Abstract
We describe here the development and characterization of the physicochemical and pharmacokinetic properties of a novel liposomal formulation for FTY720 delivery, LP-FTY720. The mean diameter of LP-FTY720 was ~157 nm, and the FTY720 entrapment efficiency was ~85%. The liposomal formulation protected FTY720 from degradation in aqueous buffer and showed toxicity in CLL patient B cells comparable to that of free FTY720. Following intravenous injection in ICR mice, LP-FTY720 had an increased elimination phase half-life (~28 vs. ~19 hr) and decreased clearance (235 vs. 778 mL/h/kg) compared to the free drug. Antibodies against CD19, CD20 and CD37 were incorporated into LP-FTY720, which provided targeted delivery to CLL patient B cells and thus achieved higher killing efficacy. The novel liposomal carrier of FTY720 demonstrated improved pharmacokinetic properties, comparable activity, and a potential platform for targeted delivery to CLL by overcoming the limited application of free FTY720 to B malignancy treatment.
Keywords: FTY720, Liposome, Leukemia, Drug delivery, CD37, Nanotechnology
FTY720 (Fingolimod) is a synthetic compound based on modification of the natural immunosuppressant, myriocin (ISP-1).1,2 It was approved by the U.S. Food and Drug Administration in 2010 as the first orally bioavailable drug for patients with multiple sclerosis (MS) to reduce relapse and delay disease progression to disability.3
In addition to MS, FTY720 showed promising in vitro and in vivo preclinical activity in leukemia and lymphoma disease models, including chronic lymphocytic leukemia (CLL), chronic myelogenous leukemia (CML), acute lymphocytic leukemia (ALL), NK-cell leukemia, and mantle cell lymphoma (MCL).4–8 FTY720 has also been tested in phase III clinical trials as an immunosuppressant for kidney transplantation,9,10 and is a candidate drug for therapy of heart failure and arrhythmias due to its cardio-protective effects.11,12 Mechanistic studies have shown that FTY720 functions as an immune-modulator by affecting lymphocyte production, trafficking, infiltration and apoptosis.13 Several studies have provided evidence that FTY720 induces T cell apoptosis both in vitro and in vivo,8,14–17 which is unfavorable for treatment of B-cell malignancies. Previous in vitro studies indicated that FTY720 induces down-modulation of Mcl-1 but not Bcl-2 in CLL cells, and its toxicity in CLL cells is dependent on activation of PP2a but not S1P receptor. This alternative mechanism of FTY720-induced apoptosis in CLL differs from the mechanism of FTY720 in MS.18
FTY720 is sparingly soluble in aqueous buffer. Maximum solubility of 0.2 mg/ml in 1:1 ethanol/PBS (pH 7.2) is achieved by dissolving the drug first in ethanol and then diluting with aqueous buffer. Notably, free FTY720 is not stable in aqueous buffer/solution and requires daily fresh preparation.16 The current available capsule formulation has high oral bioavailability of ~90%19 and enables daily administration with dose proportional pharmacokinetics to achieve active steady-state levels in MS patients at 0.5 mg daily. However, to be clinically effective in B-cell malignancies, higher steady-state levels will need to be achieved as effective lymphophenia is achieved in humans at 2.5 mg/day.20,21 Furthermore, alternative formulations that enable targeting of tumor cells with reduced impact on T lymphocytes and other non-target tissues will be necessary.22,23
Liposomes are nanoparticles formed by self-assembly of phospholipid molecules in water with a hydrophilic core and a lipid-bilayer membrane. This structure has proven to be an ideal delivery vehicle for several drugs.24 The modification at the surface of conventional liposomes with polymers, such as polyethylene glycol (PEG), reduces interaction between liposomes and serum proteins, prolongs circulation in blood, and lowers immunogenicity. This strategy therefore provides a biologically inert and safe platform for the design of drug delivery systems.25–27
In this study we developed a liposomal carrier of FTY720 (LP-FTY720) and characterized its physicochemical, morphological and pharmacokinetic properties. Compared to free FTY720, LP-FTY720 showed improved aqueous stability and prolonged circulation time in mice. We also explored the effects of incorporating antibodies (anti-CD19, anti-CD20 and anti-CD37) into the formulation (ILP-FTY720) for targeting tumor specific cell surface receptors, which demonstrated enhanced delivery and killing efficiency in primary CLL cells ex vivo. These results demonstrate that immunoliposome formulation strategy for FTY720 is a viable approach for effective targeting in B cell malignancies.
Methods
Materials
FTY720 was synthesized at The Ohio State University Comprehensive Cancer Center Medicinal Chemistry Core Facility.28 Egg phosphatidylcholine (Egg PC) and methoxypolyethylene glycol (MW ~2,000 Da)-distearoylphosphatidy-lethanolamine (PEG-DSPE) were obtained from Lipoid (Newark, NJ). Cholesterol (Chol), Maleimide 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (Mal-PEG-DSPE) and Sphingosine C17 (Sph-17) were purchased from Avanti Polar Lipids (Alabaster, AL). 2-Iminothiolane (Traut’s reagent) and other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Experimental animals
ICR mice with weight ranging from 25 g to 30 g (age 6 weeks) were purchased from Charles River Lab (Vilmington, MA). All work performed on animals was approved by the IACUC at The Ohio State University.
