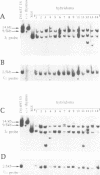
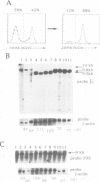
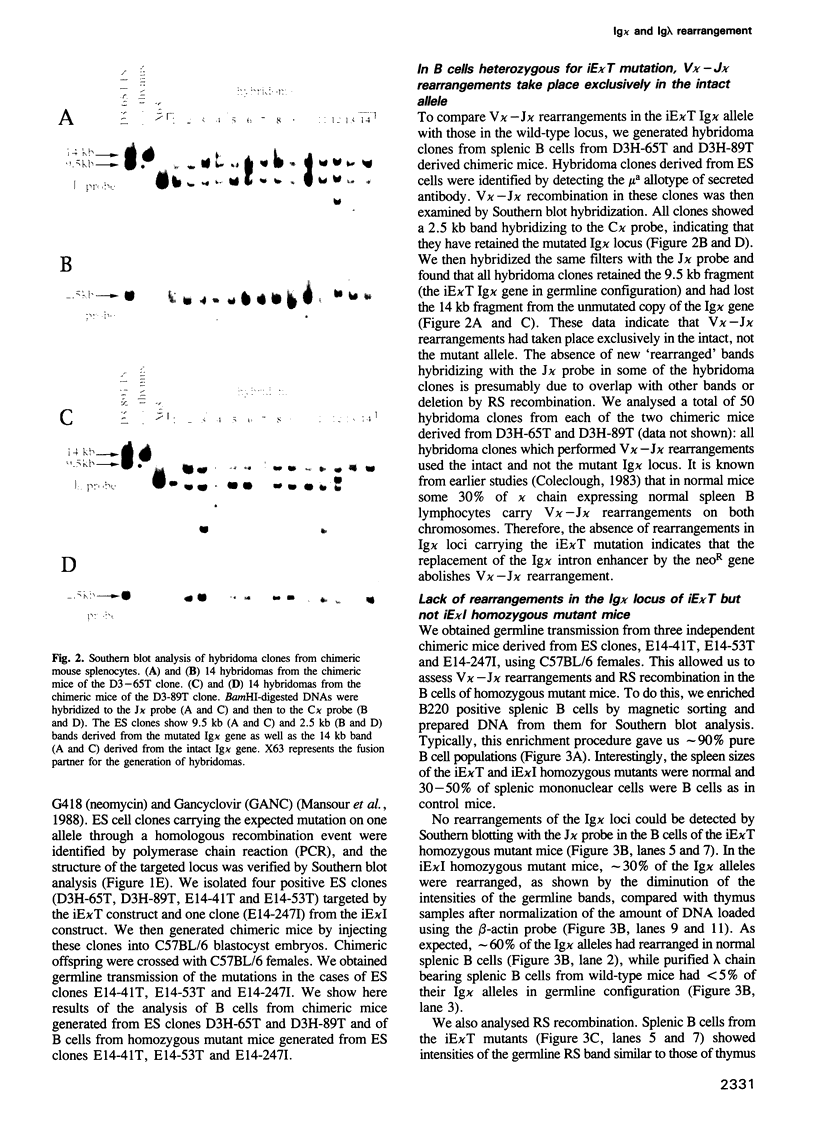
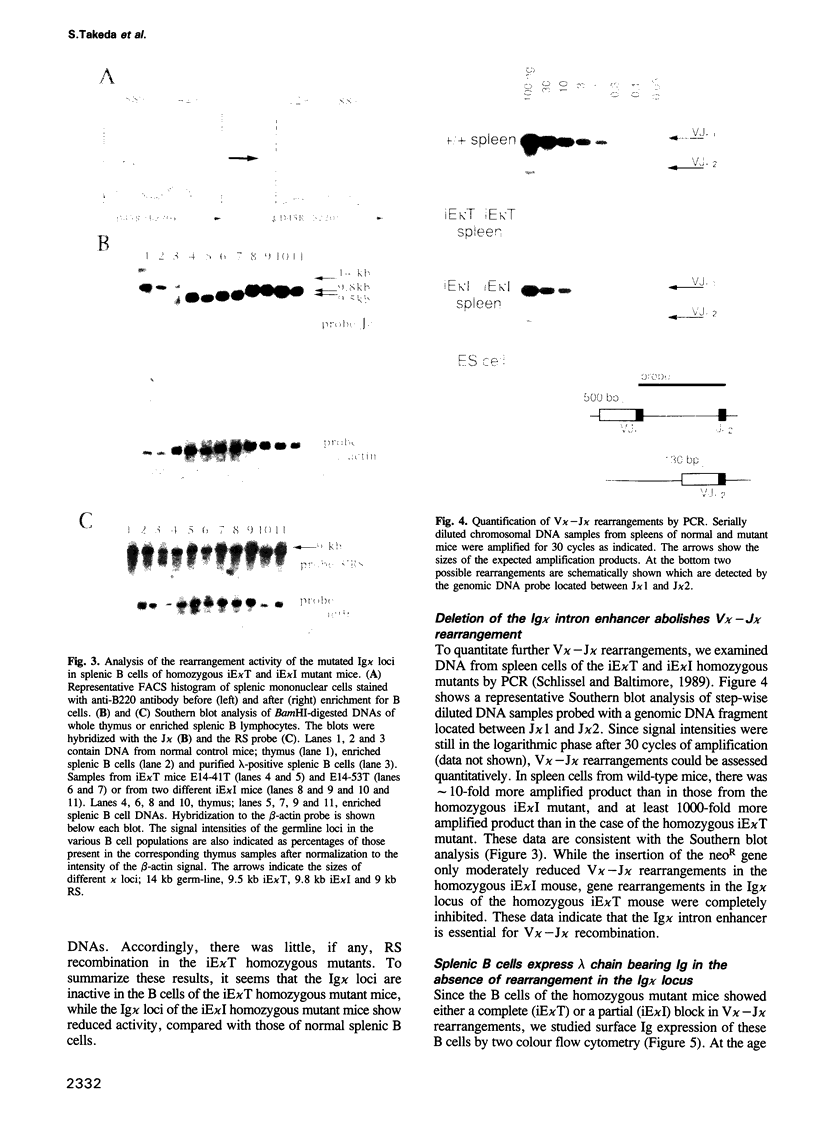
Abstract
Immunoglobulins (Ig) secreted from a plasma cell contain either kappa or lambda light chains, but not both. This phenomenon is termed isotypic kappa-lambda exclusion. While kappa-producing cells have their lambda chain genes in germline configuration, in most lambda-producing cells the kappa chain genes are either non-productively rearranged or deleted. To investigate the molecular mechanism for isotypic kappa-lambda exclusion, in particular the role of the Ig kappa intron enhancer, we replaced this enhancer by a neomycin resistance (neoR) gene in embryonic stem (ES) cells. B cells heterozygous for the mutation undergo V kappa-J kappa recombination exclusively in the intact Ig kappa locus but not in the mutated Ig kappa locus. Homozygous mutant mice exhibited no rearrangements in their Ig kappa loci. However, splenic B cell numbers were only slightly reduced as compared with the wild-type, and all B cells expressed lambda chain bearing surface Ig. These findings demonstrate that rearrangement in the Ig kappa locus is not essential for lambda gene rearrangement. We also generated homozygous mutant mice in which the neoR gene was inserted at the 3' end of the Ig kappa intron enhancer. Unexpectedly, mere insertion of the neoR gene showed some suppressive effect on V kappa-J kappa recombination. However, the much more pronounced inhibition of V kappa-J kappa recombination by the replacement of the Ig kappa intron enhancer suggests that this enhancer is essential for V kappa-J kappa recombination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Enea V., Bothwell A. L., Baltimore D. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 1980 Aug;21(1):1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenburger W., Neumaier P. S., Steinmetz M., Zachau H. G. DNA sequence of the constant gene region of the mouse immunoglobulin kappa chain. Nucleic Acids Res. 1981 Feb 25;9(4):971–981. doi: 10.1093/nar/9.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artelt P., Grannemann R., Stocking C., Friel J., Bartsch J., Hauser H. The prokaryotic neomycin-resistance-encoding gene acts as a transcriptional silencer in eukaryotic cells. Gene. 1991 Mar 15;99(2):249–254. doi: 10.1016/0378-1119(91)90134-w. [DOI] [PubMed] [Google Scholar]
- Atchison M. L., Perry R. P. Complementation between two cell lines lacking kappa enhancer activity: implications for the developmental control of immunoglobulin transcription. EMBO J. 1988 Dec 20;7(13):4213–4220. doi: 10.1002/j.1460-2075.1988.tb03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison M. L., Perry R. P. The role of the kappa enhancer and its binding factor NF-kappa B in the developmental regulation of kappa gene transcription. Cell. 1987 Jan 16;48(1):121–128. doi: 10.1016/0092-8674(87)90362-x. [DOI] [PubMed] [Google Scholar]
