Abstract
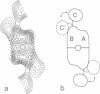
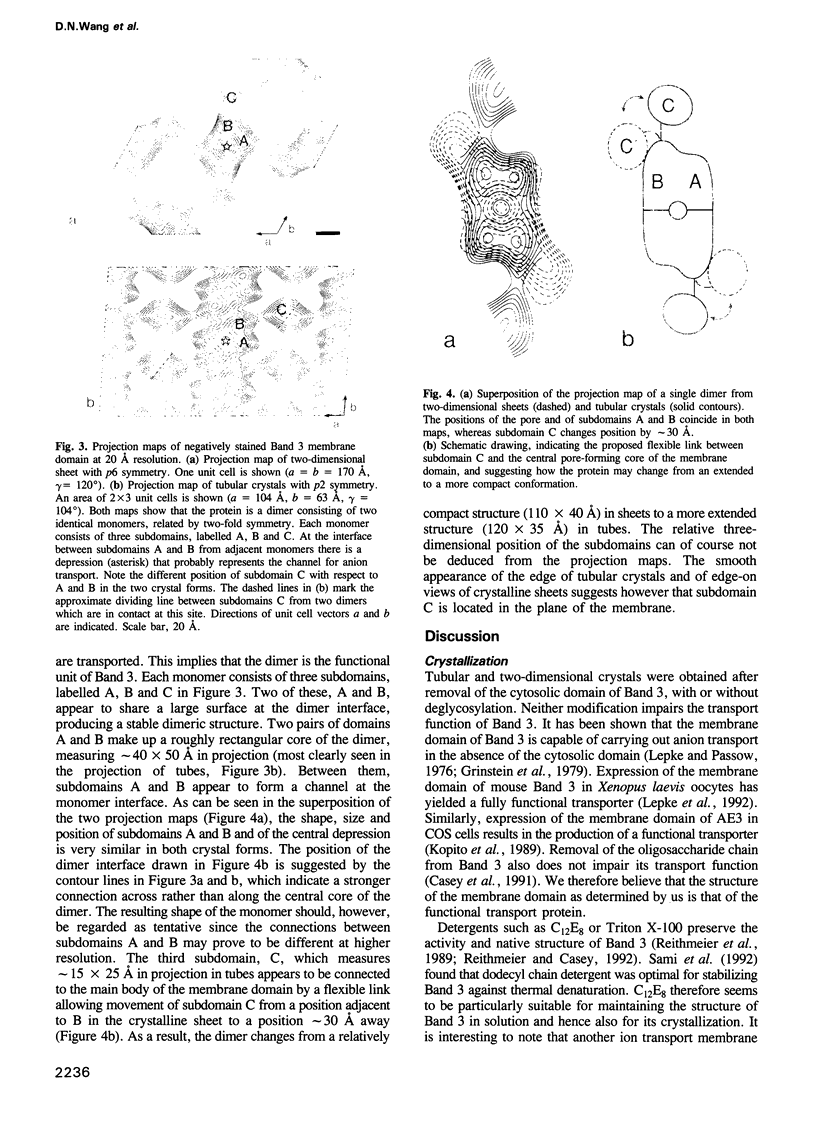
The membrane domain of human erythrocyte Band 3 protein (M(r) 52,000) was reconstituted with lipids into two-dimensional crystals in the form of sheets or tubes. Crystalline sheets were monolayers with six-fold symmetry (layer group p6, a = b = 170 A, gamma = 60 degrees), whereas the symmetry of the tubular crystals was p2 (a = 104 A, b = 63 A, gamma = 104 degrees). Electron image analysis of negatively stained specimens yielded projection maps of the protein at 20 A resolution. Maps derived from both crystal forms show that the membrane domain is a dimer of two monomers related by two-fold symmetry, with each monomer consisting of three subdomains. In the dimer, two subdomains of each monomer form a roughly rectangular core (40 x 50 A in projection), surrounding a central depression. The third subdomain of the monomer measures approximately 15 x 25 A in projection and appears to be connected to the other two by a flexible link. We propose that the central depression may represent the channel for anion transport while the third subdomain appears not to be directly involved in channel formation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper S. L., Kopito R. R., Libresco S. M., Lodish H. F. Cloning and characterization of a murine band 3-related cDNA from kidney and from a lymphoid cell line. J Biol Chem. 1988 Nov 15;263(32):17092–17099. [PubMed] [Google Scholar]
- Alper S. L. The band 3-related anion exchanger (AE) gene family. Annu Rev Physiol. 1991;53:549–564. doi: 10.1146/annurev.ph.53.030191.003001. [DOI] [PubMed] [Google Scholar]
- Amos L. A., Henderson R., Unwin P. N. Three-dimensional structure determination by electron microscopy of two-dimensional crystals. Prog Biophys Mol Biol. 1982;39(3):183–231. doi: 10.1016/0079-6107(83)90017-2. [DOI] [PubMed] [Google Scholar]
- Appell K. C., Low P. S. Partial structural characterization of the cytoplasmic domain of the erythrocyte membrane protein, band 3. J Biol Chem. 1981 Nov 10;256(21):11104–11111. [PubMed] [Google Scholar]
- Boodhoo A., Reithmeier R. A. Characterization of matrix-bound Band 3, the anion transport protein from human erythrocyte membranes. J Biol Chem. 1984 Jan 25;259(2):785–790. [PubMed] [Google Scholar]
- Brosius F. C., 3rd, Alper S. L., Garcia A. M., Lodish H. F. The major kidney band 3 gene transcript predicts an amino-terminal truncated band 3 polypeptide. J Biol Chem. 1989 May 15;264(14):7784–7787. [PubMed] [Google Scholar]
- Casey J. R., Lieberman D. M., Reithmeier R. A. Purification and characterization of band 3 protein. Methods Enzymol. 1989;173:494–512. doi: 10.1016/s0076-6879(89)73034-2. [DOI] [PubMed] [Google Scholar]
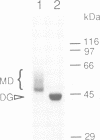
- Casey J. R., Pirraglia C. A., Reithmeier R. A. Enzymatic deglycosylation of human Band 3, the anion transport protein of the erythrocyte membrane. Effect on protein structure and transport properties. J Biol Chem. 1992 Jun 15;267(17):11940–11948. [PubMed] [Google Scholar]