Liposomal FTY720 preparation
LP-FTY720 was prepared by ethanol injection method. FTY720 was dissolved in absolute ethanol at 10 μM. Lipids with composition of Chol: Egg-PC: PEG-DSPE (molar ratio = 33.5: 65: 1.5) was dissolved in ethanol at 40 mg/ml. Fully mixed lipids and FTY720 at a weight ratio of 20:1 were rapidly injected into phosphate buffered saline (PBS, pH 7.4) with 9 times more volume to form LP-FTY720. Empty liposomes were prepared with equal volume of ethanol as substitute for FTY720. The liposomes were then dialyzed against HEPES-buffered saline (HBS, 145 mM NaCl, 20 mM HEPES pH 7.4) overnight using a DispoDialyzer (Spectrum Labs, Rancho Dominguez, CA) with a molecular weight cut-off of 10,000 Da to remove non-encapsulated FTY720. Before and after dialysis, the volume and concentration of FTY720 in dialysis bag were measured. The entrapment efficacy was determined by the ratio of total amount of FTY720 after and before dialysis. For fluorescent liposomes, 6-fluorescein modified Bcl-2 antisense oligodeoxynucleotide G3139 (FAM-G3139) (Alpha DNA, Inc., Montreal, Canada) dissolved in PBS was mixed with lipids and FTY720 in ethanol at G3139: lipids (weight ratio) = 1: 12.5 followed by dialysis against PBS to remove free G3139 and FTY720. Immunoliposomes (ILPs) with monoclonal antibodies (anti-CD19, anti-CD20 or anti-CD37) (anti-CD19 and anti-CD37 from BD Biosciences Pharmingen, San Jose, CA; anti-CD20 used was Rituximab) and IgG (negative control) immobilized onto the surface of LP-FTY720 were prepared as described previously with a post-insertion method.29 Briefly, antibody (Ab) was reacted with 10× Traut’s reagent (2 h, room temperature) to yield Ab-SH at pH 8.0. Ab-SH was then reacted to form micelles of Mal-PEG-DSPE at a molar ratio of 1:10 at pH 6.8, and then incubated with LP-FTY720 for 1 h at 37 °C. ILP-FTY720 with antibody to FTY720 weight ratio of 1:4500 was thus prepared. The ILPs were further purified on a Sepharose CL-4B size exclusion column to remove free antibody.
Characterization of LP-FTY720
The size distribution of LP-FTY720 was determined by dynamic light scattering (DLS) (Brookhaven Instruments Corporation BI200SM, Holtsville, NY) using the laser wavelength of 632.8 nm at 90° detection angle. Three batches of LP-FTY720 were measured independently and the mean diameter by volume ± standard deviation was reported. The zeta-potential of LP-FTY720 was evaluated by a ZetaPALS zeta-potential analyzer (Brookhaven Instruments Corporation, Holtsville, NY). Liposomes were diluted in 1× PBS buffer and measured independently with 10 runs each at room temperature. The Smoluchowski model30 was used to calculate the zeta-potential and the mean ± standard deviation was reported.
Morphology study of LP-FTY720
The morphology of LP-FTY720 was observed with a cryogenic transmission electron microscope (Cryo-TEM). Briefly, the LP-FTY720 was applied to a glow-discharged holey carbon/formvar film coated grid in a controlled environment vitrification system at constant humidity and temperature of 25 °C. Samples were immediately plunged into a bath of liquid ethane for vitrification after gently blotting. Maintained at −172 °C, the prepared grid was transferred to Gatan cryo-holder and imaged in low-dose mode with FEI Tecnai G2 Spirit TEM equipped with bioTWIN optics operating at 120 kV. Images were recorded using a Gatan CCD camera.
Liquid chromatography-tandem mass spectrometry analysis
A rapid and robust high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method was developed for the quantification of FTY720 in mouse plasma on a Shimudzu LC system (comprising two LC-20AD pumps, a SIL-20AC autosampler, DGU-20A5 degasser and a CBM-20A system controller) coupled to a TSQ Quantum Ultra mass spectrometer with electrospray ionization source. HPLC separation was performed on a ZORBAX Extend C18 column (50 × 2.1 mm I.D, 3.5 μm particle size) at room temperature with a total run time of 6 min for each sample. A linear gradient elution was used with water/0.1% formic acid as mobile phase A and methanol/0.1%formic acid as mobile phase B at a flow rate of 200 μL/min. The gradient was as follows: 0.0 min (30% A and 70% B), 1.0 min (30% A and 70% B), 3.0 mi6n (100% B), 4.5 min (100% B), 4.6 min (30% A and 70% B) and 6.0 min (30% A and 70% B). MS monitoring was carried out in positive ion mode with selected reaction monitoring. The monitored ion transition channels were m/z 308.00 → 255.00 for FTY720 and 286.00 → 238.00 for Sph-17 with 30 ms scan times. MS parameters were optimized by a direct infusion of 10 μg/mL FTY720 and internal standard Sph-17 at a flow rate of 10 μL/ min via a syringe pump. The ion spray voltage, skimmer offset and tube lens offset were set to 5000, 10 and 100 V, respectively. Argon collision gas pressure was 1.5 mTorr, and nitrogen sheath and auxiliary gas were set to 30 and 15 (arbitrary unit), respectively. Collision energy was 15% for FTY720 and 16% for Sph-17. The ion transfer tube was maintained at 325 °C. This quantification method has been fully validated and all inter-day and intra-day accuracy of FTY720 samples ranged narrowly from 98% to 112% while the relative standard deviations of both intra-day and inter-day were below 10% (data not shown).
