Abstract
Purpose
Understanding the determinants of fatigue worsening may help distinguish between different fatigue phenotypes and inform clinical trial designs.
Patients and Methods
Patients with invasive cancer of the breast, prostate, colon/rectum, or lung were enrolled from multiple sites. At enrollment during an outpatient visit and 4–5 weeks later patients rated their symptoms on a 0–10 numerical rating scale. A 2-point change was considered clinically significant for fatigue change. Effects of demographic and clinical factors on patient-reported fatigue were examined using logistic regression models.
Results
3123 patients were enrolled at baseline and 3032 were analyzable for fatigue change. At baseline, 23% had no fatigue, 35% mild, 25% moderate, and 17% severe. Key parameters in our model of fatigue worsening includes fatigue at baseline (OR 0.75), disease status (OR 1.99), performance status (OR 1.38), history of depression (OR 1.28), patient perception of bother due to comorbidity (OR 1.26) and treatment exposures including recent cancer treatment (OR 1.77), and use of corticosteroids (1.37). The impact of gender was examined only in colorectal and lung cancer patients, and it was a significant factor with men most likely to experience worsening of fatigue (OR=1.46).
Conclusions
Predictors of fatigue worsening include multiple factors that are difficult to modify: baseline fatigue level, gender, disease status, performance status, recent cancer treatment, bother due to comorbidity, and history of depression. Future fatigue prevention and treatment trial designs should account for key predictors of worsening fatigue.
Keywords: cancer fatigue, symptom management, medical oncology, ambulatory care
INTRODUCTION
Fatigue is the most prevalent symptom experienced by patients and confronted by healthcare providers in the outpatient oncology setting, and it is clearly a symptom with major impact on patients. Despite the publication of dozens of original research studies and review articles every year, progress has been disappointing in terms of understanding the various biological underpinnings of cancer fatigue and establishing evidence-based standards and a strong consensus about how patients should be assessed and treated1–5. There are no less than 170 published interventional studies intended to improve cancer fatigue6, and these are most often negative trials7–14. One of the challenges is considerable diversity in the conceptual and operational definition of fatigue in cancer, with no fewer than 24 conceptual definitions posed in the literature.5 Most self-report scales address the sensation and the impact domains of fatigue, and some scales include additional domains.15 The popular National Comprehensive Cancer Network (NCCN) definition refers to cancer-related fatigue as a “distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.”16
Several authors have discussed the importance of the pre-existing (baseline) levels of fatigue as an important determinant of response to an intervention12, 17–20, but data regarding the impact of baseline fatigue and other fixed and modifiable factors are limited. We sought to evaluate the determinants of worsening self-reported fatigue levels in a secondary analysis of a prospective study describing symptom expression and practice patterns in ambulatory patients with common solid tumors. In these data, much like in ordinary clinical practice, we do not have clear attributions for individual symptoms such as fatigue that are causing functional interference. Indeed, patients with various comorbidities often have multiple causes of fatigue. As such, we focused this report on the construct of patient-reported fatigue rather than cancer-related fatigue. We hypothesized that through analyses of these prospective data, we could explore a wide set of key variables, including disease type, cancer stage, cancer treatment status and type, supportive care medication exposures, and other clinical and demographic variables in order to identify key predictors of worsening fatigue. Finding such factors would clearly have implications for the design and conduct of clinical trials aimed at the treatment or prevention of fatigue in ambulatory solid tumor patients.
METHODS
Study Design and Subjects
From March 3, 2006, to May 19, 2008, we enrolled oncology outpatients at any point in the trajectory of their care for invasive breast, lung, prostate, or colorectal cancer. Patients were registered at 38 institutions, including 6 academic sites and 32 community clinics. Patients treated in academic centers were enrolled from disease site-specific clinics. In contrast, patients treated in community clinics were enrolled from general oncology clinics. Eligible patients had to be at least 18 years of age, receiving care at an Eastern Cooperative Oncology Group-affiliated institution, willing to complete the follow-up survey, and judged by the study screener to have cognitive function adequate for completing study surveys. Patients were recruited when they checked in for their clinic appointments, and patient information was collected before their visit with a clinician. Patients and their treating clinicians were surveyed at the initial visit and at follow-up 28–35 days later. Further details about the study cohort have been published.21 The protocol was approved by the institutional review boards at each registering institution. All patients provided written informed consent.
Study Procedures
Patients were recruited when they checked in for their clinic appointments, and patients’ information was collected before their visit with a clinician. Patients and their treating clinicians were surveyed at the initial visit and at follow-up 28–35 days later.
Patients were asked to read the instructions at the beginning of each questionnaire and complete all items in terms of their experience during the preceding 24 hours. Reasons for incomplete forms were documented on the Assessment Compliance Form. Patients who could not complete the follow-up questionnaire because of acute illness were given the option to mail the forms to the treating clinic by day 42 after the initial visit.
Study Measures
The initial survey was used to collect patients’ basic clinical and demographic information, including cancer treatment history and current therapies. At the initial and follow-up visits, patients reported symptom intensity and functional interference using a modification of The University of Texas MD Anderson Cancer Center Symptom Inventory (MDASI), a validated measure that is very similar to the Brief Fatigue Inventory in terms of structure and patient burden assessment.22, 23 Patients used the MDASI to rate “fatigue (tiredness) at its worst” along with symptoms and functional interference items that they most frequently experienced in the previous 24 hours using an 11-point Likert scale ranging from 0 (“not present”) to 10 (“as bad as you can imagine”). Clinicians reported patients’ specific medications, including those that were newly prescribed. A clinician-specific survey was used to ascertain symptom prioritization and symptom attribution. The protocol and case report forms are accessible on the study web site24.
