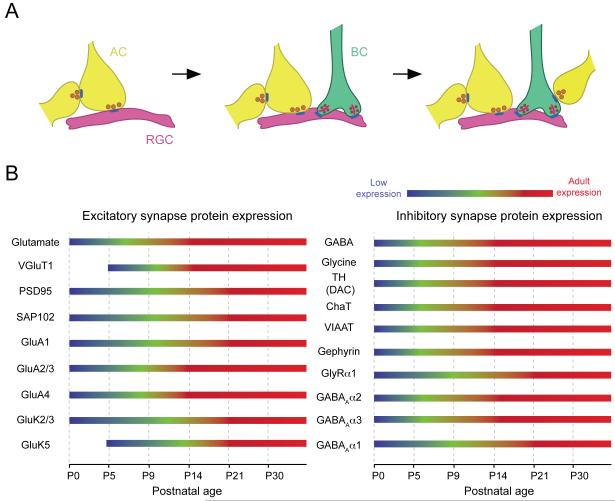
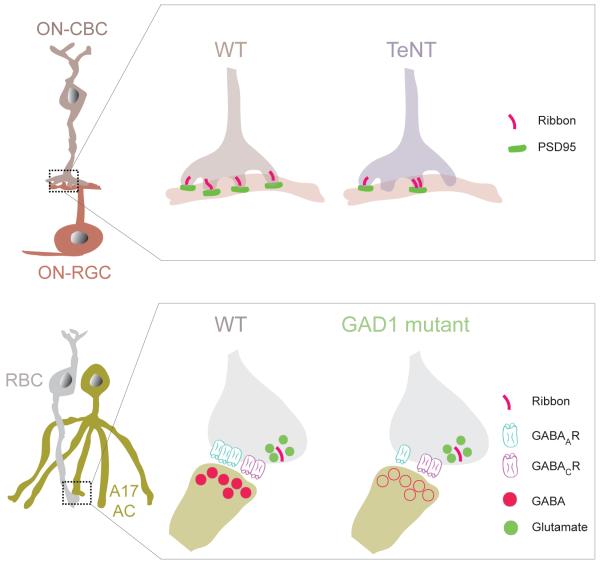
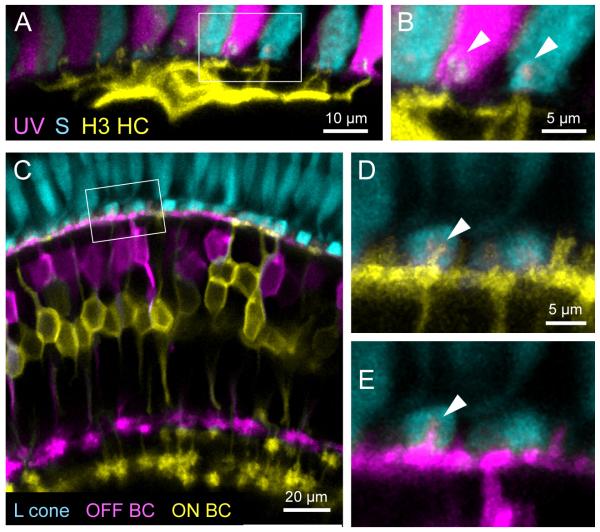
Abstract
Structure and function are highly correlated in the vertebrate retina, a sensory tissue that is organized into cell layers with microcircuits working in parallel and together to encode visual information. All vertebrate retinas share a fundamental plan, comprising five major neuronal cell classes with cell body distributions and connectivity arranged in stereotypic patterns. Conserved features in retinal design have enabled detailed analysis and comparisons of structure, connectivity and function across species. Each species, however, can adopt structural and/or functional retinal specializations, implementing variations to the basic design in order to satisfy unique requirements in visual function. Recent advances in molecular tools, imaging and electrophysiological approaches have greatly facilitated identification of the cellular and molecular mechanisms that establish the fundamental organization of the retina and the specializations of its microcircuits during development. Here, we review advances in our understanding of how these mechanisms act to shape structure and function at the single cell level, to coordinate the assembly of cell populations, and to define their specific circuitry. We also highlight how structure is rearranged and function is disrupted in disease, and discuss current approaches to re-establish the intricate functional architecture of the retina.