In vitro release of FTY720 from the liposomes
Rates of drug release from liposomes were studied by measuring retention of FTY720 in the liposome fraction. LP-FTY720 was dialyzed against PBS (pH 7.4) or 10% fetal bovine serum (FBS) in RPMI 1640 media at 37 °C. Samples were collected at different time points (0, 30 min, 1, 2, 4, 8, 12 and 24 h), and liposomes were lysed with methanol. Concentration of FTY720 was measured by HPLC-MS/MS as described above. The percentage of drug remaining in the liposome fractions was calculated by comparing to the original total drug amount.
Apoptosis assay
Primary B-cells from CLL patients were separated and incubated as previously described.5 Apoptosis activity was determined in primary CLL cells after 24-h incubation with indicated agents by annexin V/PI staining. The degree of apoptosis induction is displayed as the total percentage of annexin V and PI double negative cells normalized to untreated condition.
Binding efficiency study
CLL B-cells were incubated with 1 μM FAM G3139 encapsulated ILPs at 37 °C for 1 h and washed twice with cold PBS. Cells then were analyzed by flow cytometry using a FL1 filter (Beckman coulter cytomics FC 500, air-cooled Argon ion laser, 488 nm). IgG-ILPs were used as negative control.
Immunoblot analyses
Whole cell lysates were prepared using lysis buffer (10 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% deoxycholic acid, 0.1% SDS, 5 mM EDTA) containing protease and phosphatase inhibitors. Proteins were analyzed by immunoblot following standard procedures18 using anti-Mcl-1 antibody (S-19) from Santa Cruz Biotech (Santa Cruz, CA). Digital quantification was completed using a ChemiDocstation (Bio-rad, Hercules, CA).
Pharmacokinetic studies
Plasma pharmacokinetics of free FTY720 and LP-FTY720 were evaluated in ICR mice (Charles River Lab, Vilmington, MA). In groups of five, mice were intravenously injected with FTY720 at a dosage of 5 mg/kg body weight through tail vein. Blood samples were collected in EDTA-containing tubes at various time points, and plasma was immediately isolated by centrifugation and stored at −80 °C. FTY720 in 50 μL plasma was mixed with 50 μL of blank mouse plasma and internal standard (final concentration 1 μM), extracted with ethyl acetate, dried and reconstituted in 120 μL of 50% methanol. Samples were analyzed using LC-MS/MS as described above. WinNonlin Version 5.2 (Pharsight Co., Mountain View, CA) was used to determine non-compartmental pharmacokinetic parameters of each data set.
Statistical analysis
Data were analyzed by Student’s t tests using MiniTAB software (Minitab Inc., State College, PA). P < 0.05 was used as the cutoff for statistically significant differences.
Results
Characterization of LP-FTY720
The lipids chosen for our study (Chol: Egg-PC: PEG-DSPE; molar ratio = 33.5: 65: 1.5) are widely used to encapsulate small molecule drugs. Compared to this cholesterol based formulation, another formulation composed of cholesteryl hemisuccinate: Egg-PC: PEG-DSPE; molar ratio = 33.5: 65: 1.5 induced more cytotoxicity and had less favorable properties (data not shown). Characteristics of both empty liposomes and LP-FTY720 are shown in Table 1. The mean diameter by volume of nanoparticles did not change following addition of FTY720 into the composition. FTY720 increased the zeta potential of nanoparticles from −4.10 ± 0.34 to 3.99 ± 1.67 mV. By measuring and calculating the amount of FTY720 before and after dialysis against PBS with LC-MS/MS, the drug entrapment efficiency was 85.2 ± 5.72%. Morphological analysis of LP-FTY720 was carried out by using Cryo-TEM (Figure 1). The empty liposome nanoparticles showed uniform structures with one lipid-bilayer, while the LP-FTY720 exhibited a bilamellar structure. FTY720 was presumably incorporated into the lipid-bilayer, which is typical for hydrophobic small molecules.
Table 1.
Characterization of liposomes and LP-FTY720.
| Formulation | Size (nm) | Polydispersity index (PI) | Entrapment efficiency (EE) (%) | Zeta potential (mV) |
|---|---|---|---|---|
| Empty Liposome | 159.1 ± 5.40 | 0.186 ± 0.038 | N/A | −4.10 ± 0.34 |
| LP-FTY720 | 157.5 ± 2.91 | 0.193 ± 0.074 | 85.2 ± 5.72 | 3.99 ± 1.67 |
Data represent the mean ± S.D. of three separate experiments (n = 3).
Figure 1.