- Casey J. R., Reithmeier R. A. Analysis of the oligomeric state of Band 3, the anion transport protein of the human erythrocyte membrane, by size exclusion high performance liquid chromatography. Oligomeric stability and origin of heterogeneity. J Biol Chem. 1991 Aug 25;266(24):15726–15737. [PubMed] [Google Scholar]
- Davio S. R., Low P. S. Characterization of the calorimetric C transition of the human erythrocyte membrane. Biochemistry. 1982 Jul 20;21(15):3585–3593. doi: 10.1021/bi00258a009. [DOI] [PubMed] [Google Scholar]
- Demuth D. R., Showe L. C., Ballantine M., Palumbo A., Fraser P. J., Cioe L., Rovera G., Curtis P. J. Cloning and structural characterization of a human non-erythroid band 3-like protein. EMBO J. 1986 Jun;5(6):1205–1214. doi: 10.1002/j.1460-2075.1986.tb04348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix J. A., Verkman A. S., Solomon A. K., Cantley L. C. Human erythrocyte anion exchange site characterised using a fluorescent probe. Nature. 1979 Nov 29;282(5738):520–522. doi: 10.1038/282520a0. [DOI] [PubMed] [Google Scholar]
- Dux L., Pikula S., Mullner N., Martonosi A. Crystallization of Ca2+-ATPase in detergent-solubilized sarcoplasmic reticulum. J Biol Chem. 1987 May 15;262(14):6439–6442. [PubMed] [Google Scholar]
- Eidelman O., Yani P., Englert H. C., Lang H. G., Greger R., Cabantchik Z. I. Macromolecular conjugates of transport inhibitors: new tools for probing topography of anion transport proteins. Am J Physiol. 1991 May;260(5 Pt 1):C1094–C1103. doi: 10.1152/ajpcell.1991.260.5.C1094. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Dell A., Oates J. E., Fukuda M. N. Structure of branched lactosaminoglycan, the carbohydrate moiety of band 3 isolated from adult human erythrocytes. J Biol Chem. 1984 Jul 10;259(13):8260–8273. [PubMed] [Google Scholar]
- Grinstein S., Ship S., Rothstein A. Anion transport in relation to proteolytic dissection of band 3 protein. Biochim Biophys Acta. 1978 Feb 21;507(2):294–304. doi: 10.1016/0005-2736(78)90424-8. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Jap B. K., Walian P. J., Gehring K. Structural architecture of an outer membrane channel as determined by electron crystallography. Nature. 1991 Mar 14;350(6314):167–170. doi: 10.1038/350167a0. [DOI] [PubMed] [Google Scholar]
- Jennings M. L. Oligomeric structure and the anion transport function of human erythrocyte band 3 protein. J Membr Biol. 1984;80(2):105–117. doi: 10.1007/BF01868768. [DOI] [PubMed] [Google Scholar]
- Jennings M. L. Structure and function of the red blood cell anion transport protein. Annu Rev Biophys Biophys Chem. 1989;18:397–430. doi: 10.1146/annurev.bb.18.060189.002145. [DOI] [PubMed] [Google Scholar]
- Kang D., Okubo K., Hamasaki N., Kuroda N., Shiraki H. A structural study of the membrane domain of band 3 by tryptic digestion. Conformational change of band 3 in situ induced by alkali treatment. J Biol Chem. 1992 Sep 25;267(27):19211–19217. [PubMed] [Google Scholar]