Keywords: Retinal development, Mouse retina, Zebrafish retina, Primate retina, Retinal cell mosaics, Retinal synapses, Retinal repair
1. Introduction
The vertebrate retina is a layered structure with a large diversity of component cells that form morphologically and functionally distinct circuits that work in parallel, and in combination, to produce a complex visual output. Developmental mechanisms that establish the structure and function of retinal neurons are increasingly understood, largely due to advances in molecular biology, electrophysiological methods and imaging techniques. Here, we discuss our current knowledge of the cellular and molecular mechanisms that (i) shape the morphology of retinal neurons, (ii) organize their spatial distributions across the retina, and (iii) regulate the assembly of their circuitry. We will also compare the development of different cell types, and of similar circuits across species to highlight common and disparate strategies employed to attain optimal structure and function. In particular, we will discuss findings primarily in three well-studied species, each with its own advantages: (i) Mouse, for which there is an increasing availability of transgenic animals and is the current focus of ‘connectomics’, (ii) Monkey (primarily macaque retina), with structure and function closest to humans, and (iii) Zebrafish, that like mice are highly amenable to genetic manipulation, but have the added advantage of possessing a capacity for tissue-regeneration. We will end the review with a brief discussion of how retinal structure and function are disrupted in common retinal diseases, and postulate how studies of retinal development could contribute to future therapeutic interventions.
2. Organization of the vertebrate retina
The fundamental plan of the retina is conserved across vertebrates; five major neuronal cell classes (Fig. 1) with Müller glial cells providing metabolic and homeostatic support. Coding of visual information begins with conversion of light energy to membrane potential changes in photoreceptors that alters neurotransmitter release. Photoreceptors can be broadly categorized into rods and cones (Fig. 1). Rods have exquisite sensitivity to light and can detect even a single photon (Rieke, 2000; Sampath and Rieke, 2004). Rods are thus responsible for dim-light vision. Cones are 100 times less sensitive than rods, but exhibit much faster response kinetics during phototransduction. Furthermore, each cone photoreceptor type is most sensitive to a specific wavelength of light. Thus, cones are engaged in bright-light, high acuity color vision.
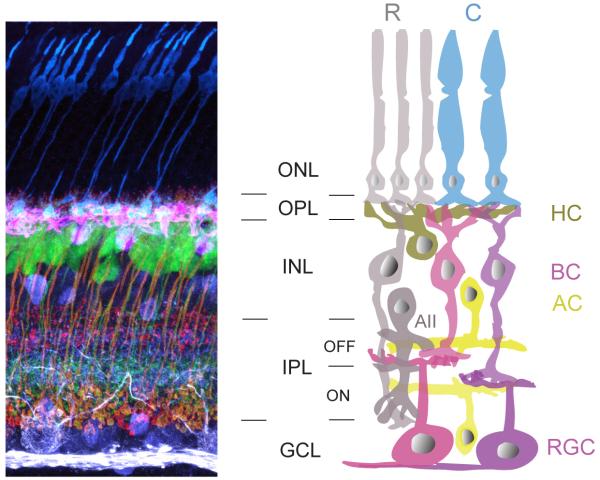
Figure 1. Schematic organization of neurons in the mammalian retina.
(Left) Vertical section of mouse retina showing labeling of the major neuronal cell types. Immunostaining for cone photoreceptors (anti-cone arrestin, blue), horizontal cells (anti-calbindin, pink), bipolar cell terminals (anti-synatotagmin2 and anti-PKC, red), amacrine cells (anti-calretinin, purple), and ganglion cells (SMI-32, white). Immunolabeling was performed on a retina from a transgenic line in which a subtype of bipolar cell (ON-type) express yellow fluorescent protein (green). ONL: outer nuclear layer, OPL: outer plexiform layer, INL: inner nuclear layer, IPL: inner plexiform layer and GCL: ganglion cell layer.
(Right) Schematic of the retina. R: rod photoreceptor, C: cone photoreceptor, HC: horizontal cell, BC: bipolar cell, AC: amacrine cell, RGC: retinal ganglion cell. The rod pathway (cells shaded in grey) conveys scotopic information to the photopic cone pathway, via the AII amacrine cell. Colored cells represent cone pathways. Neurons that are depolarized by light increments restrict their synaptic connectivity to the ON sublamina of the IPL, whereas connections of cells that are hyperpolarized instead form the OFF sublamima.