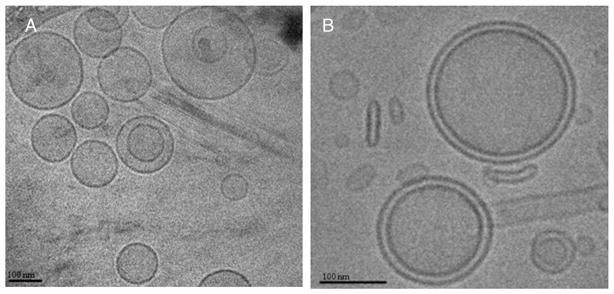
Morphological analysis of LP-FTY720 by Cryo-TEM. Empty liposomes were unilamellar nanoparticles (A) while LP-FTY720 had a unique bilamellar structure (B).
In vitro release studies
The in vitro release kinetics of FTY720 from liposomes was determined. As shown in Figure 2, the formulation was stable in PBS and in serum at 37 °C. The release over a time period of 12 h was <40%, while at 24 h the liposomes still retained over 50% FTY720 in both media. The results indicated LP-FTY720 was adequately stable in PBS and in serum.
Figure 2.
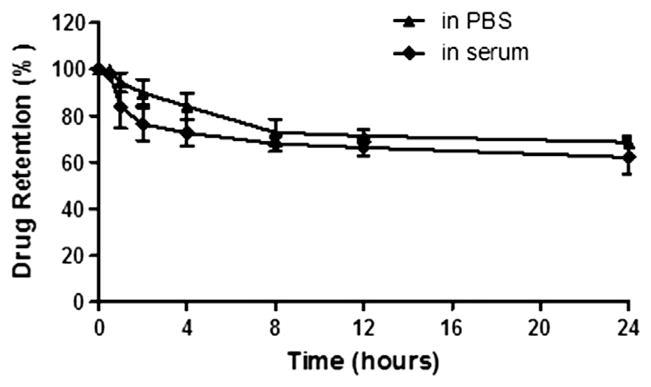
In vitro release of FTY720 from liposome nanoparticles. FTY720 entrapped liposome in PBS was incubated at 37 °C and dialyzed against PBS or 10% serum. At different time points, the liposomes were collected and disintergrated by methanol. The amount of drug retained and released from liposomes were measured by HPLC-MS/MS (n = 3).
Cytotoxicity of LP-FTY720
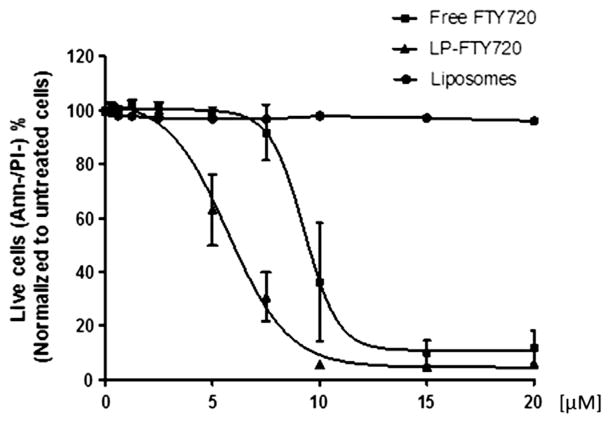
The cytotoxicity of LP-FTY720 was evaluated in 106 B-CLL patient cells. In addition, equivalent empty liposomes and free FTY720 diluted in PBS were included as controls. After incubating with different concentrations (0.3, 0.6, 1.25, 2.5, 5, 7.5, 10, 15, 20 μM) of FTY720 or empty liposomes, cells were stained with Annexin V/PI for flow cytometry analysis. The sigmoidal dose-response curve (Figure 3) indicates that cells treated with increasing concentrations of FTY720 or LP-FTY720 showed a dose-dependent decrease in viability corresponding to a concomitant decreased amount of annexin V-/PI- cells. The EC50 values of free FTY720 and LP-FTY720 were 9.3 μM and 5.7 μM, respectively. The obtained EC50 values of LP-FTY720 provided an optimal dose level that we can use for further evaluation of LP-FTY720 with targeting reagents.
Figure 3.
Comparison of EC50 of free FTY720 and liposomal FTY720 in B-CLL patient cells (n = 6). Purified CD19+ lymphocytes from six patients with CLL were incubated with various concentrations of empty liposomes, free FTY720 or liposomal FTY720 for 24 h and were evaluated by flow cytometry with annexin V/PI staining. The data shown represent percentages of annexin V-/PI- viable cells with SD that are normalized to untreated media control.
Previous study showed that FTY720 induces down-modulation of Mcl-1 in CLL B cells,18 therefore the expression of Mcl-1 protein was evaluated in CLL patient B cells after the LP-FTY720 treatment at FTY720 concentration of 5 μM and compared with free FTY720. The Mcl-1 protein expression was successfully reduced by 40% and 43% by free FTY720 in DMSO and LP-FTY720, respectively, compared to untreated samples (Figure 4).
Figure 4.
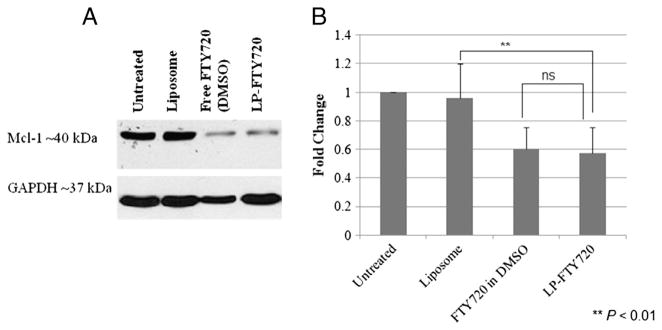
LP-FTY720 down-regulates Mcl-1 in CLL patient cells. (A) Representative western blot of Mcl-1 protein level after treating CLL cells at 106 cell/mL with 5 μM FTY720 for 20 h. (B) Relative percentage of Mcl-1 down-regulation levels of CLL patient cells (n = 4).