Statistical Analysis
Patients were grouped into four categories based on their fatigue level at baseline measured by the fatigue item of the MDASI: no fatigue (0), mild fatigue (1–3), moderate fatigue (4–6) and severe fatigue (7–10). The association between fatigue severity at baseline and patient demographic and disease characteristics was examined using the Chi-square test.
A 2-point change in the fatigue item of the MDASI between the initial and follow up assessments was considered clinically significant.25 This study focused on fatigue worsening, defined as a 2-point increase in fatigue score between the two assessments. Fisher's exact tests were used for the association between fatigue change and fatigue severity at baseline. Univariate and multivariable logistic regression models were used to examine the unadjusted and adjusted effects of demographic and clinical factors on fatigue worsening in patients with baseline levels of fatigue in the range of 0–8. A total of 31 variables were included as covariates in the logistic models. These variables either have been shown to be associated with fatigue worsening in previous studies or there are possibilities for such association based on biology. For cancers affecting both men and women at similar rates (lung and colorectal cancer), sex was also evaluated as a potential predictor of fatigue worsening. The Variance Inflation Factor (VIF) was used to check multicollinearity among these variables in the multivariable regression model. The largest VIF value was 1.9. A separate category for missing data was generated for categorical covariates if the proportion of missingness was no less than 5 percent. For categorical covariates with less than 5 percent missing data and continuous covariates, patients with missing data were excluded from the logistic models. Robust standard errors of mean (i.e., clustered sandwich estimator) were used in the logistic regression models to account for the clustering effect of institutions (i.e., patients enrolled in the same institution might not be independent).
All P values were two-sided and P < 0.05 was considered statistically significant. STATA 11.0 software (2009; StataCorp, College Station, TX) was used for all data analysis.
Results
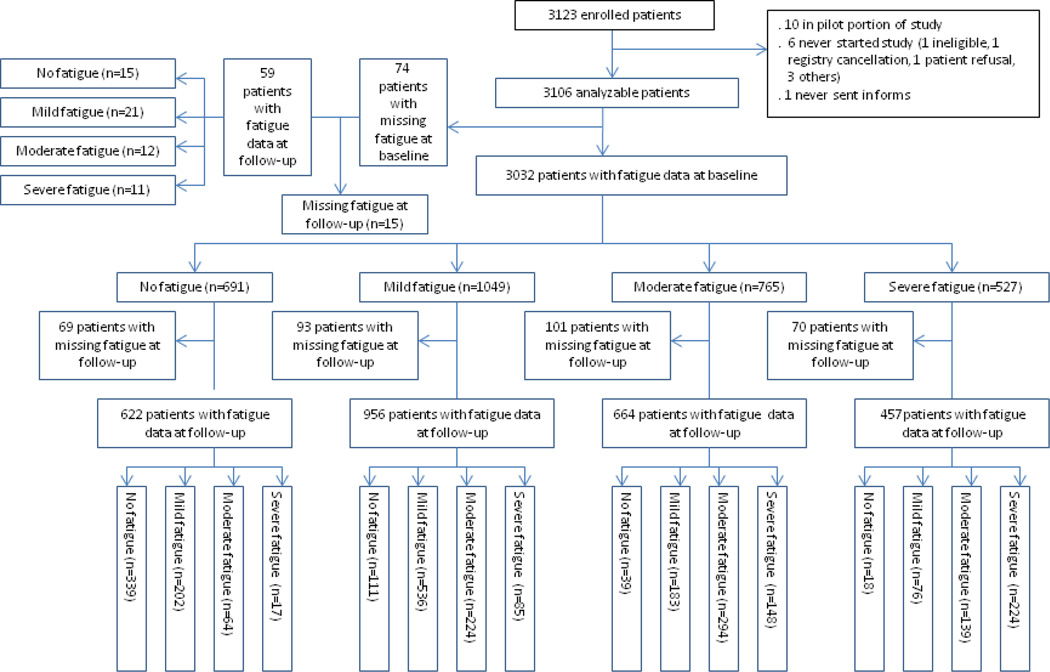
The study flow diagram is summarized in Figure 1. Of the 3,123 patients enrolled in this study, we found 3,032 with a self-reported fatigue item available at enrollment (97%). No fatigue (score = 0) was reported by 691 patients (23%), mild fatigue by 1,049 (35%), moderate fatigue by 765 (25%), and severe fatigue by 527 (17%).
Figure 1.
Study flow diagram. Mild fatigue: fatigue scores of 1–3, moderate fatigue: fatigue scores of 4–6, and severe fatigue: fatigue scores of 7–10. A total of 2699 patients reported a fatigue score at both initial and follow-up assessments.
Patient demographics and disease characteristics at the initial assessments are summarized in Table 1. Strong associations between fatigue severity and indicators of disease complexity, such as advanced stage, number of metastatic sites, and perceived degree of care difficulty, were found. Also noted are unilateral associations between worse fatigue severity and black-race- and minority-based institutions.
Table 1.
