Abstract
During the last decade, along with its explosive growth globally, biomedical photoacoustics has become a rapidly growing research field in China as well. In particular, photoacoustic tomography (PAT), capable of imaging intact biological tissue in vivo at great depths, has generated intense interest among Chinese researchers. This review briefly summarizes the current status and recent progress of the research in PAT in China. The focus is on the technology development and biomedical applications of three representative embodiments of PAT: photoacoustic microscopy, photoacoustic computed tomography, and photoacoustic endoscopy. In addition, recent development and studies in other related areas are also reviewed shortly.
Keywords: Biomedical photoacoustics, Photoacoustic microscopy, Photoacoustic computed tomography, Photoacoustic endoscopy, Molecular imaging
1. Introduction
When scientists worldwide started to explore the feasibility of photoacoustic (optoacoustic) detection of diseased tissue in the 1980s [1], researchers in China also made attempts to exploit the photoacoustic effect for biomedical applications [2]. Afterwards, the development of biomedical photoacoustics in China had been slow for a long period. However, during the past decade, this process has accelerated significantly, when photoacoustic tomography (PAT) emerged and started to find important applications in biomedical imaging [3–5]. The unique multi-contrast, multi-scale in vivo imaging capability of PAT [6], together with its initial success in a variety of biomedical applications, has generated intense interest among Chinese researchers from different research communities, including optics, ultrasonics, biology, and medicine. So far, demonstrated representative applications of PAT include label-free imaging of vascular morphology and functions [7,8], study of tumor angiogenesis [9,10], molecular imaging of cancer [11,12], intravascular imaging of atherosclerosis [13,14], and endoscopic imaging of internal organs [15]. In China, since the development of a linear-array-based photoacoustic computed tomography (PACT) system at the South China Normal University in 2004 [16], the pursuit of research in photoacoustic imaging has rapidly spread to more than 20 universities and/or research institutes over the last decade. With a significantly increased funding support from both the national and regional governments, the number of publications on biomedical photoacoustics from China has increased dramatically. For example, a search in the Web of Knowledge (Thomson Reuters) with either “photoacoustic” or “optoacoustic” in “Title” and “China” as the “Countries/Territories” has returned 109 articles published in 2012, about twice the number of articles published in 2009 (the search was conducted on May 20, 2013). Meanwhile, this number has amounted for ∼14% of the total number of publications in the field in 2012 (searched with either “photoacoustic” or “optoacoustic” in “Title”).
In the following sections, we will review the current status and recent research progress of biomedical photoacoustics in China. The focus will be on the technology development and biomedical applications of three representative embodiments of PAT, namely, photoacoustic microscopy (PAM), photoacoustic computed tomography (PACT), and photoacoustic endoscopy (PAE). In addition, research progress in other related areas in China will also be reviewed shortly.
2. Photoacoustic microscopy
Based on how the lateral resolution is determined – either by optical focusing or acoustic focusing, photoacoustic microscopy (PAM) can be classified into optical-resolution photoacoustic microscopy (OR-PAM) and acoustic-resolution photoacoustic microscopy (AR-PAM). Our review on PAM below is organized according to the above mentioned two forms: OR-PAM and AR-PAM.
OR-PAM provides excellent optical-absorption contrast with optical-diffraction limited resolution that can be as fine as sub-micrometers. Owing to its unique capability of label-free imaging of microvascular morphology and functions at high resolution in vivo, OR-PAM has become a vital imaging tool for microcirculation studies, including the study of tumor angiogenesis [9,10], diabetic retinopathy [17], and other vascular complications [18]. The schematic of a reflection-mode OR-PAM system developed at the Shenzhen Institutes of Advanced Technology (SIAT), the Chinese Academy of Sciences, is shown in Fig. 1(A) [19]. In this system, the output laser beam from a 532-nm pulsed Nd:YAG laser (SPOT-532, Elforlight) was first coupled into a single mode fiber, and then collimated using a microscope objective (PLN4X, Olympus, NA: 0.1). Upon reflecting by a mirror, the collimated laser beam was focused by another identical objective to provide micrometer-scale optical illumination on the tissue surface. A custom-developed optical-ultrasonic beam combiner was used to enable reflection-mode photoacoustic imaging [19]. Images of mouse ear, back, and cerebral vasculature can be acquired in vivo using this system, without any exogenous contrast agents (Fig. 1(B)). The system's lateral resolution was ∼5.7 μm, which can be further improved to ∼3.0 μm using a blind-deconvolution algorithm, without the need of physically increasing the numerical aperture (NA) of the objective [19].