Rod and cone photoreceptors use glutamate as a neurotransmitter and synapse onto second order glutamatergic bipolar cells at the outer plexiform layer (OPL). Synaptic transmission between photoreceptors and bipolar cells is modulated by horizontal cells (Fig. 1). Bipolar cells can be divided into two major classes, rod and cone bipolar cells. Rod bipolar cells contact primarily rod photoreceptors whereas cone bipolar cells mostly synapse with cone photoreceptors (Fig. 1). In addition, bipolar cells form two functional subclasses: Those that depolarize (ON) and those that hyperpolarize (OFF) to increments in light intensity. Rod bipolar cells are ON-bipolar cells whereas cone bipolar cells can either be ON- or OFF-type. Thus, at the very first synapse of the retina coding of visual information diverges into distinct parallel pathways: rod and cone, ON and OFF.
Cone bipolar cells contact retinal ganglion cells and amacrine cells within the inner plexiform layer (IPL). Ganglion cells are the sole output neurons of the retina, projecting their axons to higher visual centers. Excitation of retinal ganglion cells is modulated in two ways by amacrine cells, either directly by feedforward inhibition from amacrine cell synapses onto retinal ganglion cell dendrites or by feedback inhibition, in which amacrine cells contact axon terminals of bipolar cells. Inhibition is mediated largely by two fast neurotransmitters, γ-aminobutyric acid (GABA) and glycine. Within the IPL, synaptic connections are further organized in two structurally and functionally distinct layers. The inner lamina of the IPL comprises synapses between ON-bipolar cells and retinal ganglion cells and amacrine cells, whereas the outer lamina contains synaptic connections of OFF-bipolar cells with amacrine and retinal ganglion cells (Fig. 1). However, retinal ganglion cells with dendritic arbors in both ON and OFF layers are commonly found (Dacey, 2000; Dacey and Lee, 1994; Masland, 2001). Also, subsets of bipolar cells in fish retina commonly have terminal boutons in both the ON and OFF layers (Li et al., 2012; Wong and Dowling, 2005; Wu et al., 2000).
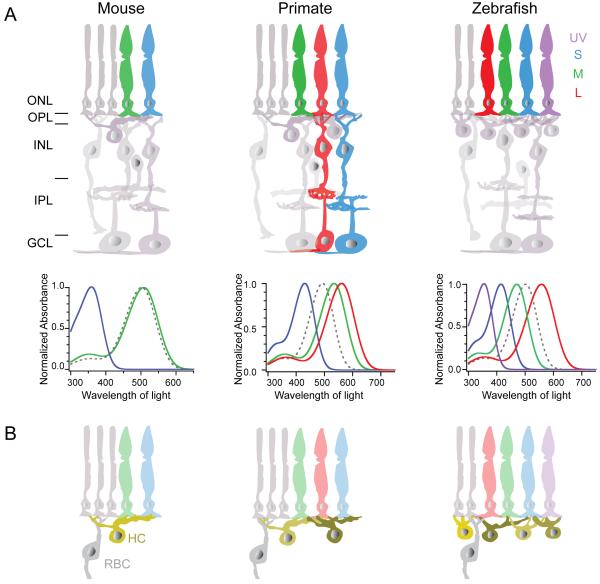
In addition to the common structural-functional relationships within the retina, there are specializations in circuit design across species (Fig. 2). One of the most striking specializations across species is the variation in the composition of retinal photoreceptors and their associated circuitry (Fig. 2A). The mouse retina comprises two kinds of cone photoreceptors: 5% that express only a short wavelength sensitive opsin (S opsin), whereas 95% of the cones co-express a middle wavelength sensitive opsin (M) together with S opsin (Applebury et al., 2000; Haverkamp et al., 2005; Nikonov et al., 2006; Rohlich et al., 1994). In contrast, macaque retina has three cone photoreceptor types, with peak sensitivity to short (S, blue), middle (M, green) or long (L, red) wavelengths of light. Zebrafish retina possesses a fourth cone photoreceptor type with peak sensitivity to ultraviolet (UV) light. Apart from distinct chromatic pathways, the composition of the retinal cell classes and their connectivity also differs across species (Fig. 2B). The diversity of horizontal cell types across species is noteworthy, and they vary both in their morphology and their connectivity patterns (Fig. 2B). Mouse retina has only one horizontal cell type (Peichl and Gonzalez-Soriano, 1994), whereas macaque retina has two types (Dacey, 1999; Wässle and Boycott, 1991) and zebrafish retina four types (Li et al., 2009). Zebrafish retina is also specialized in that the rod bipolar cell does not get input solely from the rod photoreceptors but also information from L cones (Li et al., 2012), thus unlike mouse and monkey retina the zebrafish retina does not have a ‘pure’ rod photoreceptor – rod bipolar cell channel (see 3.1.2. and 3.6., further discussion of retinal specializations in 2.3.).