Demographic and Disease Characteristics by Severity of Fatigue at Baseline
| Demographic and disease characteristics |
Number of patients |
Fatigue score at baseline (%) |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 1–3 | 4–6 | 7–10 | P value | |||
| Demographic characteristics | |||||||
| Age (years) | >=55 | 2075 | 74.24 | 67.78 | 69.15 | 61.1 | < 0.001 |
| <55 | 957 | 25.76 | 32.22 | 30.85 | 38.9 | ||
| Sex | Female | 2,126 | 22.3 | 35.6 | 24.5 | 17.6 | 0.186 |
| Male | 906 | 24.0 | 32.2 | 27.0 | 16.8 | ||
| Race and ethnicity | Minority | 667 | 28.6 | 26.4 | 23.2 | 21.7 | < 0.001 |
| Non-Hispanic whites | 2,137 | 20.8 | 36.9 | 25.9 | 16.5 | ||
| Disease Characteristics | |||||||
| Primary disease site | Breast | 1,509 | 24.3 | 36.7 | 23.0 | 16.0 | < 0.001 |
| Colon/rectum | 700 | 24.3 | 34.3 | 25.6 | 15.9 | ||
| Prostate | 307 | 27.4 | 30.3 | 28.7 | 13.7 | ||
| Lung | 516 | 13.6 | 31.4 | 29.3 | 25.8 | ||
| Current status of disease | CR/PR/ SD | 2,583 | 24.3 | 35.1 | 24.7 | 15.9 | < 0.001 |
| PD | 432 | 13.9 | 31.3 | 27.8 | 27.1 | ||
| Current stage of disease | Non-advanced | 1,877 | 29.1 | 34.9 | 21.9 | 14.1 | < 0.001 |
| Advanced | 1,145 | 12.5 | 34.2 | 30.5 | 22.8 | ||
| ECOG performance status | 0 | 1,718 | 29.9 | 38.7 | 21.0 | 10.5 | < 0.001 |
| 1–4 | 1,300 | 13.5 | 29.2 | 30.9 | 26.4 | ||
| Duration of cancer | Median | 3,032 | 1.7 | 1.2 | 1.1 | 1.1 | 0.002 |
| Treatment of Cancer | |||||||
| Institution type | Academic | 296 | 29.7 | 25.7 | 25.0 | 19.6 | 0.001 |
| Community | 2,736 | 22.0 | 35.6 | 25.3 | 17.1 | ||
| Prior chemo/immuno/hormonal therapy | No | 1,160 | 22.8 | 35.7 | 24.7 | 16.8 | 0.740 |
| Yes | 1,871 | 22.7 | 33.9 | 25.6 | 17.7 | ||
| Prior radiation therapy | No | 1,737 | 24.1 | 34.5 | 24.9 | 16.5 | 0.208 |
| Yes | 1,269 | 21.0 | 35.1 | 25.5 | 18.4 | ||
| Current therapy (any type) | No | 783 | 32.6 | 32.4 | 21.1 | 13.9 | < 0.001 |
| Yes | 2,249 | 19.4 | 35.4 | 26.7 | 18.6 | ||
| Number of medications currently taking | 0–4 | 882 | 28.3 | 37.6 | 20.2 | 13.8 | < 0.001 |
| 5–9 | 1,180 | 21.0 | 35.3 | 27.4 | 16.3 | ||
| >=10 | 668 | 13.9 | 29.9 | 31.0 | 25.2 | ||
| Receive individual counseling | No | 2,736 | 23.1 | 35.1 | 25.2 | 16.7 | 0.009 |
| Yes | 291 | 20.3 | 29.6 | 26.1 | 24.1 | ||
| Participate support group | No | 2,829 | 22.9 | 34.6 | 25.2 | 17.3 | 0.762 |
| Yes | 199 | 20.1 | 34.2 | 26.6 | 19.1 | ||
| Quality of Life | |||||||
| Clinician perception of being bothered by difficulty related to co-morbidities | Not at all/ A little bit | 2,273 | 25.7 | 36.3 | 23.7 | 14.4 | < 0.001 |
| Moderately/Quite a bit/Extremely | 741 | 13.8 | 29.6 | 30.1 | 26.6 | ||
| Patient perception of being bothered by difficulty related to co-morbidities | Not at all/ A little bit | 2,039 | 27.2 | 37.6 | 21.8 | 13.4 | < 0.001 |
| Moderately/Quite a bit/Extremely | 986 | 13.6 | 28.5 | 32.4 | 25.6 | ||
| Weight loss in the previous 6 months | < 5% | 2,571 | 24.8 | 35.5 | 24.5 | 15.2 | < 0.001 |
| >=5% | 427 | 11.0 | 29.7 | 29.3 | 30.0 | ||
| Patient reported overall quality of life | Good/ Excellent | 2126 | 28.9 | 39.8 | 21.1 | 10.2 | < 0.001 |
| Fair /Poor/ Very poor | 897 | 8.4 | 22.1 | 35.1 | 34.5 | ||
| History of depression | No | 2,147 | 26.0 | 35.8 | 24.2 | 14.0 | < 0.001 |
| Yes | 881 | 15.0 | 31.6 | 27.8 | 25.7 | ||
| Overall | 3,032 | 22.8 | 34.6 | 25.2 | 17.4 | ||
Abbreviations: ECOG, Eastern Cooperative Oncology Group
Notes:
1) Significance was tested by chi-square test for binary and categorical variables, and Fisher's exact test of the equality of the medians was used for continuous variables. Significance level was set at 0.05. 2) Seventy-four patients had missing fatigue data at baseline.
3) The total number of patients did not add up to 3032 for some variables due to missing values.
The proportion of patients with initial fatigue scores of 1–3, 4–6, or 7–8 whose fatigue level worsened by 2 or more points varied significantly by initial fatigue level. Changes in fatigue severity according to initial fatigue expression levels and separated by cancer treatment exposure are summarized in Table 2. The logistical regression model for fatigue worsening is summarized in Table 3. Key parameters in this model include fatigue at baseline (odds ratio [OR] 0.75), disease status (OR 1.99), performance status (OR 1.38), history of depression (OR 1.28), patient perception of bother due to comorbidity (OR 1.26), and treatment exposures, including recent cancer treatment (OR 1.77) and use of corticosteroids (1.37).