- Bergman Y., Rice D., Grosschedl R., Baltimore D. Two regulatory elements for immunoglobulin kappa light chain gene expression. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7041–7045. doi: 10.1073/pnas.81.22.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Hozumi N., Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978 Dec;15(4):1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Chen J., Trounstine M., Kurahara C., Young F., Kuo C. C., Xu Y., Loring J. F., Alt F. W., Huszar D. B cell development in mice that lack one or both immunoglobulin kappa light chain genes. EMBO J. 1993 Mar;12(3):821–830. doi: 10.1002/j.1460-2075.1993.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Coffman R. L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Coleclough C. Chance, necessity and antibody gene dynamics. Nature. 1983 May 5;303(5912):23–26. doi: 10.1038/303023a0. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Currie R. A., Roeder R. G. Identification of an octamer-binding site in the mouse kappa light-chain immunoglobulin enhancer. Mol Cell Biol. 1989 Oct;9(10):4239–4247. doi: 10.1128/mcb.9.10.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson I., Xiao J. H., Rosales R., Staub A., Chambon P. The HeLa cell protein TEF-1 binds specifically and cooperatively to two SV40 enhancer motifs of unrelated sequence. Cell. 1988 Sep 23;54(7):931–942. doi: 10.1016/0092-8674(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Doetschman T., Maeda N., Smithies O. Targeted mutation of the Hprt gene in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8583–8587. doi: 10.1073/pnas.85.22.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durdik J., Moore M. W., Selsing E. Novel kappa light-chain gene rearrangements in mouse lambda light chain-producing B lymphocytes. Nature. 1984 Feb 23;307(5953):749–752. doi: 10.1038/307749a0. [DOI] [PubMed] [Google Scholar]
- Engler P., Haasch D., Pinkert C. A., Doglio L., Glymour M., Brinster R., Storb U. A strain-specific modifier on mouse chromosome 4 controls the methylation of independent transgene loci. Cell. 1991 Jun 14;65(6):939–947. doi: 10.1016/0092-8674(91)90546-b. [DOI] [PubMed] [Google Scholar]
- Ferrier P., Krippl B., Blackwell T. K., Furley A. J., Suh H., Winoto A., Cook W. D., Hood L., Costantini F., Alt F. W. Separate elements control DJ and VDJ rearrangement in a transgenic recombination substrate. EMBO J. 1990 Jan;9(1):117–125. doi: 10.1002/j.1460-2075.1990.tb08087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromental C., Kanno M., Nomiyama H., Chambon P. Cooperativity and hierarchical levels of functional organization in the SV40 enhancer. Cell. 1988 Sep 23;54(7):943–953. doi: 10.1016/0092-8674(88)90109-2. [DOI] [PubMed] [Google Scholar]