Pharmacokinetic study
Pharmacokinetics of LP-FTY720 was determined in ICR mice with free FTY720 as comparison. As shown in the plasma FTY720 concentration vs. time plot (Figure 5), the FTY720 in the liposomes was cleared at a slower rate compared to free FTY720. Plasma concentration data were evaluated with non-compartment methods, and resulting pharmacokinetic parameters are displayed in Table 2. The highest observed concentration (Cmax) of LP-FTY720 was twice that of free drug, suggesting LP-FTY720 was retained in plasma and did not distribute as extensively to other compartments. LP-FTY720 clearance was approximately one third that of free drug, and terminal phase half-life was approximately 50% greater with LP-FTY720. These data are consistent with expectations that LP-FTY720 has increased plasma exposure compared to free drug.
Figure 5.
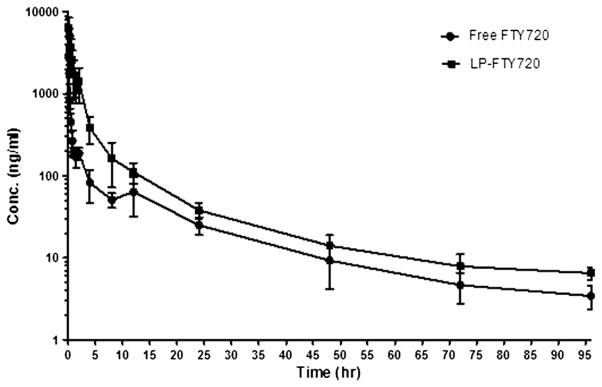
Plasma concentration-time curve of FTY720 and LP-FTY720 after i.v. bolus injection in mice. FTY720 was injected through the tail vein (5 mg/kg) in groups of 5 mice each. At indicated time points mouse plasma was collected and FTY720 was extracted for analysis by LC-MS/MS. Error bars stand for standard deviations (n = 5).
Table 2.
FTY720 and LP-FTY720 non-compartmental PK parameters.
| Parameter | Units | Free FTY720 | LP-FTY720 |
|---|---|---|---|
| Dose | mg/kg | 5 | 5 |
| λz | 1/h | 0.0357 | 0.0244 |
| T1/2λz | h | 19.44 | 28.36 |
| Cmax | mg/L | 5.572 | 12.505 |
| Vd | L/kg | 21.81 | 9.60 |
| AUC0-∞_obs | h * mg/mL | 6.24 | 20.78 |
| CL | mL/h/kg | 777.81 | 234.61 |
Table values are non-compartmental model parameter estimates. λz Elimination rate constant; T1/2λz Elimination half life; Cmax observed maximum concentration; Vd apparent volume of distribution; AUC0-∞_obs area under the observed concentration-time curve estimated to infinity.
Targeted delivery and cytotoxicity of Ab-conjugated LP-FTY720
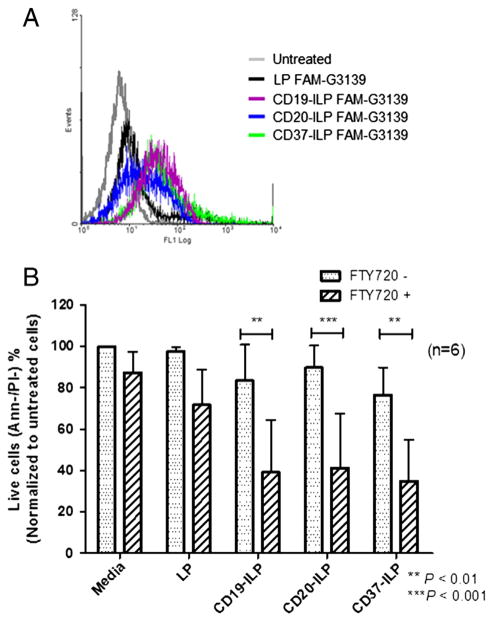
Antibody-targeted ILPs have shown improved drug delivery outcomes in therapy across multiple cancers.29,31–33 ILPs utilize antibodies for specific targeting of therapeutic reagents, efficiently and specifically delivering the encapsulated drug to its targeted cell or tissue. We chose three monoclonal antibodies, anti-CD19, anti-CD20 and anti-CD37, which are commonly used in CLL characterization or treatment.34–36 FAM-G3139 was encapsulated in ILPs to evaluate the delivery efficiency in CLL patient cells. Figure 6, A displays a typical example of improved binding efficiency of targeting ILPs. Compared to the negative control, ILPs with each of the three antibodies promoted binding efficiency to CLL cells. To further investigate the efficacy of targeted delivery of ILP-FTY720, CLL cells were treated with 3 μM FTY720 at a concentration of antibodies of 0.2 μg/mL for 24 h. These antibody concentrations are known to be only mildly cytotoxic when the antibodies are administered alone (data not shown). Free FTY720 was also added to the test cultures as controls to ensure that under suboptimal concentrations only minimal effects were observed with the individual reagents, while targeted delivery of FTY720 into the cells with antibody was able to achieve appreciable benefit. As shown in Figure 6, B, CD19-ILP-FTY720, CD20-ILP-FTY720 and CD37-ILP-FTY720 mediated significantly increased cytotoxicity to CLL B cells compared to non-targeted LP-FTY720 (n = 5, P < 0.05). Additionally, in contrast to their corresponding ILPs without payload, all ILPs with FTY720 significantly increased cytotoxicity (n = 5, P < 0.01).