Fig. 1.
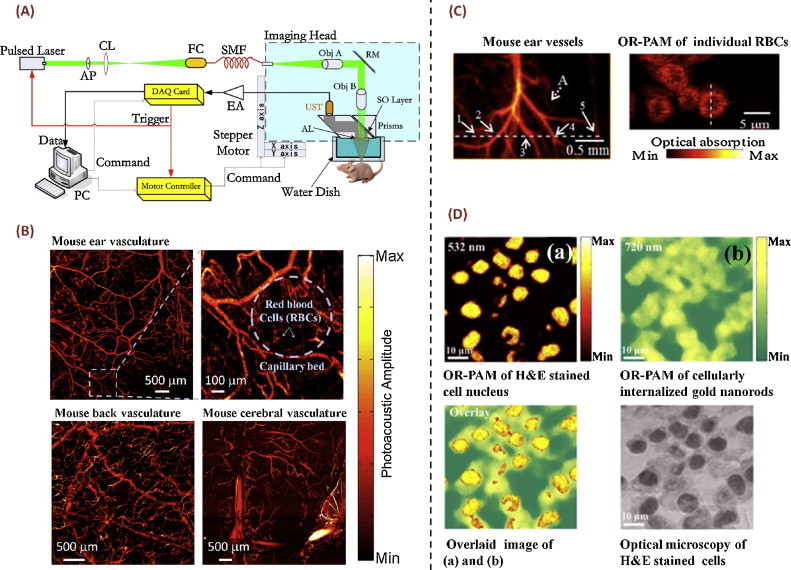
OR-PAM and its representative applications. (A) Schematic of a typical reflection-mode OR-PAM system [19]; (B) in vivo images of the ear, back, and cerebral vasculature of mice acquired with the system shown in (A); (C) exemplary images from a transmission-mode OR-PAM system [21]; (D) OR-PAM of H&E stained cell nucleus and internalized gold nanorods in the cytoplasm [22].
Reprinted with permission from Refs. [19,21,22].
Using a custom-made hollow focused ultrasonic transducer, another reflection-mode OR-PAM system was developed at the South China Normal University (SCNU). In this system, the laser beam was focused by an objective lens through a small hole in the center of the ultrasonic transducer [20]. In another study, by adding galvanometer-based 2D optical scanning to a conventional optical microscope, a transmission-mode OR-PAM system was also developed at SCNU, which was capable of imaging individual red blood cells ex vivo with a lateral resolution of ∼500 nm (Fig. 1(C)) [21]. Moreover, using this system, intracellular structures, such as H&E stained cell nucleus and internalized gold nanorods in the cytoplasm, were photoacoustically imaged (Fig. 1(D)) [22]. Other implementations and applications of transmission-mode OR-PAM were widely explored as well, including the development of laser-diode-based OR-PAM [23] and label-free photoacoustic imaging of zebrafish larvae in vivo [24].