Table 2.
Change in Fatigue Severity by Fatigue Severity Level at Baseline
| A. All patients | ||||
|---|---|---|---|---|
| Fatigue score at baseline |
No. of patients |
Better | No change | Worse |
| 0 | 622 | 0 (0.0) | 439 (70.6) | 183 (29.4) |
| 1–3 | 956 | 82 (8.6) | 542 (56.7) | 332 (34.7) |
| 1 | 356 | 0 (0.0) | 224 (62.9) | 132 (37.1) |
| 2–3 | 600 | 82 (13.7) | 318 (53.0) | 200 (33.3) |
| 4–6 | 664 | 208 (31.3) | 308 (46.4) | 148 (22.3) |
| 7–10 | 457 | 245 (53.6) | 186 (40.7) | 26 (5.7) |
| 7–8 | 301 | 151 (50.2) | 124 (41.2) | 26 (8.6) |
| 9–10 | 156 | 94 (60.3) | 62 (39.7) | 0 (0.0) |
| Total | 2699 | 535 (19.8) | 1475 (54.6) | 689 (25.5) |
| B. Patients with no current therapy | ||||
|---|---|---|---|---|
| Fatigue score at baseline |
No. of patients |
Better | No change | Worse |
| 0 | 230 | 0 (0.0) | 182 (79.1) | 48 (20.9) |
| 1–3 | 222 | 22 (9.9) | 128 (57.7) | 72 (32.4) |
| 1 | 86 | 0 (0.0) | 56 (65.1) | 30 (34.9) |
| 2–3 | 136 | 22 (16.2) | 72 (52.9) | 42 (30.9) |
| 4–6 | 135 | 45 (33.3) | 66 (48.9) | 24 (17.8) |
| 7–10 | 88 | 55 (62.5) | 27 (30.7) | 6 (6.8) |
| 7–8 | 55 | 31 (56.4) | 18 (32.7) | 6 (10.9) |
| 9–10 | 33 | 24 (72.7) | 9 (27.3) | 0 (0.0) |
| Total | 675 | 122 (18.1) | 403 (59.7) | 150 (22.2) |
| C. Patient with current therapy within 1 month | ||||
|---|---|---|---|---|
| Fatigue score at baseline |
No. of patients |
Better | No change | Worse |
| 0 | 104 | 0(0.0) | 55 (52.9) | 49 (47.2) |
| 1–3 | 195 | 16 (8.2) | 88 (45.1) | 91 (46.7) |
| 1 | 76 | 0(0.0) | 37 (48.7) | 39 (51.3) |
| 2–3 | 119 | 16 (13.4) | 51 (42.9) | 52 (43.7) |
| 4–6 | 122 | 31 (25.4) | 55 (45.1) | 36 (29.5) |
| 7–10 | 98 | 58 (59.2) | 35 (35.7) | 5 (5.1) |
| 7–8 | 64 | 34 (53.1) | 25 (39.1) | 5 (7.8) |
| 9–10 | 34 | 24 (70.6) | 10 (29.4) | 0 (0.0) |
| Total | 519 | 105 (20.2) | 233 (44.9) | 181 (34.9) |
| D. Patients with current therapy between 1 month and 1 year | ||||
|---|---|---|---|---|
| Fatigue score at baseline |
No. of patients |
Better | No change | Worse |
| 0 | 173 | 0(0.0) | 112 (64.7) | 61 (35.3) |
| 1–3 | 386 | 31 (8.0) | 224 (58.0) | 131 (33.9) |
| 1 | 140 | 0(0.0) | 91 (65.0) | 49 (35.0) |
| 2–3 | 246 | 31 (12.6) | 133 (54.1) | 82 (33.3) |
| 4–6 | 312 | 98 (31.4) | 139 (44.6) | 75 (24.0) |
| 7–10 | 207 | 94 (45.4) | 100 (48.3) | 13 (6.3) |
| 7–8 | 141 | 62 (44.0) | 66 (46.8) | 13 (9.2) |
| 9–10 | 66 | 32 (48.5) | 34 (51.5) | 0(0.0) |
| Total | 1,078 | 223 (20.7) | 575 (53.3) | 280 (26.0) |
| E. Patients with current therapy beyond 1 year | ||||
|---|---|---|---|---|
| Fatigue score at baseline |
No. of patients |
Better | No change | Worse |
| 0 | 112 | 0(0.0) | 87 (77.7) | 25 (22.3) |
| 1–3 | 151 | 13 (8.6) | 101 (66.9) | 37 (24.5) |
| 1 | 52 | 0(0.0) | 39 (75.0) | 13 (25.0) |
| 2–3 | 99 | 12 (13.1) | 62 (62.6) | 24 (26.2) |
| 4–6 | 92 | 32 (34.8) | 47 (51.1) | 13 (14.1) |
| 7–10 | 62 | 37 (59.7) | 23 (37.1) | 2 (3.2) |
| 7–8 | 39 | 23 (59.0) | 14 (35.9) | 2 (5.1) |
| 9–10 | 23 | 14 (60.9) | 9 (39.1) | 0(0.0) |
| Total | 417 | 82 (19.7) | 258 (61.9) | 77 (18.5) |
Note: Change in fatigue severity: worse was defined as >=2 point increase; better was defined as >=2 point decrease; no change was defined as <2 points change (either increase or decrease)
Table 3.