- Förster I., Vieira P., Rajewsky K. Flow cytometric analysis of cell proliferation dynamics in the B cell compartment of the mouse. Int Immunol. 1989;1(4):321–331. doi: 10.1093/intimm/1.4.321. [DOI] [PubMed] [Google Scholar]
- Hagman J., Lo D., Doglio L. T., Hackett J., Jr, Rudin C. M., Haasch D., Brinster R., Storb U. Inhibition of immunoglobulin gene rearrangement by the expression of a lambda 2 transgene. J Exp Med. 1989 Jun 1;169(6):1911–1929. doi: 10.1084/jem.169.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Johnston R. F., Pickett S. C., Barker D. L. Autoradiography using storage phosphor technology. Electrophoresis. 1990 May;11(5):355–360. doi: 10.1002/elps.1150110503. [DOI] [PubMed] [Google Scholar]
- Kawakami T., Takahashi N., Honjo T. Complete nucleotide sequence of mouse immunoglobulin mu gene and comparison with other immunoglobulin heavy chain genes. Nucleic Acids Res. 1980 Sep 11;8(17):3933–3945. doi: 10.1093/nar/8.17.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kendall C., Ionescu-Matiu I., Dreesman G. R. Utilization of the biotin/avidin system to amplify the sensitivity of the enzyme-linked immunosorbent assay (ELISA). J Immunol Methods. 1983 Feb 11;56(3):329–339. doi: 10.1016/s0022-1759(83)80022-2. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992 May 29;69(5):823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Rajewsky K. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992 Mar 12;356(6365):154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- Klobeck H. G., Zachau H. G. The human CK gene segment and the kappa deleting element are closely linked. Nucleic Acids Res. 1986 Jun 11;14(11):4591–4603. doi: 10.1093/nar/14.11.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Leclercq L., Butkeraitis P., Reth M. A novel germ-line JK transcript starting immediately upstream of JK1. Nucleic Acids Res. 1989 Sep 12;17(17):6809–6819. doi: 10.1093/nar/17.17.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S., Rosenberg N., Alt F., Baltimore D. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982 Oct;30(3):807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Manz J., Denis K., Witte O., Brinster R., Storb U. Feedback inhibition of immunoglobulin gene rearrangement by membrane mu, but not by secreted mu heavy chains. J Exp Med. 1988 Oct 1;168(4):1363–1381. doi: 10.1084/jem.168.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. B., Neuberger M. S. The immunoglobulin kappa locus contains a second, stronger B-cell-specific enhancer which is located downstream of the constant region. EMBO J. 1989 Jul;8(7):1959–1964. doi: 10.1002/j.1460-2075.1989.tb03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltenyi S., Müller W., Weichel W., Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11(2):231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- Nadel B., Cazenave P. A., Sanchez P. Murine lambda gene rearrangements: the stochastic model prevails over the ordered model. EMBO J. 1990 Feb;9(2):435–440. doi: 10.1002/j.1460-2075.1990.tb08128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Caskey H. M., Pettersson S., Williams G. T., Surani M. A. Isotype exclusion and transgene down-regulation in immunoglobulin-lambda transgenic mice. Nature. 