Compared with OR-PAM, AR-PAM provides greater imaging depth up to several centimeters, with a scalable ultrasonic resolution ranging from tens to hundreds of micrometers. Reflection-mode dark-field AR-PAM systems were developed at multiple universities and/or research institutes in China, including the Huazhong University of Science and Technology (HUST) [25], Peking University (PKU) [26], and SIAT of the Chinese Academy of Sciences [27]. The schematic of a representative system is shown in Fig. 2(A). In one study, the cerebral hemodynamics and oxygen metabolism associated with ischemic stroke in a rat model were imaged in vivo, using AR-PAM in combination with laser speckle techniques [28] (Fig. 2(B)). In another study, AR-PAM was applied to performing molecular imaging of cancer, with intravenously administrated nanosized, reduced graphene oxide (nano-rGO) as contrast agents (Fig. 2(C)) [27]. Meanwhile, because of significantly enhanced photothermal effect by nano-rGO in the tumor region, cancer cells were effectively ablated in vivo using a continuous-wave near-infrared laser. In addition to the above mentioned studies, using a long focal ultrasonic transducer, a circular scanning AR-PAM system was developed at Fujian Normal University, which was utilized to image acute myocardial ischemia and thyroid disease [29,30].
Fig. 2.
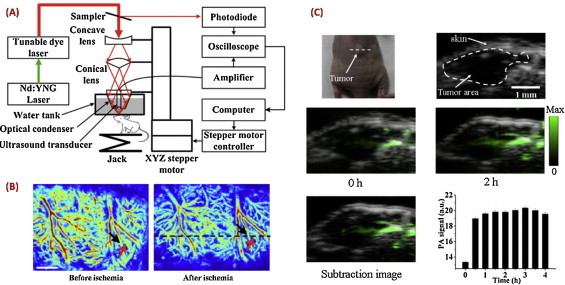
AR-PAM and its representative applications. (A) A typical AR-PAM system [28]; (B) AR-PAM of ischemic stroke in vivo in a rat model [28]; (C) molecular AR-PAM of tumor in vivo with intravenously administrated nano-rGO [27].
Reprinted with permission from Refs. [27,28].
3. Photoacoustic computed tomography
Unlike PAM, photoacoustic computed tomography (PACT) requires an inverse reconstruction algorithm to form the final image. In general, PACT can be implemented using either single-element scanning or ultrasonic array detection. Compared with single-element scanning PACT, PACT with ultrasonic array detection provides faster data acquisition, and may be better suited for clinical studies.
Using a linear ultrasonic array, a circular-scanning PACT system was developed at SCNU (Fig. 3(A)) [31]. While circular ultrasonic arrays almost always require customization, linear ultrasonic arrays are widely used in the clinics with much better accessibility. On the other hand, compared with the use of a single-element ultrasonic transducer, the use of the linear array significantly reduced the required scanning steps to form a full-view cross sectional image. In another work, using an 8×8 planar ultrasonic array (center frequency: 7.5 MHz; element size: 0.98 mm; element spacing: 1.25 mm), a high-speed PACT system was developed, which was able to acquire 3D photoacoustic images at a speed as fast as 10 Hz (limited by the laser repetition rate) [32].
Fig. 3.
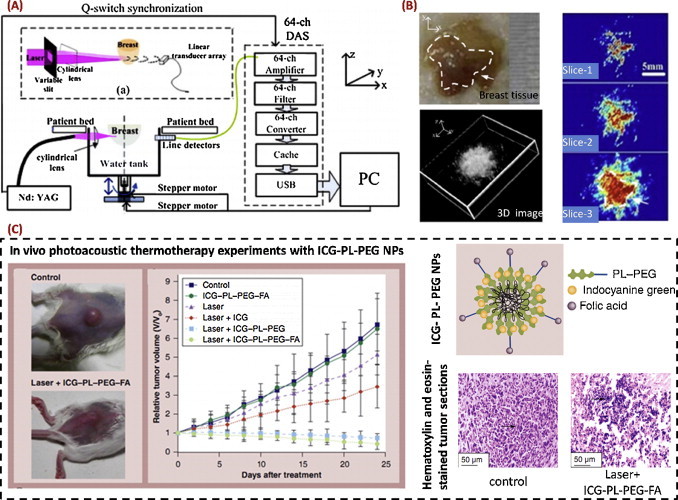
PACT and its representative applications. (A) Schematic of a circular-scanning PACT system using a linear ultrasonic array [31]; (B) PACT images of excised human breast cancer tissue [31]; (C) in vivo treatment of tumor-bearing mouse with laser-induced large photoacoustic effect of ICG–PL-PEG–FA [44].