Figure 6.
Enhanced delivery and cytotoxicity of Ab-based ILP-FTY720. (A) Representative binding efficiency of anti-CD19, anti-CD20 or anti-CD37 based ILPs encapsulating FAM-G3139 to CLL B cells with IgG-ILPs as negative control. (B) Improved cytotoxicity effect of ILP-FTY720 with antibodies as targeting reagents to CLL B cells (n = 6).
Discussion
Compared to free drugs, liposomal drugs may provide an advantage by decreasing the toxicity and increasing the circulation time.37–39 In this study, we loaded FTY720 into a liposomal carrier and characterized its physicochemical, biological and pharmacokinetic properties. Additionally, immune-targeted LP-FTY720 for CLL was investigated.
The structure of FTY720 shares the amphiphilic feature of phospholipids and hence the classic ethanol injection method was employed to synthesize LP-FTY720. Studies related to size measurement, encapsulation efficiency evaluation and in vitro drug release kinetics revealed that the composition of lipids and loading method resulted in nanoparticles suitable for delivery.
The development of LP-FTY720 provides a more favorable efficacy/toxicity profile for this promising agent. Cytotoxicity studies showed that LP-FTY720 was slightly more potent than free FTY720 in CLL patient cells. Moreover, empty liposomes were not toxic to cells indicating that the observed cytotoxicity with LP-FTY720 was due to the encapsulated FTY720 and not the liposome formulation components. The modulation of Mcl-1 protein expression was evaluated as a representative readout for the LP-FTY720 pharmacodynamic parameter. As expected, LP-FTY720 successfully decreased Mcl-1 protein expression to the same level as free FTY720. Further studies will ensure if free drug and LP-FTY720 operate through the same mechanisms.40 Detailed mechanistic studies will reveal the basis of cellular uptake of LP-FTY720. The detailed characterization of cellular uptake mechanisms via endocytosis and conventional clathrin-coated pits remains to be tested. The intracellular trafficking of liposomal nanoparticles has been shown to be affected by each component involved in the formulation.41 Additionally, ligands such as antibodies modifying the surface of liposomes may also result in alteration of intracellular trafficking.22,23,42
Pharmacokinetic data demonstrated LP-FTY720 had higher plasma Cmax and overall exposure (AUC0-∞_obs) of FTY720 compared to free drug. This will be clearly beneficial for targeting hematologic diseases where the target is located in the blood compartment. PEG-lipids are widely used to increase the in vivo stability and circulation half-life of liposomal drug. The in vitro release studies indicated that FTY720 had been well encapsulated in liposomes with slow release under simulated physiological conditions. This has ensured that the drug in LP-FTY720 remained longer in circulation and cleared slower than free drug, thus exhibiting a longer terminal phase half-life. Although we did not evaluate in vivo pharmacokinetics of ILPs, we anticipate exposures would be similar or potentially even greater since antibodies will target ILPs to circulating blood cells.
We explored in vitro the potential of three common surface markers, CD19, CD20 and CD37, of B or CLL cells as targets for selective LP-FTY720 delivery. Though the chosen antibodies were also used as single killing drugs in pre-clinical or clinical studies,34,36,43 sub-toxic concentrations of antibodies were chosen as targeting reagents in these proof of principal studies. Compared to non-targeted LP-FTY720, ILPs with each of the antibody conjugated formulation improved potency and delivery of FTY720 to CLL patient B cells. Further optimization of FTY720 and antibody concentration and antibody type will be necessary to optimize activity and in vivo efficacy. Moreover, rationale choice of targets and antibodies will enable expansion of the LP-FTY720 formulation into other diseases where FTY720 has been shown to have a therapeutic benefit. Ongoing studies using cell lines and humanized mouse models will allow detailed characterization of the in vitro release and in vivo pharmacokinetic studies of ILP-FTY720 for future clinical application.
In conclusion, an efficient liposomal FTY720 formulation has been designed and evaluated. The formulation exhibits high drug loading ratio, prolonged in vitro release rate and beneficial pharmacokinetic properties in vivo. It also provides an alternative degradable vehicle for administration of FTY720 with increased efficacy and decreased potential for off-target effects. Further preclinical studies are warranted to define the safety, mechanism and therapeutic efficacy of this novel formulation.
Acknowledgments
Sources of support: NSF Nanoscale Science and Engineering Center (NSEC) grant EEC-0425626, NIH (R01 CA135332, RO1 CA159296, R01 CA135243 and P50-CA140158), Leukemia Lymphoma Society and D. Warren Brown Foundation.
