Abstract
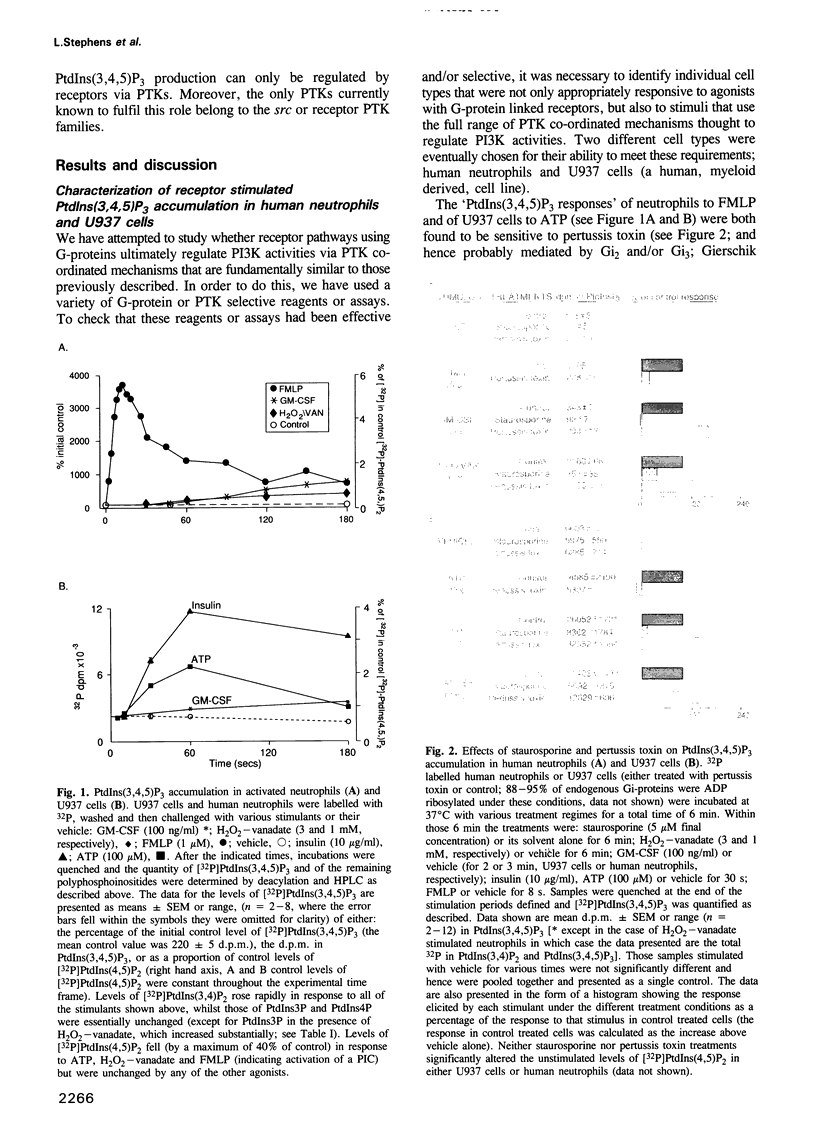
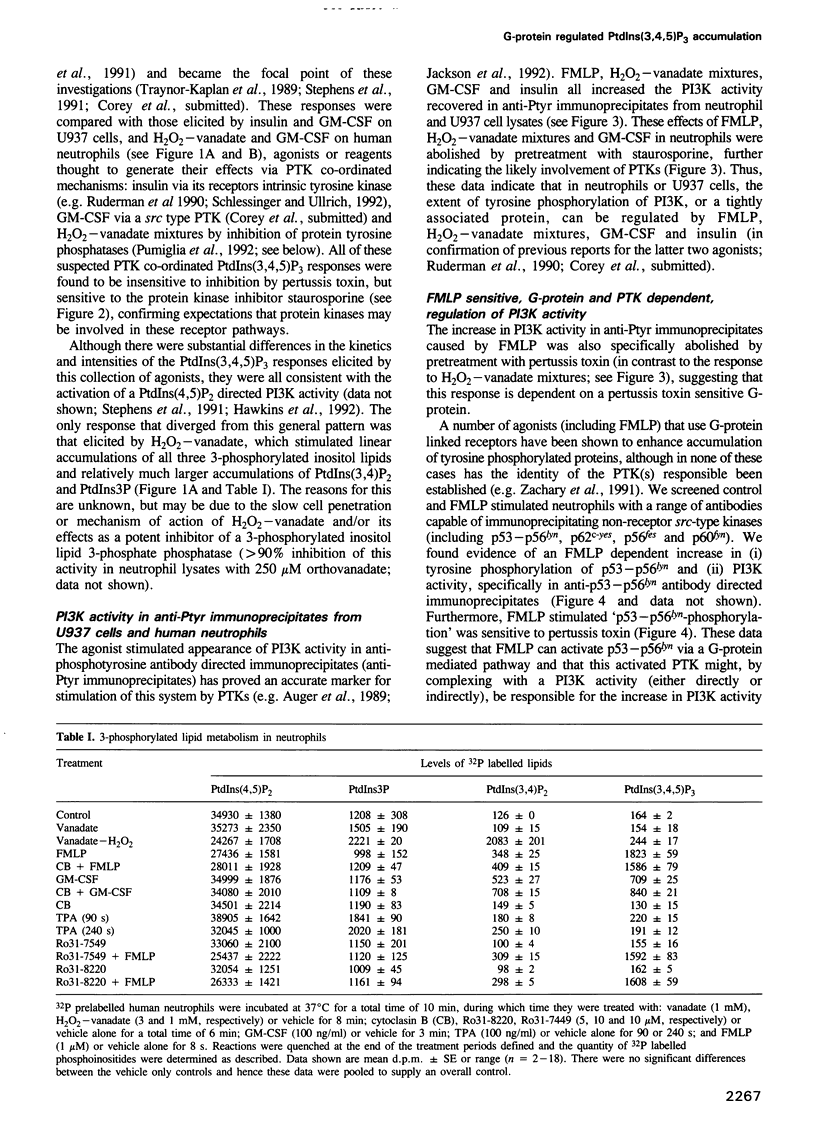
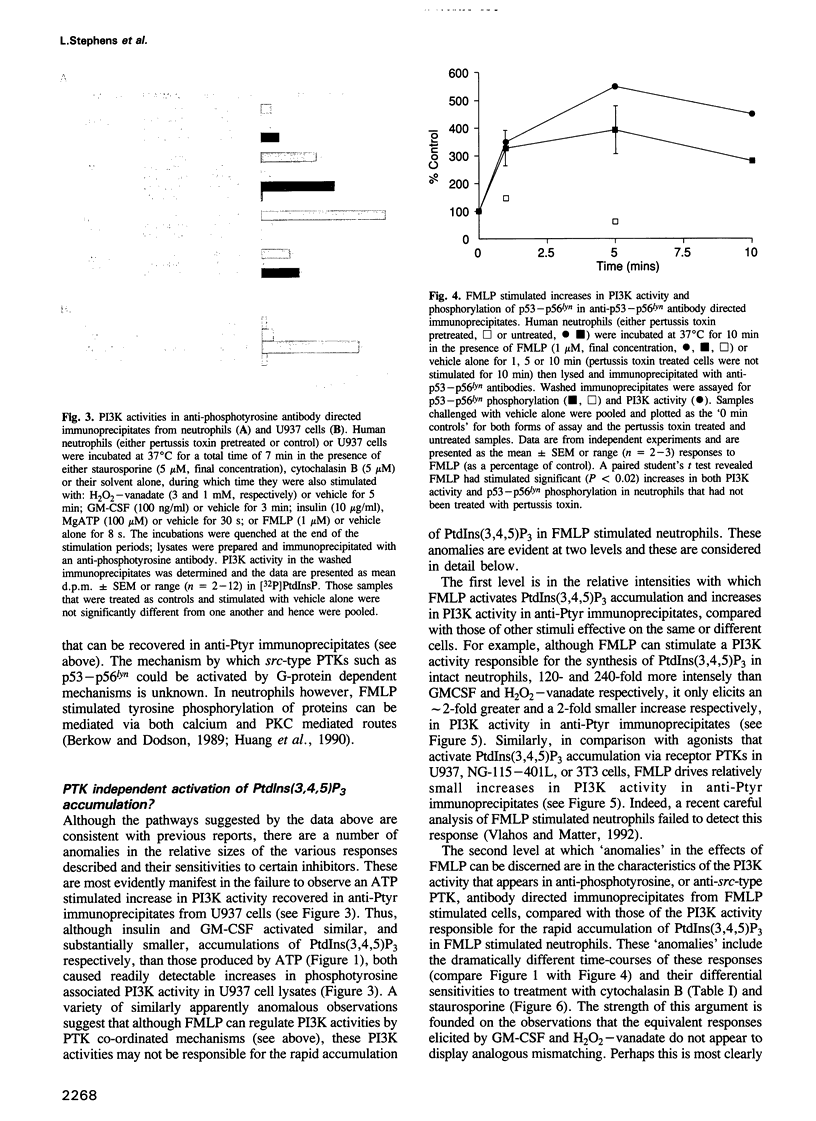
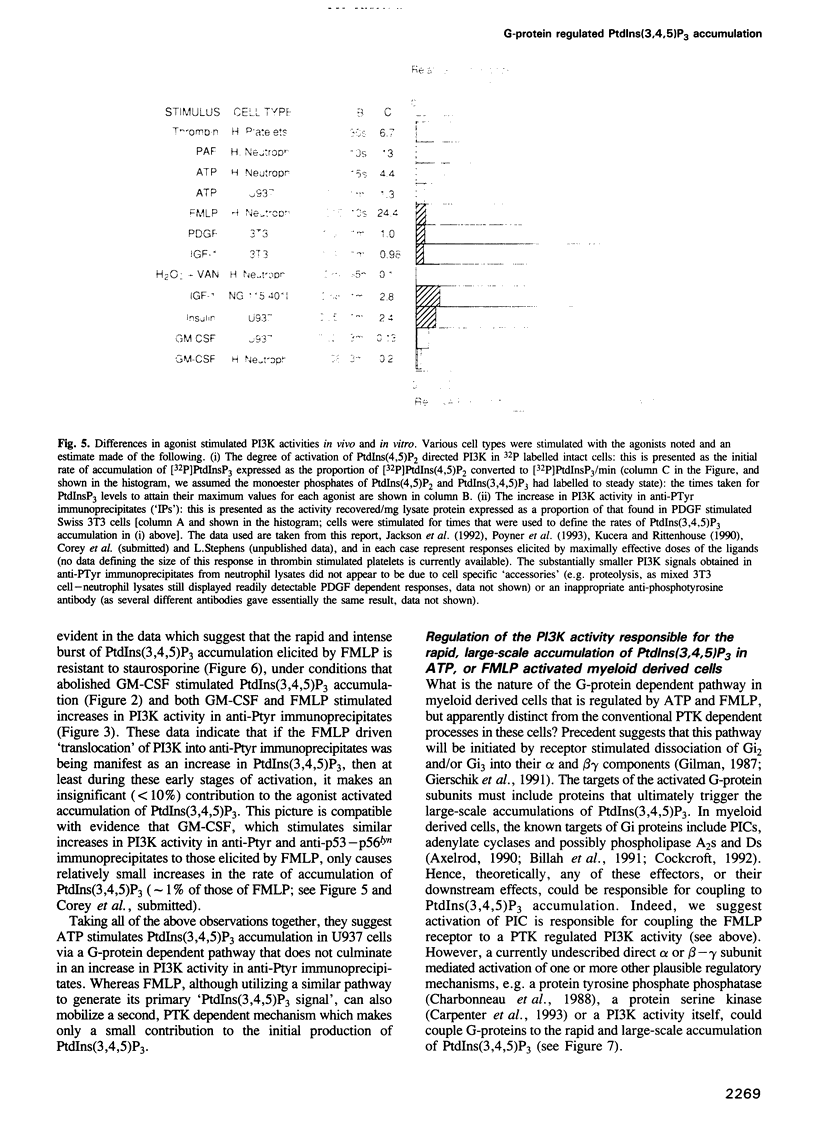
Phosphoinositide 30H-kinase (PI3K) activities are thought to be critical regulatory enzymes in a new intracellular signalling pathway, the activation of which results in the rapid accumulation of a putative signalling molecule, phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5) P3]. To date, activation of PI3K has always correlated with its recruitment into complexes containing protein tyrosine kinases (PTK). Here we report that agonists which utilize G-protein mediated transduction pathways can stimulate very rapid and large accumulations of PtdIns(3,4,5)P3 via a novel mechanism, possibly involving direct coupling between the G-protein and a PI3K activity. In addition, some of these agonists also stimulate small increases in PI3K activity in anti-phosphotyrosine and anti-src-type PTK antibody directed immunoprecipitates, indicating activation of PI3K via a 'conventional' PTK mediated mechanism; these pathways however, play only a minor role in the initial, agonist sensitive production of PtdIns(3,4,5)P3 in myeloid derived cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Axelrod J. Receptor-mediated activation of phospholipase A2 and arachidonic acid release in signal transduction. Biochem Soc Trans. 1990 Aug;18(4):503–507. doi: 10.1042/bst0180503. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkow R. L., Dodson R. W. Tyrosine-specific protein phosphorylation during activation of human neutrophils. Blood. 1990 Jun 15;75(12):2445–2452. [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C., Mullmann T. J. Receptor-coupled phospholipase D: regulation and functional significance. Biochem Soc Trans. 1991 Apr;19(2):324–329. doi: 10.1042/bst0190324. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Thompson P. A., Eiseman E., Horak I. D. Expression and interactions of the Src family of tyrosine protein kinases in T lymphocytes. Adv Cancer Res. 1991;57:103–149. doi: 10.1016/s0065-230x(08)60997-5. [DOI] [PubMed] [Google Scholar]