Reprinted with permission from references [31,44].
Besides the development of different PACT systems, several novel reconstruction algorithms were also developed to improve the data acquisition speed and image quality of PACT [33–37]. For example, compressed sensing photoacoustic reconstruction algorithms with partially known support were developed for both acoustic-resolution PACT and optical-resolution PACT, enabling new possibilities to develop high-speed, low-cost PACT using a sparse ultrasonic array [33,34]. In another work, to compensate the acoustic scattering in heterogeneous biological tissue, a time-reversal technique was developed to improve the image quality of PACT [38].
With the above mentioned PACT systems, many biomedical studies were conducted, including: (1) feasibility studies of breast cancer detection and osteosarcoma diagnosis, with excised human tissue and a rat model, respectively (Fig. 3(B)) [31,39]; (2) molecular PACT of integrin-αvβ3 positive U87 glioblastoma in a mouse model, using intravenously injected antibody-functionalized single-walled carbon nanotubes (SWNTs) [40]; (3) multispectral quantitative PACT of osteoarthritis in finger joints [41]; (4) photoacoustic velocimetry for flow-field measurement in phantoms [42]; and (5) tissue microstructure analysis using PACT spectra [43].
4. Photoacoustic endoscopy
Photoacoustic endoscopy (PAE) is emerging as a novel technology for imaging internal organs and/or tissue, such as esophagus, colon, and/or coronary atherosclerotic plaques. PAE provides valuable functional and spectroscopic information of biological tissue at depth, which effectively complements existing endoscopic imaging technologies that primarily detect structural abnormalities. To improve the imaging speed of PAE, ultrasonic-arrays have been exploited for proof-of-concept studies. In one study, a ring ultrasonic array in combination with a taper reflector was used to construct an optical-acoustic co-axial photoacoustic endoscopic catheter (outer diameter: 10 mm) [45]. In another work, the feasibility of developing PAE using a linear ultrasonic array was also studied [46]. With the use of ultrasonic array detection, these PAE systems can potentially provide very high imaging speed (currently limited by the laser repetition rate). However, the fabrication of miniature high-frequency ultrasonic arrays can be challenging and expensive.
An alternative approach, of course, is to construct a PAE probe using a single-element high-frequency ultrasonic transducer. With this approach, both rotational and pull-back mechanical scanning are required to acquire a 3D image. To characterize atherosclerosis at high spatial resolution, a single-element PAE system with optical-diffraction limited lateral resolution was developed at SIAT, offering a spatial resolution as high as ∼19 μm [47]. The outer diameter of the developed catheter was ∼1.1 mm, the smallest among reported PAE probes up to the preparation of this review. Feasibility studies on intravascular imaging of atherosclerotic plaques and image-guided stent deployment were conducted with this system. Presently, one challenge for single-element PAE to image in vivo is the relatively slow imaging speed, limited by both the laser repetition rate and the need of mechanical scanning for a cross-section image. The development and employment of high-repetition-rate tunable lasers, however, may present as a potential solution to this issue.
5. Multi-modality imaging and others
Multi-modality imaging can provide complementary information of biological tissue, which may improve the diagnostic sensitivity and specificity over a single imaging modality alone. There are several notable multimodality imaging technologies developed in China that incorporate PAT as a major modality. One such system has combined X-ray imaging with PAT to perform anatomic and molecular imaging of tumor simultaneously, which can potentially improve the accuracy of both the diagnosis and image-guided therapy of cancer [48]. Another system has integrated PAM with laser speckle imaging [28]. Using the flow speed obtained from laser speckle imaging and the oxygen saturation from PAM, the cerebral hemodynamics and oxygen metabolism associated with ischemic stroke were quantitatively imaged in a rat model in vivo. To acquire complementary information for cellular imaging applications, a multimodality system combing PAM and laser scanning confocal microscopy was also developed, offering a lateral resolution as fine as 1.25 μm [49].