Logistic Regression Modeling Fatigue Worsening for Patients with Baseline Fatigue Level of 0–8 (n=2,367)
| Variable | Univariate regression | Multivariable regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||||
| Demographics | |||||||||
| Age | < 55 v > = 55 | 0.98 | 0.81 | 1.19 | 0.858 | 1.10 | 0.86 | 1.41 | 0.448 |
| Race | Non-Hispanic white v other | 0.95 | 0.80 | 1.13 | 0.564 | 0.96 | 0.79 | 1.16 | 0.673 |
| Disease Characteristics | |||||||||
| Duration of cancer | Continuous (years) | 0.96 | 0.94 | 0.98 | 0.001 | 0.97 | 0.94 | 1.00 | 0.032 |
| Disease status | PD v other | 1.60 | 1.27 | 2.03 | <0.001 | 1.99 | 1.53 | 2.58 | <0.001 |
| Disease site | Colorectal v breast | 0.89 | 0.68 | 1.17 | 0.424 | 0.77 | 0.57 | 1.04 | 0.090 |
| Prostate v breast | 0.91 | 0.62 | 1.33 | 0.633 | 0.93 | 0.59 | 1.45 | 0.744 | |
| Lung v breast | 1.36 | 1.10 | 1.67 | 0.004 | 1.18 | 0.90 | 1.55 | 0.222 | |
| Advanced disease | Yes v no | 1.04 | 0.84 | 1.29 | 0.704 | 0.92 | 0.69 | 1.22 | 0.564 |
| Treatment of Cancer | |||||||||
| Exposure to corticosteroids | Yes v no | 1.55 | 1.21 | 1.98 | 0.001 | 1.37 | 1.03 | 1.83 | 0.031 |
| Current treatment | < 1 m v no treatment | 1.91 | 1.48 | 2.46 | <0.001 | 1.77 | 1.32 | 2.38 | <0.001 |
| > 1 m and <1 yr v no treatment | 1.24 | 0.99 | 1.55 | 0.066 | 1.28 | 0.95 | 1.72 | 0.104 | |
| >1 yr v no treatment | 0.97 | 0.56 | 1.03 | 0.080 | 0.75 | 0.53 | 1.07 | 0.112 | |
| Number of medicines currently taken | 5–9 v 1–4 | 1.29 | 1.04 | 1.59 | 0.018 | 1.18 | 0.91 | 1.52 | 0.203 |
| > = 10 v 1–4 | 1.52 | 1.19 | 1.95 | 0.001 | 1.33 | 0.92 | 1.94 | 0.131 | |
| Missing v 1–4 | 1.44 | 1.05 | 1.97 | 0.022 | 1.26 | 0.91 | 1.73 | 0.158 | |
| Exposure to SSRI/newer antidepressants | Yes v no | 1.33 | 1.10 | 1.60 | 0.003 | 1.29 | 0.99 | 1.68 | 0.055 |
| Exposure to other antidepressants | Yes v no | 0.93 | 0.48 | 1.80 | 0.824 | 0.77 | 0.38 | 1.55 | 0.456 |
| Exposure to anxiolytics | Yes v no | 1.38 | 1.11 | 1.73 | 0.004 | 1.22 | 0.94 | 1.58 | 0.133 |
| Exposure to sedative | Yes v no | 1.04 | 0.76 | 1.43 | 0.790 | 0.83 | 0.57 | 1.20 | 0.319 |
| Exposure to beta blockers | Yes v no | 1.12 | 0.93 | 1.35 | 0.226 | 1.17 | 0.96 | 1.43 | 0.120 |
| Undertreatment of pain | Yes v no | 1.45 | 1.17 | 1.79 | 0.001 | 1.09 | 0.85 | 1.41 | 0.491 |
| Missing v no | 1.13 | 0.67 | 1.91 | 0.648 | 1.13 | 0.61 | 2.11 | 0.699 | |
| Prior chemo/immuno/hormonal therapy | Yes v no | 0.81 | 0.71 | 0.94 | 0.004 | 0.88 | 0.71 | 1.09 | 0.233 |
| Prior radiation | Yes v no | 0.90 | 0.74 | 1.09 | 0.270 | 1.05 | 0.84 | 1.31 | 0.677 |
| Type of institute | Community v academic | 1.01 | 0.73 | 1.39 | 0.968 | 1.17 | 0.72 | 1.91 | 0.533 |
| Receive counseling | Yes v no | 1.14 | 0.86 | 1.51 | 0.373 | 1.08 | 0.73 | 1.58 | 0.701 |
| Participate in support group | Yes v no | 0.74 | 0.51 | 1.07 | 0.111 | 0.77 | 0.52 | 1.14 | 0.187 |
| Quality of Life | |||||||||
| Fatigue at baseline | Continuous | 0.85 | 0.83 | 0.88 | <0.001 | 0.75 | 0.72 | 0.78 | <0.001 |
| History of depression | Yes v no | 1.27 | 1.05 | 1.54 | 0.015 | 1.28 | 1.02 | 1.61 | 0.033 |
| ECOG performance status | > = 1 v 0 | 1.28 | 1.06 | 1.54 | 0.009 | 1.38 | 1.13 | 1.69 | 0.002 |
| Patient's perception of being bothered by comorbidity | Yes v no | 1.12 | 0.94 | 1.33 | 0.224 | 1.26 | 1.03 | 1.54 | 0.028 |
| Clinician's perception of being bothered by comorbidity | Yes v no | 1.10 | 0.90 | 1.33 | 0.370 | 1.14 | 0.87 | 1.49 | 0.346 |
| Patient-reported quality of life | Poor v good | 1.01 | 0.80 | 1.28 | 0.930 | 1.13 | 0.86 | 1.47 | 0.383 |
| Weight loss | > = 5% v < 5% | 1.04 | 0.77 | 1.41 | 0.785 | 1.09 | 0.78 | 1.53 | 0.608 |
| Cognitive difficulty | > = 5 v < 5 | 0.92 | 0.69 | 1.22 | 0.554 | 1.34 | 0.97 | 1.85 | 0.081 |
| Drowsy at baseline | > = 5 v < 5 | 0.67 | 0.53 | 0.86 | 0.002 | 1.03 | 0.74 | 1.44 | 0.852 |
| Sleep at baseline | > = 5 v < 5 | 0.84 | 0.68 | 1.04 | 0.104 | 1.15 | 0.86 | 1.54 | 0.346 |
| Pain at baseline | > = 5 v < 5 | 0.72 | 0.54 | 0.95 | 0.019 | 1.04 | 0.69 | 1.56 | 0.845 |
The role of sex could only be explored in lung and colorectal cancer patients, as they are diseases that affect both men and women in significant proportions. Men had higher odds of worsening fatigue during the study period than women, after adjusting for other covariates (OR = 1.46; 95% confidence interval [CI]: 1.06–2.02, P value = 0.0201).