- Kaplan J. H., Scorah K., Fasold H., Passow H. Sidedness of the inhibitory action of disulfonic acids on chloride equilibrium exchange and net transport across the human erythrocyte membrane. FEBS Lett. 1976 Feb 15;62(2):182–185. doi: 10.1016/0014-5793(76)80048-8. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Membrane protein oligomeric structure and transport function. Nature. 1981 Apr 9;290(5806):449–454. doi: 10.1038/290449a0. [DOI] [PubMed] [Google Scholar]
- Kopito R. R., Lee B. S., Simmons D. M., Lindsey A. E., Morgans C. W., Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell. 1989 Dec 1;59(5):927–937. doi: 10.1016/0092-8674(89)90615-6. [DOI] [PubMed] [Google Scholar]
- Kudrycki K. E., Newman P. R., Shull G. E. cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl-/HCO3- exchanger. J Biol Chem. 1990 Jan 5;265(1):462–471. [PubMed] [Google Scholar]
- Kudrycki K. E., Shull G. E. Primary structure of the rat kidney band 3 anion exchange protein deduced from a cDNA. J Biol Chem. 1989 May 15;264(14):8185–8192. [PubMed] [Google Scholar]
- Köhne W., Haest C. W., Deuticke B. Mediated transport of anions in band 3-phospholipid vesicles. Biochim Biophys Acta. 1981 Jun 9;644(1):108–120. doi: 10.1016/0005-2736(81)90065-1. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W. Two-dimensional crystallization of membrane proteins. Q Rev Biophys. 1992 Feb;25(1):1–49. doi: 10.1017/s0033583500004716. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N. Three-dimensional structure of plant light-harvesting complex determined by electron crystallography. Nature. 1991 Mar 14;350(6314):130–134. doi: 10.1038/350130a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., McIntyre G. F., Parks D. J., Yates J. C., Massenburg D. Bilayer curvature and certain amphipaths promote poly(ethylene glycol)-induced fusion of dipalmitoylphosphatidylcholine unilamellar vesicles. Biochemistry. 1992 Mar 17;31(10):2643–2653. doi: 10.1021/bi00125a003. [DOI] [PubMed] [Google Scholar]
- Lepke S., Becker A., Passow H. Mediation of inorganic anion transport by the hydrophobic domain of mouse erythroid band 3 protein expressed in oocytes of Xenopus laevis. Biochim Biophys Acta. 1992 Apr 29;1106(1):13–16. doi: 10.1016/0005-2736(92)90215-8. [DOI] [PubMed] [Google Scholar]
- Lepke S., Passow H. Effects of incorporated trypsin on anion exchange and membrane proteins in human red blood cell ghosts. Biochim Biophys Acta. 1976 Dec 2;455(2):353–370. doi: 10.1016/0005-2736(76)90311-4. [DOI] [PubMed] [Google Scholar]
- Lindenthal S., Schubert D. Monomeric erythrocyte band 3 protein transports anions. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6540–6544. doi: 10.1073/pnas.88.15.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey A. E., Schneider K., Simmons D. M., Baron R., Lee B. S., Kopito R. R. Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5278–5282. doi: 10.1073/pnas.87.14.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. S., Allen D. P., Zioncheck T. F., Chari P., Willardson B. M., Geahlen R. L., Harrison M. L. Tyrosine phosphorylation of band 3 inhibits peripheral protein binding. J Biol Chem. 1987 Apr 5;262(10):4592–4596. [PubMed] [Google Scholar]