- Booker G. W., Breeze A. L., Downing A. K., Panayotou G., Gout I., Waterfield M. D., Campbell I. D. Structure of an SH2 domain of the p85 alpha subunit of phosphatidylinositol-3-OH kinase. Nature. 1992 Aug 20;358(6388):684–687. doi: 10.1038/358684a0. [DOI] [PubMed] [Google Scholar]
- Camps M., Hou C., Sidiropoulos D., Stock J. B., Jakobs K. H., Gierschik P. Stimulation of phospholipase C by guanine-nucleotide-binding protein beta gamma subunits. Eur J Biochem. 1992 Jun 15;206(3):821–831. doi: 10.1111/j.1432-1033.1992.tb16990.x. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter C. L., Auger K. R., Duckworth B. C., Hou W. M., Schaffhausen B., Cantley L. C. A tightly associated serine/threonine protein kinase regulates phosphoinositide 3-kinase activity. Mol Cell Biol. 1993 Mar;13(3):1657–1665. doi: 10.1128/mcb.13.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. L., Cantley L. C. Phosphoinositide kinases. Biochemistry. 1990 Dec 25;29(51):11147–11156. doi: 10.1021/bi00503a001. [DOI] [PubMed] [Google Scholar]
- Carpenter C. L., Duckworth B. C., Auger K. R., Cohen B., Schaffhausen B. S., Cantley L. C. Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem. 1990 Nov 15;265(32):19704–19711. [PubMed] [Google Scholar]
- Chan A. C., Irving B. A., Fraser J. D., Weiss A. The zeta chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9166–9170. doi: 10.1073/pnas.88.20.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K., Walsh K. A., Fischer E. H. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7182–7186. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke N. G., Dawson R. M. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem J. 1981 Apr 1;195(1):301–306. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S. G-protein-regulated phospholipases C, D and A2-mediated signalling in neutrophils. Biochim Biophys Acta. 1992 Aug 14;1113(2):135–160. [PubMed] [Google Scholar]
- Downes C. P., Carter A. N. Phosphoinositide 3-kinase: a new effector in signal transduction? Cell Signal. 1991;3(6):501–513. doi: 10.1016/0898-6568(91)90027-r. [DOI] [PubMed] [Google Scholar]