Discussion
This prospective study of patient-report fatigue in outpatient oncology in the United States provides strong point estimates concerning the prevalence of fatigue in the most common settings for cancer care and the distribution of fatigue according to levels of severity and by key clinical and demographic attributes. We found that 60% of patients have mild-to-moderate levels of fatigue and 17% of patients had severe fatigue. These data take us beyond summaries of fatigue prevalence with wide ranges (typically 50%–90%) that have been typically reported for a variety of different care settings. The existing fatigue data have been predominantly derived from breast cancer patients during their adjuvant treatment with radiation and/or chemotherapy, with some studies evaluating other patient cohorts after therapy or during treatment or groups of advanced cancer patients referred for specialized care6. Although these data focus on the construct of patient-reported fatigue rather than cancer-related fatigue, our findings are consistent with data related to cancer-related fatigue from other researchers who have found that approximately 30%–60% of patients have mild to moderate fatigue, approximately 20% of patients have severe levels of fatigue during or shortly after treatment for cancer, and approximately 10%–15% have fatigue as a chronic effect of treatment26–31.
In the existing fatigue literature, from a variety of different cohorts, predictors of fatigue have included most prominently co-existing psychological symptoms and mental health factors (including the tendency toward catastrophizing)29, 30, 32–35, co-existing pain36, 37, duration of cancer treatment38, primary disease site39, comorbidity32, 40–42, and a variety of other factors. As shown in Table 2, we found that the worsening in fatigue levels varied according to baseline levels of fatigue, and our model of fatigue worsening included baseline fatigue as a significant predictor. It is therefore important to take into account the baseline level of fatigue when trying to decipher the underlying basis for fatigue change. We also found that improvement in fatigue varied according to the baseline level, and this trend was consistent across all categories of timing in relation to cancer treatment. Overall, more than half of the patients who reported a fatigue level of 7–8 at study enrollment improved by 2 or more points, whereas less than one-third improved significantly when they reported fatigue in the 4–6 range at enrollment. There are a variety of endpoints that have been used in fatigue trials, and most of these trials define the minimal clinically meaningful change as a 0.3–0.5 effect size. In this dataset, the standard deviation for the MDASI fatigue item at study enrollment was 2.9. Thus, a 2-point change in this item represents a moderate effect size of 0.69 that is considered clinically meaningful.
It is notable that most of the significant predictors of fatigue change cannot easily be modified, although one factor, prescription of corticosteroids, is modifiable. This category of medication is often directed at symptom management and is sometimes intended specifically for the treatment of fatigue. It is most likely that the short-term prescription of corticosteroids for symptom control is not what was reflected in this logistical regression. Rather, the model more likely reflects other uses of corticosteroids, such as prolonged treatment of pain or chronic underlying inflammatory conditions.
These findings have important implications. It is clear that interventions to intervene on the prevalent symptom of fatigue as a clinical trial outcome is of great interest, but it continues to be an evolving field5, 43. Fatigue interventions in patients with complex chronic illness most often target two assessments 3–4 weeks apart44, and there are multiple examples of such trial intervals.10, 45–47 For these studies, the trends at 4 weeks are highly correlated with the 6 and 8 week results. Moreover, there are also a number of high profile fatigue trials that are focused on shorter intervals in the 2–4 week range.8, 9, 14, 19, 48 We believe that our data about fatigue change during this 28–35 day interval is highly relevant to clinicians and trialists. We acknowledge that further longitudinal data would also be relevant and such data will be needed in future studies to better understand the trajectory of this symptom. Future fatigue studies should account for levels of baseline fatigue, baseline and longitudinal prescription of corticosteroids as well as consideration of comorbidity (including history of depression). Data from this study should provoke clinicians to consider the possibility that some interventions may be undervalued (or overvalued), based on the existing literature, which often does not account for these key variables20. Furthermore, clinicians should be alert to the fact that men are especially vulnerable to fatigue worsening, and explicit discussion of fatigue risk may provide care providers opportunities to manage it proactively.