Figure 2. Retinal architecture across species.
The retinas of mouse, primate (macaque) and zebrafish exhibit a common basic architecture, but with functional variations. Notably, cone composition (A) varies across these species. UV: ultraviolet, S: short, M: medium, L: long wavelength cones. Primate retina has pathways dedicated for color processing as shown for L and S cone pathways. Illustrations depict the absorption spectra of the various cone opsins across species (bold colored lines) compared to rhodopsin (dotted line) (summarized from: Baylor et al., 1987; Cameron, 2002; Chinen et al., 2003; Govardovskii et al., 2000; Imai et al., 2007; Robinson et al., 1993; Wang et al., 2011). Note that the spectrum of S opsin in mouse retina closely resembles that of UV opsin in zebrafish retina. In addition the cellular distribution and connectivity patterns also vary across species. Shown in B are species differences in the number of horizontal cell (HC) types and the connectivity of rod bipolar cells (RBC). ONL: outer nuclear layer, OPL: outer plexiform layer, INL: inner nuclear layer, IPL: inner plexiform layer and GCL: ganglion cell layer.
Nonetheless, the overall consistency in the basic organization of the retina across species has facilitated investigations into the relationship between structure and function of the adult retina, as well as investigations of the mechanisms underlying its development and maintenance. In the next section, we will explore in greater detail recent work that has provided insight into the developmental mechanisms that shape neuronal structure and function in the retina, from individual cell morphologies to the spatial organization of cell populations with specific functions.
2.1. Morphogenesis of retinal neurons
The major classes of retinal neurons can be divided into subtypes according to their characteristic morphologies and function (Masland, 2001; Wässle, 2004; Wässle and Boycott, 1991). The mechanisms responsible for patterning neuronal arbors unique to each subtype are only beginning to be unraveled. Retinal ganglion cells cultured in isolation, without any afferent inputs, target tissue or neighbors, recapitulate complex dendritic branching patterns found in vivo (Montague and Friedlander, 1989, 1991). This observation argues for the presence of intrinsic cues dictating dendritic morphology. However, it is also increasingly clear that cell-cell interactions, i.e. extrinsic factors, are also important. For instance, growth factors belonging to the neurotrophin family like BDNF (brain derived neurotrophic factor) can regulate retinal ganglion cell arborizations (Cohen-Cory and Lom, 2004). With the aid of mouse mutants, recent experiments have identified several other key molecules within the retina that pattern the arbors of retinal neurons in both a cell-autonomous and non-autonomous manner.
The dendritic arbors of many amacrine cells and retinal ganglion cells exhibit the feature of isoneuronal ‘self-avoidance’, a term reflecting minimal crossings of sister dendrites from the same cell. Minimal branch overlap ensures that the neuronal arbor of the cell covers more space and reduces the probability of receiving redundant inputs (Grueber and Sagasti, 2010). The neurites of retinal cells of the same subtype also tend to spatially avoid each other, a process called heteroneuronal self-avoidance. Molecules involved in ensuring isoneuronal and heteroneuronal self-avoidance have now been identified using targeted genetic manipulations and loss of function analyses. There are some instances, however, of an increase in cell number also causing self-avoidance deficits (Keeley et al., 2012).
The protein Down-syndrome cell adhesion molecule (Dscam) is expressed by a subpopulation of cells in the inner nuclear layer (INL) and by cells in the ganglion cell layer (GCL) of the mouse retina. Dopamine-containing amacrine cells and brain nitric-oxide synthase (bNOS)-positive amacrine cells, but not cholinergic starburst amacrine cells or glycinergic AII amacrine cells (Fuerst et al., 2008) express Dscam. In Dscam knockout (KO) mice, dendrites of dopaminergic amacrine cells exhibit isoneuronal and heteroneuronal fasciculation instead of avoidance (Fig. 3A). The dendritic fasciculation observed in the Dscam KO is accompanied by a clumping of dopaminergic amacrine cell somata (Fig. 3A). bNOS-positive amacrine cells, melanopsin-containing retinal ganglion cells (M1 and M2 retinal ganglion cells) and SMI-32-positive alpha-type retinal ganglion cells all show a similar fasciculation phenotype. In all affected cell types, fasciculation of dendrites and clumping of somata occur only amongst cells of the same type (Fuerst et al., 2009). Dscam-negative starburst amacrine cells and AII amacrine cells maintain normal dendritic morphology in the Dscam KO mouse. However, AII amacrine cells, along with rod bipolar cells, do express the closely related Dscam molecule, Dscaml1 (Fuerst et al., 2009). Loss of Dscaml1 function results in neurite fasciculation and somatal clumping of rod bipolar cells and AII amacrine cells. Together, these studies emphasize a central role for Dscam and Dscam-like proteins in patterning the arbors of individual retinal neurons as well as their cell populations.