- Escobedo J. A., Navankasattusas S., Kavanaugh W. M., Milfay D., Fried V. A., Williams L. T. cDNA cloning of a novel 85 kd protein that has SH2 domains and regulates binding of PI3-kinase to the PDGF beta-receptor. Cell. 1991 Apr 5;65(1):75–82. doi: 10.1016/0092-8674(91)90409-r. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fry M. J. Defining a new GAP family. Curr Biol. 1992 Feb;2(2):78–80. doi: 10.1016/0960-9822(92)90207-q. [DOI] [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Kathuria S. Characterization of receptor tyrosine-specific protein kinases by the use of inhibitors. Staurosporine is a 100-times more potent inhibitor of insulin receptor than IGF-I receptor. Biochem Biophys Res Commun. 1988 Dec 30;157(3):955–962. doi: 10.1016/s0006-291x(88)80967-7. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Moghtader R., Straub C., Dieterich K., Jakobs K. H. Signal amplification in HL-60 granulocytes. Evidence that the chemotactic peptide receptor catalytically activates guanine-nucleotide-binding regulatory proteins in native plasma membranes. Eur J Biochem. 1991 May 8;197(3):725–732. doi: 10.1111/j.1432-1033.1991.tb15964.x. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Sidiropoulos D., Jakobs K. H. Two distinct Gi-proteins mediate formyl peptide receptor signal transduction in human leukemia (HL-60) cells. J Biol Chem. 1989 Dec 25;264(36):21470–21473. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gout I., Dhand R., Panayotou G., Fry M. J., Hiles I., Otsu M., Waterfield M. D. Expression and characterization of the p85 subunit of the phosphatidylinositol 3-kinase complex and a related p85 beta protein by using the baculovirus expression system. Biochem J. 1992 Dec 1;288(Pt 2):395–405. doi: 10.1042/bj2880395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind J. S., Lacal P. M., Robbins K. C. Thrombin-dependent association of phosphatidylinositol-3 kinase with p60c-src and p59fyn in human platelets. Mol Cell Biol. 1990 Jul;10(7):3806–3809. doi: 10.1128/mcb.10.7.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. T., Jackson T. R., Stephens L. R. Platelet-derived growth factor stimulates synthesis of PtdIns(3,4,5)P3 by activating a PtdIns(4,5)P2 3-OH kinase. Nature. 