This work was supported by NSF Nanoscale Science and Engineering Center (NSEC) grant EEC-0425626, NIH (R01 CA135332, R01 CA159296, R01 CA135243 and P50-CA140158), Leukemia Lymphoma Society and D. Warren Brown Foundation.
References
- 1.Strader CR, Pearce CJ, Oberlies NH. Fingolimod (FTY720): a recently approved multiple sclerosis drug based on a fungal secondary metabolite. J Nat Prod. 2011;74:900–7. doi: 10.1021/np2000528. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki S, Enosawa S, Kakefuda T, Shinomiya T, Amari M, Naoe S, et al. A novel immunosuppressant, FTY720, with a unique mechanism of action, induces long-term graft acceptance in rat and dog allotransplantation. Transplantation. 1996;61:200–5. doi: 10.1097/00007890-199601270-00006. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–97. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 4.Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, et al. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117:2408–21. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q, Alinari L, Chen CS, Yan F, Dalton JT, Lapalombella R, et al. FTY720 shows promising in vitro and in vivo preclinical activity by downmodulating Cyclin D1 and phospho-Akt in mantle cell lymphoma. Clin Cancer Res. 2010;16:3182–92. doi: 10.1158/1078-0432.CCR-09-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alinari L, Mahoney E, Patton J, Zhang X, Huynh L, Earl CT, et al. FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death. Blood. 2011;118:6893–903. doi: 10.1182/blood-2011-06-363879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao A, Broeg K, Fox T, Tan SF, Watters R, Shah MV, et al. Therapeutic efficacy of FTY720 in a rat model of NK-cell leukemia. Blood. 2011;118:2793–800. doi: 10.1182/blood-2011-01-331447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuoka Y, Nagahara Y, Ikekita M, Shinomiya T. A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br J Pharmacol. 2003;138:1303–12. doi: 10.1038/sj.bjp.0705182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skerjanec A, Tedesco H, Neumayer HH, Cole E, Budde K, Hsu CH, et al. FTY720, a novel immunomodulator in de novo kidney transplant patients: pharmacokinetics and exposure-response relationship. J Clin Pharmacol. 2005;45:1268–78. doi: 10.1177/0091270005279799. [DOI] [PubMed] [Google Scholar]
- 10.Braun WE. Renal transplantation: basic concepts and evolution of therapy. J Clin Apher. 2003;18:141–52. doi: 10.1002/jca.10070. [DOI] [PubMed] [Google Scholar]
- 11.Egom EE, Ke Y, Musa H, Mohamed TM, Wang T, Cartwright E, et al. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J Mol Cell Cardiol. 2010;48:406–14. doi: 10.1016/j.yjmcc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egom EE, Mohamed TM, Mamas MA, Shi Y, Liu W, Chirico D, et al. Activation of Pak1/Akt/eNOS signaling following sphingosine-1-phosphate release as part of a mechanism protecting cardiomyocytes against ischemic cell injury. Am J Physiol Heart Circ Physiol. 2011;301:H1487–95. doi: 10.1152/ajpheart.01003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meno-Tetang GM, Li H, Mis S, Pyszczynski N, Heining P, Lowe P, et al. Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2 [2-(-4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos. 2006;34:1480–7. doi: 10.1124/dmd.105.009001. [DOI] [PubMed] [Google Scholar]
- 14.Nagahara Y, Enosawa S, Ikekita M, Suzuki S, Shinomiya T. Evidence that FTY720 induces T cell apoptosis in vivo. Immunopharmacology. 2000;48:75–85. doi: 10.1016/s0162-3109(00)00181-8. [DOI] [PubMed] [Google Scholar]
- 15.Nagahara Y, Ikekita M, Shinomiya T. T cell selective apoptosis by a novel immunosuppressant, FTY720, is closely regulated with Bcl-2. Br J Pharmacol. 2002;137:953–62. doi: 10.1038/sj.bjp.0704970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4(+)CD25(+) regulatory T cells. J Immunol. 2007;178:2458–68. doi: 10.4049/jimmunol.178.4.2458. [DOI] [PubMed] [Google Scholar]
- 17.Omar HA, Chou CC, Berman-Booty LD, Ma Y, Hung JH, Wang D, et al. Antitumor effects of OSU-2S, a nonimmunosuppressive analogue of FTY720, in hepatocellular carcinoma. Hepatology. 2011;53:1943–58. doi: 10.1002/hep.24293. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Liu Q, Zhao X, Frissora F, Ma Y, Santhanam R, Jarjoura D, et al. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111:275–84. doi: 10.1182/blood-2006-10-053884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David OJ, Kovarik JM, Schmouder RL. Clinical pharmacokinetics of fingolimod. Clin Pharmacokinet. 2012;51:15–28. doi: 10.2165/11596550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Kahan BD, Karlix JL, Ferguson RM, Leichtman AB, Mulgaonkar S, Gonwa TA, et al. Pharmacodynamics, pharmacokinetics, and safety of multiple doses of FTY720 in stable renal transplant patients: a multicenter, randomized, placebo-controlled, phase I study. Transplantation. 2003;76:1079–84. doi: 10.1097/01.TP.0000084822.01372.AC. [DOI] [PubMed] [Google Scholar]