This study has several important limitations. First, these data only generalize to outpatient care settings and patients with common solid tumors over the time interval of 28–35 days. Although there is a known, high correlation between single item screening fatigue screening and multiple-item instruments,28 the use of a single item measure of fatigue severity in this study limits the comparison with studies of cancer-related fatigue that include more detailed assessment and attribution of the symptom. Another limitation is that the prescribing data concerning corticosteroid use do not reveal details related to the underlying reason for prescribing, and the data about recent cancer treatment data were also very general. Finally, it is noteworthy that no bio-specimens were collected.
In conclusion, both the proportion of patients with worsening fatigue and the set of predictors for fatigue worsening vary depending on the category of baseline fatigue and exposure to co-prescribed medications. Among patients with solid tumors for whom there is a good gender mix (e.g., patients with lung or colorectal cancer), male gender was a strong predictor of fatigue worsening. These data draw attention to clinicians and clinical trialists to the need for comprehensive assessment of ambulatory cancer patients to include multiple symptoms and all comorbidities and concomitant medications in order to unveil modifiable factors and improve on the patient experience of fatigue. Ultimately, rigorous case definitions and correlative science will be needed to improve our understanding of mechanisms for fatigue in cancer patients and improve our ability to identify clinically useful, distinct phenotypes for fatigue in ambulatory cancer patients.
Acknowledgments
Role of the sponsor: Supported in part by grants from the National Cancer Institute of the National Institutes of Health, including U10 CA37403 and U10 CA17145 to the Eastern Cooperative Oncology Group, and R01 CA026582 to C.S.C. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Financial Disclosures/Conflicts of Interest: None.
Dr. Fisch had full access to all of the data in the study and made the final decision to submit them for publication.
Previous presentation: These data were presented for the first time in a poster presentation (abstract #9112) at the Annual Meeting of the American Society of Clinical Oncology in June 2012.
References
- 1.Donovan KA, McGinty HL, Jacobsen PB. A systematic review of research using the diagnostic criteria for cancer-related fatigue. Psycho-oncology. 2012 doi: 10.1002/pon.3085. [DOI] [PubMed] [Google Scholar]
- 2.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118:2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 3.Campos MP, Hassan BJ, Riechelmann R, Del Giglio A. Cancer-related fatigue: a practical review. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:1273–1279. doi: 10.1093/annonc/mdq458. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell SA. Cancer-related fatigue: state of the science. PM & R. 2010;2:364–383. doi: 10.1016/j.pmrj.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Barsevick AM, Cleeland CS, Manning DC, et al. ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. Journal of pain and symptom management. 2010;39:1086–1099. doi: 10.1016/j.jpainsymman.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell SA. Cancer-related fatigue: state of the science. PM & R. 2010;2:364–383. doi: 10.1016/j.pmrj.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Berger AM, Kuhn BR, Farr LA, et al. One-year outcomes of a behavioral therapy intervention trial on sleep quality and cancer-related fatigue. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:6033–6040. doi: 10.1200/JCO.2008.20.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruera E, El Osta B, Valero V, et al. Donepezil for cancer fatigue: a double-blind, randomized, placebo-controlled trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:3475–3481. doi: 10.1200/JCO.2007.10.9231. [DOI] [PubMed] [Google Scholar]
- 9.Bruera E, Valero V, Driver L, et al. Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebo-controlled trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:2073–2078. doi: 10.1200/JCO.2005.02.8506. [DOI] [PubMed] [Google Scholar]
- 10.Cruciani RA, Zhang J, Manola JB, Cella D, Ansari B, Fisch MJ. Phase III randomized, placebo-controlled trial of L-carnitine supplementation for fatigue in patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(suppl):e20532. doi: 10.1200/JCO.2011.40.2180. abstr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodd MJ, Cho MH, Miaskowski C, et al. A randomized controlled trial of home-based exercise for cancer-related fatigue in women during and after chemotherapy with or without radiation therapy. Cancer nursing. 2010;33:245–257. doi: 10.1097/NCC.0b013e3181ddc58c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer. 2010;116:3513–3520. doi: 10.1002/cncr.25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrow GR, Hickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:4635–4641. doi: 10.1200/JCO.2003.04.070. [DOI] [PubMed] [Google Scholar]
- 14.Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3673–3679. doi: 10.1200/JCO.2010.28.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barsevick AM, Irwin MR, Hinds P, et al. Recommendations for high priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013 doi: 10.1093/jnci/djt242. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger AM, Abernethy AP, Atkinson A, et al. Cancer-related fatigue. Journal of the National Comprehensive Cancer Network : JNCCN. 2010;8:904–931. doi: 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]
- 17.Andrykowski MA, Donovan KA, Jacobsen PB. Magnitude and correlates of response shift in fatigue ratings in women undergoing adjuvant therapy for breast cancer. Journal of pain and symptom management. 2009;37:341–351. doi: 10.1016/j.jpainsymman.2008.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psych. 2007;26:660–667. doi: 10.1037/0278-6133.26.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yennurajalingam S, Palmer JL, Chacko R, Bruera E. Factors associated with response to methylphenidate in advanced cancer patients. The oncologist. 2011;16:246–253. doi: 10.1634/theoncologist.2010-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bower JE. Treating cancer-related fatigue: the search for interventions that target those most in need. J Clin Oncol. 2012;30:4449–4450. doi: 10.1200/JCO.2012.46.0436. [DOI] [PubMed] [Google Scholar]