1992 Jul 9;358(6382):157–159. doi: 10.1038/358157a0. [DOI] [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- Hu P., Margolis B., Skolnik E. Y., Lammers R., Ullrich A., Schlessinger J. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol Cell Biol. 1992 Mar;12(3):981–990. doi: 10.1128/mcb.12.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. K., Bonak V., Laramee G. R., Casnellie J. E. Protein tyrosine phosphorylation in rabbit peritoneal neutrophils. Biochem J. 1990 Jul 15;269(2):431–436. doi: 10.1042/bj2690431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T. R., Stephens L. R., Hawkins P. T. Receptor specificity of growth factor-stimulated synthesis of 3-phosphorylated inositol lipids in Swiss 3T3 cells. J Biol Chem. 1992 Aug 15;267(23):16627–16636. [PubMed] [Google Scholar]
- Kaziro Y., Itoh H., Kozasa T., Nakafuku M., Satoh T. Structure and function of signal-transducing GTP-binding proteins. Annu Rev Biochem. 1991;60:349–400. doi: 10.1146/annurev.bi.60.070191.002025. [DOI] [PubMed] [Google Scholar]
- King W. G., Kucera G. L., Sorisky A., Zhang J., Rittenhouse S. E. Protein kinase C regulates the stimulated accumulation of 3-phosphorylated phosphoinositides in platelets. Biochem J. 1991 Sep 1;278(Pt 2):475–480. doi: 10.1042/bj2780475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kucera G. L., Rittenhouse S. E. Human platelets form 3-phosphorylated phosphoinositides in response to alpha-thrombin, U46619, or GTP gamma S. J Biol Chem. 1990 Apr 5;265(10):5345–5348. [PubMed] [Google Scholar]
- Nasmith P. E., Mills G. B., Grinstein S. Guanine nucleotides induce tyrosine phosphorylation and activation of the respiratory burst in neutrophils. Biochem J. 1989 Feb 1;257(3):893–897. doi: 10.1042/bj2570893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgauer J., Eberle M., Lemke H. D., Aktories K. Activation of human neutrophils by mastoparan. Reorganization of the cytoskeleton, formation of phosphatidylinositol 3,4,5-trisphosphate, secretion up-regulation of complement receptor type 3 and superoxide anion production are stimulated by mastoparan. Biochem J. 1992 Mar 1;282(Pt 2):393–397. doi: 10.1042/bj2820393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Marth J. D., Ziegler S. F., Garvin A. M., Pawar