- 21.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Yu B, Mao Y, Yuan Y, Yue C, Wang X, Mo X, et al. Targeted drug delivery and cross-linking induced apoptosis with anti-CD37 based dual-ligand immunoliposomes in B chronic lymphocytic leukemia cells. Biomaterials. 2013;34:6185–93. doi: 10.1016/j.biomaterials.2013.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu B, Mao Y, Bai LY, Herman SE, Wang X, Ramanunni A, et al. Targeted nanoparticle delivery overcomes off-target immunostimulatory effects of oligonucleotides and improves therapeutic efficacy in chronic lymphocytic leukemia. Blood. 2013;121:136–47. doi: 10.1182/blood-2012-01-407742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 25.Hansen CB, Kao GY, Moase EH, Zalipsky S, Allen TM. Attachment of antibodies to sterically stabilized liposomes: evaluation, comparison and optimization of coupling procedures. Biochim Biophys Acta. 1995;1239:133–44. doi: 10.1016/0005-2736(95)00138-s. [DOI] [PubMed] [Google Scholar]
- 26.Chen JL, Wang H, Gao JQ, Chen HL, Liang WQ. Liposomes modified with polycation used for gene delivery: preparation, characterization and transfection in vitro. Int J Pharm. 2007;343:255–61. doi: 10.1016/j.ijpharm.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 27.Bendas G. Immunoliposomes: a promising approach to targeting cancer therapy. BioDrugs. 2001;15:215–24. doi: 10.2165/00063030-200115040-00002. [DOI] [PubMed] [Google Scholar]
- 28.Hung JH, Lu YS, Wang YC, Ma YH, Wang DS, Kulp SK, et al. FTY720 induces apoptosis in hepatocellular carcinoma cells through activation of protein kinase C delta signaling. Cancer Res. 2008;68:1204–12. doi: 10.1158/0008-5472.CAN-07-2621. [DOI] [PubMed] [Google Scholar]
- 29.Hertlein E, Triantafillou G, Sass EJ, Hessler JD, Zhang X, Jarjoura D, et al. Milatuzumab immunoliposomes induce cell death in CLL by promoting accumulation of CD74 on the surface of B cells. Blood. 2010;116:2554–8. doi: 10.1182/blood-2009-11-253203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sze A, Erickson D, Ren L, Li D. Zeta-potential measurement using the Smoluchowski equation and the slope of the current-time relationship in electroosmotic flow. J Colloid Interface Sci. 2003;261:402–10. doi: 10.1016/S0021-9797(03)00142-5. [DOI] [PubMed] [Google Scholar]
- 31.Park JW, Benz CC, Martin FJ. Future directions of liposome- and immunoliposome-based cancer therapeutics. Semin Oncol. 2004;31:196–205. doi: 10.1053/j.seminoncol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–40. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 33.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8:1172–81. [PubMed] [Google Scholar]
- 34.Awan FT, Lapalombella R, Trotta R, Butchar JP, Yu B, Benson DM, Jr, et al. CD19 targeting of chronic lymphocytic leukemia with a novel Fc-domain-engineered monoclonal antibody. Blood. 2010;115:1204–13. doi: 10.1182/blood-2009-06-229039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao X, Lapalombella R, Joshi T, Cheney C, Gowda A, Hayden-Ledbetter MS, et al. Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood. 2007;110:2569–77. doi: 10.1182/blood-2006-12-062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapalombella R, Yeh YY, Wang L, Ramanunni A, Rafiq S, Jha S, et al. Tetraspanin CD37 directly mediates transduction of survival and apoptotic signals. Cancer Cell. 2012;21:694–708. doi: 10.1016/j.ccr.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11:1029–33. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 38.Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4:95–9. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, Zhao X, Phelps MA, Piao L, Rozewski DM, Liu Q, et al. A novel liposomal formulation of flavopiridol. Int J Pharm. 2009;365:170–4. doi: 10.1016/j.ijpharm.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schweingruber N, Haine A, Tiede K, Karabinskaya A, van den Brandt J, Wust S, et al. Liposomal encapsulation of glucocorticoids alters their mode of action in the treatment of experimental autoimmune encephalomyelitis. J Immunol. 2011;187:4310–8. doi: 10.4049/jimmunol.1101604. [DOI] [PubMed] [Google Scholar]
- 41.Un K, Sakai-Kato K, Oshima Y, Kawanishi T, Okuda H. Intracellular trafficking mechanism, from intracellular uptake to extracellular efflux, for phospholipid/cholesterol liposomes. Biomaterials. 2012;33:8131–41. doi: 10.1016/j.biomaterials.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 42.Mao Y, Triantafillou G, Hertlein E, Towns W, Stefanovski M, Mo X, et al. Milatuzumab-conjugated liposomes as targeted dexamethasone carriers for therapeutic delivery in CD74+ B-cell malignancies. Clin Cancer Res. 2013;19:347–56. doi: 10.1158/1078-0432.CCR-12-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rafiq S, Cheney C, Mo X, Jarjoura D, Muthusamy N, Byrd JC. XmAb-5574 antibody demonstrates superior antibody-dependent cellular cytotoxicity as compared with CD52- and CD20-targeted antibodies in adult acute lymphoblastic leukemia cells. Leukemia. 2012;26:1720–2. doi: 10.1038/leu.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]