In addition, many other studies on biomedical photoacoustics have been carried out in China, including: (1) laser-induced large photoacoustic effect (firecracker-like explosion) with SWNTs and/or folic acid labeled ICG nanoparticles for anti-tumor studies (Fig. 3(C)) [44,50]; (2) ultrashort microwave-induced high-contrast thermoacoustic imaging [51]; (3) photoacoustic imaging of the viscoelasticity of biological tissue [52]; and (4) synthetic-aperture focusing based PAM [53].
6. Summary
During the past decade, research in biomedical photoacoustics in China has experienced unprecedented growth. The development of novel systems, reconstruction algorithms, as well as the exploration of various biological and clinical applications have all been actively pursued by Chinese researchers. With a steadily increasing funding support from the governments and more researchers joining this field, we believe that the research in biomedical photoacoustics in China will continue to enjoy rapid growth and expansion in the coming years, with many new exciting research results to be expected.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China grants: 61205203 and 61308116; the Shenzhen Science and Technology Innovation Committee grants: ZDSY20130401165820357, KQCX20120816155844962, CXZZ20120617113635699, and JCYJ20120615125857842; the Guangdong Innovation Research Team Fund for Low-cost Healthcare Technologies (GIRTF-LCHT); the Low-cost Health Engineering Research Program of the Chinese Academy of Sciences [2011].
Biographies

Liang Song is Associate Professor and Founding Director of the Research Lab for Biomedical Optics and Molecular Imaging at the Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences. Prior to joining SIAT, he studied at Washington University in St. Louis with Professor Lihong Wang and received his Ph.D. in Biomedical Engineering in 2010.

Jing Meng is a Research Fellow in the Research Lab for Biomedical Optics and Molecular Imaging at the Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences. Prior to SIAT, she received her Ph.D. in Computer Science from Soochow University and studied at The Hong Kong Polytechnic University as a visiting scholar.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Baldassarre L., Cingolani A., de Tommasi A., Vailati G. Photoacoustic determination of optical absorption properties in normal and pathologic cerebral structures. Applied Physics Communications. 1982;2:183–188. [Google Scholar]
- 2.Sun H.W., Huang M.C., Wang Q.H., Wan C.W. Photoacoustic and Photothermal Phenomena II, Proceedings of the 6th International Topical Meeting. 1990. The application of photoacoustic detection in the primary diagnosis of cancer; pp. 431–434. [Google Scholar]
- 3.Kruger R.A., Liu P.Y., Fang Y.R., Appledorn C.R. Photoacoustic ultrasound (paus) – reconstruction tomography. Medical Physics. 1995;22:1605–1609. doi: 10.1118/1.597429. [DOI] [PubMed] [Google Scholar]
- 4.Hoelen C.G.A., de Mul F.F.M., Pongers R., Dekker A. Three-dimensional photoacoustic imaging of blood vessels in tissue. Optics Letters. 1998;23:648–650. doi: 10.1364/ol.23.000648. [DOI] [PubMed] [Google Scholar]
- 5.Wang X.D., Pang Y.J., Ku G., Xie X.Y., Stoica G., Wang L.H.V. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nature Biotechnology. 2003;21:803–806. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- 6.Wang L.H.V., Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335:1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao J.J., Maslov K.I., Zhang Y., Xia Y.N., Wang L.V. Label-free oxygen-metabolic photoacoustic microscopy in vivo. Journal of Biomedical Optics. 2011;16:076003. doi: 10.1117/1.3594786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H.F., Maslov K., Stoica G., Wang L.H.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nature Biotechnology. 2006;24:848–851. doi: 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]