- Low P. S. Structure and function of the cytoplasmic domain of band 3: center of erythrocyte membrane-peripheral protein interactions. Biochim Biophys Acta. 1986 Sep 22;864(2):145–167. doi: 10.1016/0304-4157(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Kopito R. R., Lodish H. F. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1). Proc Natl Acad Sci U S A. 1989 Dec;86(23):9089–9093. doi: 10.1073/pnas.86.23.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara I. G., Cantley L. C. Interactions between transport inhibitors at the anion binding sites of the band 3 dimer. Biochemistry. 1981 Sep 1;20(18):5095–5105. doi: 10.1021/bi00521a001. [DOI] [PubMed] [Google Scholar]
- Macara I. G., Kuo S., Cantley L. C. Evidence that inhibitors of anion exchange induce a transmembrane conformational change in band 3. J Biol Chem. 1983 Feb 10;258(3):1785–1792. [PubMed] [Google Scholar]
- Maneri L. R., Low P. S. Fatty acid composition of lipids which copurify with band 3. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1012–1019. doi: 10.1016/0006-291x(89)92209-2. [DOI] [PubMed] [Google Scholar]
- Maneri L. R., Low P. S. Structural stability of the erythrocyte anion transporter, band 3, in different lipid environments. A differential scanning calorimetric study. J Biol Chem. 1988 Nov 5;263(31):16170–16178. [PubMed] [Google Scholar]
- Mawby W. J., Findlay J. B. Characterization and partial sequence of di-iodosulphophenyl isothiocyanate-binding peptide from human erythrocyte anion-transport protein. Biochem J. 1982 Sep 1;205(3):465–475. doi: 10.1042/bj2050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlebach T., Cherry R. J. Influence of cholesterol on the rotation and self-association of band 3 in the human erythrocyte membrane. Biochemistry. 1982 Aug 31;21(18):4225–4228. doi: 10.1021/bi00261a006. [DOI] [PubMed] [Google Scholar]
- Oikawa K., Lieberman D. M., Reithmeier R. A. Conformation and stability of the anion transport protein of human erythrocyte membranes. Biochemistry. 1985 Jun 4;24(12):2843–2848. doi: 10.1021/bi00333a005. [DOI] [PubMed] [Google Scholar]
- Pappert G., Schubert D. The state of association of band 3 protein of the human erythrocyte membrane in solutions of nonionic detergents. Biochim Biophys Acta. 1983 Apr 21;730(1):32–40. doi: 10.1016/0005-2736(83)90313-9. [DOI] [PubMed] [Google Scholar]
- Passow H. Molecular aspects of band 3 protein-mediated anion transport across the red blood cell membrane. Rev Physiol Biochem Pharmacol. 1986;103:61–203. doi: 10.1007/3540153330_2. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Mechanisms of cooperativity and allosteric regulation in proteins. Q Rev Biophys. 1989 May;22(2):139–237. doi: 10.1017/s0033583500003826. [DOI] [PubMed] [Google Scholar]
- Pimplikar S. W., Reithmeier R. A. Affinity chromatography of Band 3, the anion transport protein of erythrocyte membranes. J Biol Chem. 1986 Jul 25;261(21):9770–9778. [PubMed] [Google Scholar]