S., Cooke M. P., Abraham K. M. Specialized protein tyrosine kinase proto-oncogenes in hematopoietic cells. Biochim Biophys Acta. 1989 Feb;948(3):245–262. doi: 10.1016/0304-419x(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Poyner D. R., Hanley M. R., Jackson T. R., Hawkins P. T. Receptor regulation of phosphoinositide 3-hydroxykinase in the NG115-401L-C3 neuronal cell line: stimulation by insulin-like growth factor-I. Biochem J. 1993 Mar 15;290(Pt 3):901–905. doi: 10.1042/bj2900901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumiglia K. M., Lau L. F., Huang C. K., Burroughs S., Feinstein M. B. Activation of signal transduction in platelets by the tyrosine phosphatase inhibitor pervanadate (vanadyl hydroperoxide). Biochem J. 1992 Sep 1;286(Pt 2):441–449. doi: 10.1042/bj2860441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G., Choi K. D. Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem. 1992 Jun 25;267(18):12393–12396. [PubMed] [Google Scholar]
- Ruderman N. B., Kapeller R., White M. F., Cantley L. C. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Larrea F., Vicendo P., Yaish P., End P., Panayotou G., Fry M. J., Morgan S. J., Thompson A., Parker P. J., Waterfield M. D. Characterization of the bovine brain cytosolic phosphatidylinositol 3-kinase complex. Biochem J. 1993 Mar 1;290(Pt 2):609–616. doi: 10.1042/bj2900609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992 Sep;9(3):383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Shibasaki F., Homma Y., Takenawa T. Two types of phosphatidylinositol 3-kinase from bovine thymus. Monomer and heterodimer form. J Biol Chem. 1991 May 5;266(13):8108–8114. [PubMed] [Google Scholar]
- Sjölander A., Yamamoto K., Huber B. E., Lapetina E. G. Association of p21ras with phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7908–7912. doi: 10.1073/pnas.88.18.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik E. Y., Margolis B., Mohammadi M., Lowenstein E., Fischer R., Drepps A., Ullrich A., Schlessinger J. Cloning of PI3 kinase-associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell. 1991 Apr 5;65(1):83–90. doi: 10.1016/0092-8674(91)90410-z. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Hughes K. T., Irvine R. F. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991 May 2;351(6321):33–39. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- Traynor-Kaplan A. E., Thompson B. L., Harris A. L., Taylor P., Omann G. M., Sklar L. A. Transient increase in phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol trisphosphate during activation of human neutrophils. J Biol Chem. 1989 Sep 15;264(26):15668–15673. [PubMed] [Google Scholar]
- Vlahos C. J., Matter W. F. Signal transduction in neutrophil activation. Phosphatidylinositol 3-kinase is stimulated without tyrosine phosphorylation. FEBS Lett. 1992 Sep 14;309(3):242–248. doi: 10.1016/0014-5793(92)80781-b. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Cook S. J., Currie S., Palmer S., Plevin R. Regulation of the hydrolysis of phosphatidylcholine in Swiss 3T3 cells. Biochem Soc Trans. 1991 Apr;19(2):321–324. doi: 10.1042/bst0190321. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Lapetina E. G. Protein kinase C-mediated formation of phosphatidylinositol 3,4-bisphosphate in human platelets. Biochem Biophys Res Commun. 1990 Apr 30;168(2):466–472. doi: 10.1016/0006-291x(90)92344-y. [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Hasegawa-Sasaki H., Sasaki T. Suppression by staurosporine of Ca(2+)-mobilization triggered by ligation of antigen-specific receptors on t and B lymphocytes. An essential role of protein tyrosine kinase in the signal transduction. FEBS Lett. 1991 Aug 19;288(1-2):46–50. doi: 10.1016/0014-5793(91)81000-x. [DOI] [PubMed] [Google Scholar]
- Yatomi Y., Ozaki Y., Kume S. Synthesis of phosphatidylinositol 3,4-bisphosphate but not phosphatidylinositol 3,4,5-trisphosphate is closely correlated with protein-tyrosine phosphorylation in thrombin-activated human platelets. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1480–1486. doi: 10.1016/s0006-291x(05)81573-6. [DOI] [PubMed] [Google Scholar]
- Zachary I., Gil J., Lehmann W., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation in intact Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4577–4581. doi: 10.1073/pnas.88.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]