- 9.Oladipupo S.S., Hu S., Santeford A.C., Yao J., Kovalski J.R., Shohet R.V. Conditional HIF-1 induction produces multistage neovascularization with stage-specific sensitivity to VEGFR inhibitors and myeloid cell independence. Blood. 2011;117:4142–4153. doi: 10.1182/blood-2010-09-307538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oladipupo S., Hu S., Kovalski J., Yao J.J., Santeford A., Sohn R.E. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13264–13269. doi: 10.1073/pnas.1101321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kircher M.F., de la Zerda A., Jokerst J.V., Zavaleta C.L., Kempen P.J., Mittra E. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nature Medicine. 2012;18:829–835. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de La Zerda A., Zavaleta C., Keren S., Vaithilingam S., Bodapati S., Liu Z. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nature Nanotechnology. 2008;3:557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang B., Su J.L., Karpiouk A.B., Sokolov K.V., Smalling R.W., Emelianov S.Y. Intravascular photoacoustic imaging. IEEE Journal of Selected Topics in Quantum Electronics. 2010;16:588–599. doi: 10.1109/JSTQE.2009.2037023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansen K., van der Steen A.F., van Beusekom H.M., Oosterhuis J.W., van Soest G. Intravascular photoacoustic imaging of human coronary atherosclerosis. Optics Letters. 2011;36:597–599. doi: 10.1364/OL.36.000597. [DOI] [PubMed] [Google Scholar]
- 15.Yang J.M., Favazza C., Chen R.M., Yao J.J., Cai X., Maslov K. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nature Medicine. 2012;18:1297–1302. doi: 10.1038/nm.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin B.Z., Xing D., Wang Y., Zeng Y.G., Zeng Y.G., Tan Y. Fast photoacoustic imaging system based on 320-element linear transducer array. Physics in Medicine and Biology. 2004;49:1339–1346. doi: 10.1088/0031-9155/49/7/019. [DOI] [PubMed] [Google Scholar]
- 17.Jiao S.L., Jiang M.S., Hu J.M., Fawzi A., Zhou Q.F., Shung K.K. Photoacoustic ophthalmoscopy for in vivo retinal imaging. Optics Express. 2010;18:3967–3972. doi: 10.1364/OE.18.003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu S., Wang L.V. Photoacoustic imaging and characterization of the microvasculature. Journal of Biomedical Optics. 2010;15:011101. doi: 10.1117/1.3281673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J., Lin R., Wang H., Meng J., Zheng H., Song L. Blind-deconvolution optical-resolution photoacoustic microscopy in vivo. Optics Express. 2013;21:7316–7327. doi: 10.1364/OE.21.007316. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z., Yang S., Xing D. In vivo detection of hemoglobin oxygen saturation and carboxyhemoglobin saturation with multiwavelength photoacoustic microscopy. Optics Letters. 2012;37:3414–3416. doi: 10.1364/OL.37.003414. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Y., Yang S.H., Xing D. Optical-resolution photoacoustic microscopy based on two-dimensional scanning galvanometer. Applied Physics Letters. 2012;100:023702. [Google Scholar]
