.196097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaenisch R, Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33 (Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 23.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 24.Reik W, Dean W. DNA methylation and mammalian epigenetics. Electrophoresis. 2001;22:2838–2843. doi: 10.1002/1522-2683(200108)22:14<2838::AID-ELPS2838>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Alikhani-Koopaei R, Fouladkou F, Frey FJ, Frey BM. Epigenetic regulation of 11 beta-hydroxysteroid dehydrogenase type 2 expression. J Clin Invest. 2004;114:1146–1157. doi: 10.1172/JCI21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antequera F, Boyes J, Bird A. High levels of de novo methylation and altered chromatin structure at cpg islands in cell lines. Cell. 1990;62:503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- 27.Jones PL, Wolffe AP. Relationships between chromatin organization and DNA methylation in determining gene expression. Semin Cancer Biol. 1999;9:339–347. doi: 10.1006/scbi.1999.0134. [DOI] [PubMed] [Google Scholar]
- 28.Dasgupta C, Chen M, Zhang H, Yang S, Zhang L. Chronic hypoxia during gestation causes epigenetic repression of the estrogen receptor-alpha gene in ovine uterine arteries via heightened promoter methylation. Hypertension. 2012;60:697–704. doi: 10.1161/HYPERTENSIONAHA.112.198242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of pkc{epsilon} gene repression in rat hearts. Circ Res. 2010;107:365–373. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrence J, Chen M, Xiong F, Xiao D, Zhang H, Buchholz JN, Zhang L. Foetal nicotine exposure causes pkcepsilon gene repression by promoter methylation in rat hearts. Cardiovasc Res. 2011;89:89–97. doi: 10.1093/cvr/cvq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Xiao D, Yang S, Zhang L. Promoter methylation represses at2r gene and increases brain hypoxic-ischemic injury in neonatal rats. Neurobiol Dis. 2013;60:32–38. doi: 10.1016/j.nbd.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porter W, Wang F, Wang W, Duan R, Safe S. Role of estrogen receptor/sp1 complexes in estrogen-induced heat shock protein 27 gene expression. Mol Endocrinol. 1996;10:1371–1378. doi: 10.1210/mend.10.11.8923463. [DOI] [PubMed] [Google Scholar]
- 33.Gruber CJ, Gruber DM, Gruber IM, Wieser F, Huber JC. Anatomy of the estrogen response element. Trends Endocrinol Metab. 2004;15:73–78. doi: 10.1016/j.tem.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Safe S. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-sp1 interactions. Vitam Horm. 2001;62:231–252. doi: 10.1016/s0083-6729(01)62006-5. [DOI] [PubMed] [Google Scholar]
- 35.Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 36.Wade PA. Methyl cpg-binding proteins and transcriptional repression. Bioessays. 2001;23:1131–1137. doi: 10.1002/bies.10008. [DOI] [PubMed] [Google Scholar]
- 37.Campanero MR, Armstrong MI, Flemington EK. Cpg methylation as a mechanism for the regulation of e2f activity. Proc Natl Acad Sci U S A. 2000;97:6481–6486. doi: 10.1073/pnas.100340697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimoto M, Kitazawa R, Maeda S, Kitazawa S. Methylation adjacent to negatively regulating ap-1 site reactivates trka gene expression during cancer progression. Oncogene. 2005;24:5108–5118. doi: 10.1038/sj.onc.1208697. [DOI] [PubMed] [Google Scholar]
- 39.Zhu WG, Srinivasan K, Dai Z, Duan W, Druhan LJ, Ding H, Yee L, Villalona-Calero MA, Plass C, Otterson GA. Methylation of adjacent cpg sites affects sp1/sp3 binding and activity in the p21(cip1) promoter. Mol Cell Biol. 2003;23:4056–4065. doi: 10.1128/MCB.23.12.4056-4065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer KD, Zhang H, Zhang L. Prenatal cocaine exposure abolished ischemic preconditioning-induced protection in adult male rat hearts: Role of pkcepsilon. Am J Physiol Heart Circ Physiol. 2009;296:H1566–1576. doi: 10.1152/ajpheart.00898.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by tet1 promotes active DNA demethylation in the adult brain. Cell. 2011;145:423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of tet proteins in 5mc to 5hmc conversion, es-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by mll partner tet1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PA, Rappsilber J, Helin K. Tet1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473:343–348. doi: 10.1038/nature10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H, Zhang Y. Mechanisms and functions of tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 2011;25:2436–2452. doi: 10.1101/gad.179184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhutani N, Burns DM, Blau HM. DNA demethylation dynamics. Cell. 2011;146:866–872. doi: 10.1016/j.cell.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet. 2012;13:7–13. doi: 10.1038/nrg3080. [DOI] [PubMed] [Google Scholar]
- 49.Kangaspeska S, Stride B, Metivier R, Polycarpou-Schwarz M, Ibberson D, Carmouche RP, Benes V, Gannon F, Reid G. Transient cyclical methylation of promoter DNA. Nature. 2008;452:112–115. doi: 10.1038/nature06640. [DOI] [PubMed] [Google Scholar]
- 50.Metivier R, Gallais R, Tiffoche C, Le Peron C, Jurkowska RZ, Carmouche RP, Ibberson D, Barath P, Demay F, Reid G, Benes V, Jeltsch A, Gannon F, Salbert G. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- 51.Chang K, Xiao D, Huang X, Xue Z, Yang S, Longo LD, Zhang L. Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension. 2010;56:750–757. doi: 10.1161/HYPERTENSIONAHA.110.155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexander BT, Bennett WA, Khalil RA, Granger JP. Preeclampsia: linking placental ischemia with cardiovascular-renal dysfunction. News Physiol Sci. 2001;16:282–286. doi: 10.1152/physiologyonline.2001.16.6.282. [DOI] [PubMed] [Google Scholar]
- 53.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol. 2007;293:H2080–H2084. doi: 10.1152/ajpheart.00667.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.