- 21.Fisch MJ, Lee JW, Weiss M, et al. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol. 2012;30:1980–1988. doi: 10.1200/JCO.2011.39.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Eastern Cooperative Oncology Group. SOAPP (Symptom Outcomes and Patient Practice Patterns): A Survey of Disease and Treatment Related Symptoms in Patients with Invasive Cancer of the Breast, Prostate, Lung, or Colon/Rectum. http://wwwsoappcom. [Google Scholar]
- 25.Schwartz AL, Meek PM, Nail LM, et al. Measurement of fatigue. determining minimally important clinical differences. J Clin Epidemiol. 2002;55:239–244. doi: 10.1016/s0895-4356(01)00469-3. [DOI] [PubMed] [Google Scholar]
- 26.Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116:5740–5748. doi: 10.1002/cncr.25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrykowski MA, Schmidt JE, Salsman JM, Beacham AO, Jacobsen PB. Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:6613–6622. doi: 10.1200/JCO.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butt Z, Wagner LI, Beaumont JL, et al. Use of a single-item screening tool to detect clinically significant fatigue, pain, distress, and anorexia in ambulatory cancer practice. Journal of pain and symptom management. 2008;35:20–30. doi: 10.1016/j.jpainsymman.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein D, Bennett BK, Webber K, et al. Cancer-related fatigue in women with breast cancer: outcomes of a 5-year prospective cohort study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1805–1812. doi: 10.1200/JCO.2011.34.6148. [DOI] [PubMed] [Google Scholar]
- 30.Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:8296–8304. doi: 10.1200/JCO.2005.10.167. [DOI] [PubMed] [Google Scholar]
- 31.Storey DJ, Waters RA, Hibberd CJ, et al. Clinically relevant fatigue in cancer outpatients: the Edinburgh Cancer Centre symptom study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007;18:1861–1869. doi: 10.1093/annonc/mdm349. [DOI] [PubMed] [Google Scholar]
- 32.Gerber LH, Stout N, McGarvey C, et al. Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19:1581–1591. doi: 10.1007/s00520-010-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prue G, Allen J, Gracey J, Rankin J, Cramp F. Fatigue in gynecological cancer patients during and after anticancer treatment. Journal of pain and symptom management. 2010;39:197–210. doi: 10.1016/j.jpainsymman.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. European journal of cancer. 2002;38:27–43. doi: 10.1016/s0959-8049(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 35.Jacobsen PB, Andrykowski MA, Thors CL. Relationship of catastrophizing to fatigue among women receiving treatment for breast cancer. J Consult Clin Psych. 2004;72:355–361. doi: 10.1037/0022-006X.72.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escalante CP, Manzullo EF, Lam TP, Ensor JE, Valdres RU, Wang XS. Fatigue and its risk factors in cancer patients who seek emergency care. Journal of pain and symptom management. 2008;36:358–366. doi: 10.1016/j.jpainsymman.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Spratt DE, Sakae M, Riaz N, et al. Time course and predictors for cancer-related fatigue in a series of oropharyngeal cancer patients treated with chemoradiation therapy. The oncologist. 2012;17:569–576. doi: 10.1634/theoncologist.2011-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Servaes P, Gielissen MF, Verhagen S, Bleijenberg G. The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Psycho-oncology. 2007;16:787–795. doi: 10.1002/pon.1120. [DOI] [PubMed] [Google Scholar]
- 39.Hickok JT, Roscoe JA, Morrow GR, Mustian K, Okunieff P, Bole CW. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005;104:1772–1778. doi: 10.1002/cncr.21364. [DOI] [PubMed] [Google Scholar]
- 40.Dhruva A, Dodd M, Paul SM, et al. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer nursing. 2010;33:201–212. doi: 10.1097/NCC.0b013e3181c75f2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armes J, Chalder T, Addington-Hall J, Richardson A, Hotopf M. A randomized controlled trial to evaluate the effectiveness of a brief, behaviorally oriented intervention for cancer-related fatigue. Cancer. 2007;110:1385–1395. doi: 10.1002/cncr.22923. [DOI] [PubMed] [Google Scholar]
- 42.Kim SH, Son BH, Hwang SY, et al. Fatigue and depression in disease-free breast cancer survivors: prevalence, correlates, and association with quality of life. Journal of pain and symptom management. 2008;35:644–655. doi: 10.1016/j.jpainsymman.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Barsevick A, Cella D. NCI Symptom Management and Quality of Life Steering Committee Clinical Trials Planning Conference: Cancer-Related Fatigue. Bethesda, MD: National Institutes of Health; 2010. [Google Scholar]
- 44.Joly F, Vardy J, Pintilie M, Tannock IF. Quality of life and/or symptom control in randomized clinical trials for patients with advanced cancer. Ann Oncol. 2007;18:1935–1942. doi: 10.1093/annonc/mdm121. [DOI] [PubMed] [Google Scholar]
- 45.Lower EE, Fleishman S, Cooper A, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. Journal of pain and symptom management. 2009;38:650–662. doi: 10.1016/j.jpainsymman.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Lower EE, Malhotra A, Surdulescu V, Baughman RP. Armodafinil for sarcoidosis-associated fatigue: a double-blind, placebo-controlled, crossover trial. J Pain Symptom Manage. 2013;45:159–169. doi: 10.1016/j.jpainsymman.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breitbart W, Rosenfeld B, Kaim M, Funesti-Esch J. A randomized, double-blind, placebo-controlled trial of psychostimulants for the treatment of fatigue in ambulatory patients with human immunodeficiency virus disease. Arch Intern Med. 2001;161:411–420. doi: 10.1001/archinte.161.3.411. [DOI] [PubMed] [Google Scholar]
- 48.Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of Cancer-Related Fatigue With Dexamethasone: A Double-Blind, Randomized, Placebo-Controlled Trial in Patients With Advanced Cancer. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.44.4661. [DOI] [PubMed] [Google Scholar]