- 22.Yang S.H., Ye F., Xing D. Intracellular label-free gold nanorods imaging with photoacoustic microscopy. Optics Express. 2012;20:10370–10375. doi: 10.1364/OE.20.010370. [DOI] [PubMed] [Google Scholar]
- 23.Zeng L., Liu G., Yang D., Ji X. 3D-visual laser-diode-based photoacoustic imaging. Optics Express. 2012;20:1237–1246. doi: 10.1364/OE.20.001237. [DOI] [PubMed] [Google Scholar]
- 24.Ye S.Q., Yang R., Xiong J.W., Shung K.K., Zhou Q.F., Li C.H. Label-free imaging of zebrafish larvae in vivo by photoacoustic microscopy. Biomedical Optics Express. 2012;3:360–365. doi: 10.1364/BOE.3.000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H., Yang X.Q., Wang Z., Deng Z.L., Gong H., Luo Q.M. Early monitoring of cerebral hypoperfusion in rats by laser speckle imaging and functional photoacoustic microscopy. Journal of Biomedical Optics. 2012;17:061207. doi: 10.1117/1.JBO.17.6.061207. [DOI] [PubMed] [Google Scholar]
- 26.Ye J.Y.S., Xi J., Ren Q., Li C. Studying murine hindlime ischemia by photoacoustic microscopy. Chinese Optics Letters. 2012;10:121701. [Google Scholar]
- 27.Sheng Z., Song L., Zheng J., Hu D., He M., Zheng M. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials. 2013;34:5236–5243. doi: 10.1016/j.biomaterials.2013.03.090. [DOI] [PubMed] [Google Scholar]
- 28.Deng Z., Wang Z., Yang X., Luo Q., Gong H. In vivo imaging of hemodynamics and oxygen metabolism in acute focal cerebral ischemic rats with laser speckle imaging and functional photoacoustic microscopy. Journal of Biomedical Optics. 2012;17:081411–081415. doi: 10.1117/1.JBO.17.8.081415. [DOI] [PubMed] [Google Scholar]
- 29.Li Z., Li H., Chen H., Xie W. In vivo determination of acute myocardial ischemia based on photoacoustic imaging with a focused transducer. Journal of Biomedical Optics. 2011;16:076011. doi: 10.1117/1.3598314. [DOI] [PubMed] [Google Scholar]
- 30.Zeng Z.P., Xie W.M., Zhang J.Y., Li L., Chen S.Q., Li Z.F. Imaging of human thyroid in vitro using focused photoacoustic tomography. Acta Physica Sinica. 2012;61:097801. [Google Scholar]
- 31.Ye F., Yang S.H., Xing D. Three-dimensional photoacoustic imaging system in line confocal mode for breast cancer detection. Applied Physics Letters. 2010;97:054701. [Google Scholar]
- 32.Zhou Q.Z., Ji X.R., Xing D. Full-field 3D photoacoustic imaging based on plane transducer array and spatial phase-controlled algorithm. Medical Physics. 2011;38:1561–1566. doi: 10.1118/1.3555036. [DOI] [PubMed] [Google Scholar]
- 33.Meng J., Wang L.H.V., Liang D. In vivo optical-resolution photoacoustic computed tomography with compressed sensing. Optics Letters. 2012;37:4573–4575. doi: 10.1364/ol.37.004573. [DOI] [PubMed] [Google Scholar]
- 34.Meng J., Wang L.H.V., Ying L.L., Liang D., Song L. Compressed-sensing photoacoustic computed tomography in vivo with partially known support. Optics Express. 2012;20:16510–16523. [Google Scholar]
- 35.Sun M.J., Feng N.Z., Shen Y., Shen X.L., Ma L.Y., Li J.G. Photoacoustic imaging method based on arc-direction compressed sensing and multi-angle observation. Optics Express. 2011;19:14801–14806. doi: 10.1364/OE.19.014801. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y., Wang Y.Y., Zhang C. Total variation based gradient descent algorithm for sparse-view photoacoustic image reconstruction. Ultrasonics. 2012;52:1046–1055. doi: 10.1016/j.ultras.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Liu X., Peng D., Guo W., Ma X., Yang X., Tian J. Compressed sensing photoacoustic imaging based on fast alternating direction algorithm. International Journal of Biomedical Imaging. 2012;2012:206214. doi: 10.1155/2012/206214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu D., Tao C., Liu X.J. Photoacoustic tomography in scattering biological tissue by using virtual time reversal mirror. Journal of Applied Physics. 2011;109:084702. [Google Scholar]