- Rao A., Martin P., Reithmeier R. A., Cantley L. C. Location of the stilbenedisulfonate binding site of the human erythrocyte anion-exchange system by resonance energy transfer. Biochemistry. 1979 Oct 16;18(21):4505–4516. doi: 10.1021/bi00588a008. [DOI] [PubMed] [Google Scholar]
- Reithmeier R. A., Lieberman D. M., Casey J. R., Pimplikar S. W., Werner P. K., See H., Pirraglia C. A. Structure and function of the band 3 Cl-/HCO3- transporter. Ann N Y Acad Sci. 1989;574:75–83. doi: 10.1111/j.1749-6632.1989.tb25137.x. [DOI] [PubMed] [Google Scholar]
- Sami M., Malik S., Watts A. Structural stability of the erythrocyte anion transporter, band 3, in native membranes and in detergent micelles. Biochim Biophys Acta. 1992 Mar 23;1105(1):148–154. doi: 10.1016/0005-2736(92)90173-j. [DOI] [PubMed] [Google Scholar]
- Scheuring U., Lindenthal S., Grieshaber G., Haase W., Schubert D. The turnover number for band 3-mediated sulfate transport in phosphatidylcholine bilayers. FEBS Lett. 1988 Jan 18;227(1):32–34. doi: 10.1016/0014-5793(88)81407-8. [DOI] [PubMed] [Google Scholar]
- Snow J. W., Brandts J. F., Low P. S. The effects of anion transport inhibitors on structural transitions in erythrocyte membranes. Biochim Biophys Acta. 1978 Oct 4;512(3):579–591. doi: 10.1016/0005-2736(78)90167-0. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976 Mar 9;15(5):1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- Stokes D. L., Green N. M. Three-dimensional crystals of CaATPase from sarcoplasmic reticulum. Symmetry and molecular packing. Biophys J. 1990 Jan;57(1):1–14. doi: 10.1016/S0006-3495(90)82501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J., Martin P. G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988 Dec 15;256(3):703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J. Proteolytic cleavage of the anion transporter and its orientation in the membrane. Methods Enzymol. 1989;173:423–432. doi: 10.1016/s0076-6879(89)73030-5. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Mullner N., Pikula S., Dux L., Peracchia C., Varga S., Martonosi A. Electron microscope observations on Ca2+-ATPase microcrystals in detergent-solubilized sarcoplasmic reticulum. J Biol Chem. 1988 Apr 15;263(11):5287–5294. [PubMed] [Google Scholar]
- Unwin N. The structure of ion channels in membranes of excitable cells. Neuron. 1989 Dec;3(6):665–676. doi: 10.1016/0896-6273(89)90235-3. [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Dix J. A., Solomon A. K. Anion transport inhibitor binding to band 3 in red blood cell membranes. J Gen Physiol. 1983 Mar;81(3):421–449. doi: 10.1085/jgp.81.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonck J., van Bruggen E. F. Electron microscopy and image analysis of two-dimensional crystals and single molecules of alcohol oxidase from Hansenula polymorpha. Biochim Biophys Acta. 1990 Mar 29;1038(1):74–79. doi: 10.1016/0167-4838(90)90012-5. [DOI] [PubMed] [Google Scholar]
- Wang D. N., Kühlbrandt W. High-resolution electron crystallography of light-harvesting chlorophyll a/b-protein complex in three different media. J Mol Biol. 1991 Feb 20;217(4):691–699. doi: 10.1016/0022-2836(91)90526-c. [DOI] [PubMed] [Google Scholar]