1989 Mar 23;338(6213):350–352. doi: 10.1038/338350a0. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Sasaki Y., Kina T., Amagai T., Katsura Y. A monoclonal antibody against Igh6-4 determinant. Immunogenetics. 1986;23(2):137–139. doi: 10.1007/BF00377976. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M. C., Shaw A. C., Sinn E., Danner D. B., Holmes K. L., Morse H. C., 3rd, Leder P. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987 May 15;236(4803):816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- Picard D., Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. doi: 10.1038/307080a0. [DOI] [PubMed] [Google Scholar]
- Pierce J. W., Gifford A. M., Baltimore D. Silencing of the expression of the immunoglobulin kappa gene in non-B cells. Mol Cell Biol. 1991 Mar;11(3):1431–1437. doi: 10.1128/mcb.11.3.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Ramsden D. A., Wu G. E. Mouse kappa light-chain recombination signal sequences mediate recombination more frequently than do those of lambda light chain. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10721–10725. doi: 10.1073/pnas.88.23.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Petrac E., Wiese P., Lobel L., Alt F. W. Activation of V kappa gene rearrangement in pre-B cells follows the expression of membrane-bound immunoglobulin heavy chains. EMBO J. 1987 Nov;6(11):3299–3305. doi: 10.1002/j.1460-2075.1987.tb02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Schüppel R., Wilke J., Weiler E. Monoclonal anti-allotype antibody towards BALB/c IgM. Analysis of specificity and site of a V-C crossover in recombinant strain BALB-Igh-Va/Igh-Cb. Eur J Immunol. 1987 May;17(5):739–741. doi: 10.1002/eji.1830170527. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Iwasato T., Yamagishi H. Deletions of immunoglobulin C kappa region characterized by the circular excision products in mouse splenocytes. J Exp Med. 1991 May 1;173(5):1065–1072. doi: 10.1084/jem.173.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch K. A., Bakhshi A., Goldman P., Korsmeyer S. J. A uniform deleting element mediates the loss of kappa genes in human B cells. Nature. 1985 Jul 18;316(6025):260–262. doi: 10.1038/316260a0. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Zou Y. R., Takeda S., Rajewsky K. Gene targeting in the Ig kappa locus: efficient generation of lambda chain-expressing B cells, independent of gene rearrangements in Ig kappa. EMBO J. 1993 Mar;12(3):811–820. doi: 10.1002/j.1460-2075.1993.tb05721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]