- 39.Hu J., Yu M.L., Ye F., Xing D. In vivo photoacoustic imaging of osteosarcoma in a rat model. Journal of Biomedical Optics. 2011;16:020503. doi: 10.1117/1.3544502. [DOI] [PubMed] [Google Scholar]
- 40.Xiang L.Z., Yuan Y., Xing D., Ou Z.M., Yang S.H., Zhou F.F. Photoacoustic molecular imaging with antibody-functionalized single-walled carbon nanotubes for early diagnosis of tumor. Journal of Biomedical Optics. 2009;14:021008. doi: 10.1117/1.3078809. [DOI] [PubMed] [Google Scholar]
- 41.Xiao J.Y., He J.S. Multispectral quantitative photoacoustic imaging of osteoarthritis in finger joints. Applied Optics. 2010;49:5721–5727. doi: 10.1364/AO.49.005721. [DOI] [PubMed] [Google Scholar]
- 42.Ma S.B., Yang S.H., Xing D. Photoacoustic imaging velocimetry for flow-field measurement. Optics Express. 2010;18:9991–10000. doi: 10.1364/OE.18.009991. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y.Q., Wang S.H., Tao C., Wang X.D., Liu X.J. Photoacoustic tomography of tissue subwavelength microstructure with a narrowband and low frequency system. Applied Physics Letters. 2012;101:034105. [Google Scholar]
- 44.Zhong J., Yang S., Zheng X., Zhou T., Xing D. In vivo photoacoustic therapy with cancer-targeted indocyanine green-containing nanoparticles. Nanomedicine. 2012;8:903–919. doi: 10.2217/nnm.12.123. [DOI] [PubMed] [Google Scholar]
- 45.Yuan Y., Yang S., Xing D. Preclinical photoacoustic imaging endoscope based on acousto-optic coaxial system using ring transducer array. Optics Letters. 2010;35:2266–2268. doi: 10.1364/OL.35.002266. [DOI] [PubMed] [Google Scholar]
- 46.Yuan Y., Yang S.H., Xing D. Three-dimensional endoscopic photoacoustic imaging based on multielement linear transducer array. Journal of Applied Physics. 2011;110:054701. [Google Scholar]
- 47.Bai X.S., Zheng J.X., Lin R.Q., Gong X.J., Song L. Intravascular optical-resolution photoacoustic microscopy. Optics in Cardiology, (Rotterdam, Netherlands, 2013) 2013 [Google Scholar]
- 48.Huang G.J., Yang S.H., Yuan Y., Xing D. Combining X-ray and photoacoustics for in vivo tumor imaging with gold nanorods. Applied Physics Letters. 2011;99:123701. [Google Scholar]
- 49.Tan Z.L., Tang Z.L., Wu Y.B., Liao Y.F., Dong W., Guo L.N. Multimodal subcellular imaging with microcavity photoacoustic transducer. Optics Express. 2011;19:2426–2431. doi: 10.1364/OE.19.002426. [DOI] [PubMed] [Google Scholar]
- 50.Zhou F.F., Wu S.N., Yuan Y., Chen W.R., Xing D. Mitochondria-targeting photoacoustic therapy using single-walled carbon nanotubes. Small. 2012;8:1543–1550. doi: 10.1002/smll.201101892. [DOI] [PubMed] [Google Scholar]
- 51.Lou C.G., Yang S.H., Ji Z., Chen Q., Xing D. Ultrashort microwave-induced thermoacoustic imaging: a breakthrough in excitation efficiency and spatial resolution. Physical Review Letters. 2012;109:218101. doi: 10.1103/PhysRevLett.109.218101. [DOI] [PubMed] [Google Scholar]
- 52.Gao G.D., Yang S.H., Xing D. Viscoelasticity imaging of biological tissues with phase-resolved photoacoustic measurement. Optics Letters. 2011;36:3341–3343. doi: 10.1364/OL.36.003341. [DOI] [PubMed] [Google Scholar]
- 53.Deng Z.L., Yang X.Q., Gong H., Luo Q.M. Adaptive synthetic-aperture focusing technique for microvasculature imaging using photoacoustic microscopy. Optics Express. 2012;20:7555–7563. doi: 10.1364/OE.20.007555. [DOI] [PubMed] [Google Scholar]