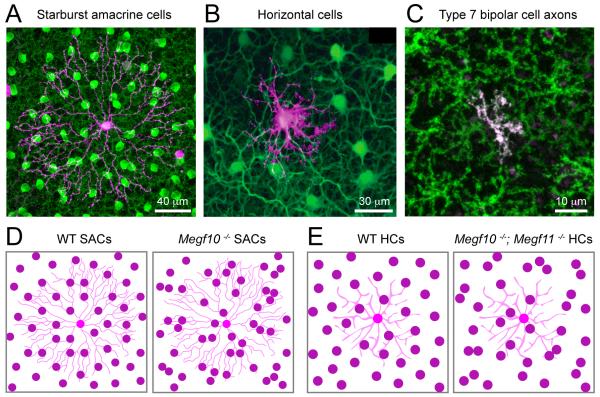
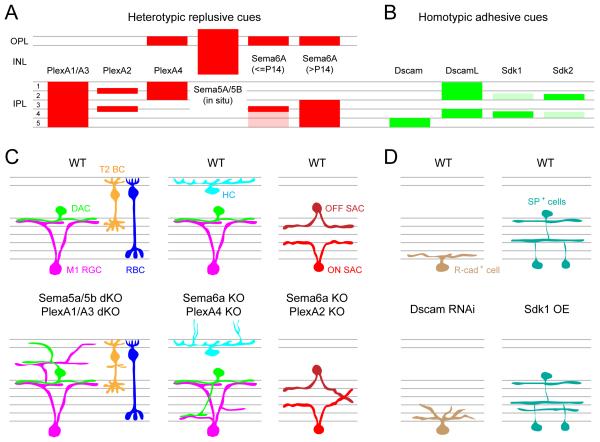
Figure 3. Molecular regulation of the branching patterns of amacrine cell neurites.
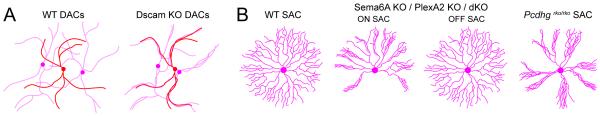
Schematics illustrating the lack of dendritic self-avoidance of two amacrine cell types in mouse mutants. (A) Dopaminergic amacrine cells (DACs) in wildtype (WT) and Dscam knockout (KO) animals. (B) Starburst amacrine cell (SAC) processes in wildtype (WT), Semaphorin6A (Sema6A) KO, plexinA2 (PlexA2) KO, Sema6A-PlexA2 double KO mice or protocadherin KO (Pcdhgrko/rko). Summarized from: Fuerst et al., 2008; Lefebvre et al., 2012; Sun et al., 2013.
Repulsive interactions mediated by semaphorins (Sema) and their receptors plexins (Plex) also regulate dendritic self-avoidance in the retina. Mouse horizontal cells express Sema6A and its receptor, PlexA4, and the loss of either molecule leads to an increased self-crossing of horizontal cell dendrites (Matsuoka et al., 2012). Similarly, both ON and OFF populations of starburst amacrine cells express PlexA2, but only ON-starburst amacrine cells express its ligand, Sema6A (Sun et al., 2013). Consequently, in the Sema6A KO mouse, ON-starburst amacrine cells develop asymmetric arbors with abundant self-crossovers, whereas OFF-starburst amacrine cell dendrites maintain their normal symmetry and field area (Sun et al., 2013 and see Fig. 3B). Thus, self-avoidance is mediated by Sema6A specifically in cells expressing PlexA2. Why only one population of starburst amacrine cells is regulated by this molecular cue is unknown, but it is highly intriguing. There are, however, molecules that control the arborization patterns of both ON- and OFF-starburst amacrine cells. A cadherin-like transmembrane protein, protocadherin (Pcdh), has recently been shown to shape the branching pattern of both starburst amacrine cell populations. The Pcdh locus in the mouse encodes 58 isoforms, which are distributed in three sub-clusters (Lefebvre et al., 2008). One of these subclusters, γPcdh (Pcdhg), encodes 22 Pcdh isoforms (Lefebvre et al., 2008). In the absence of all 22 isoforms, ON- and OFF-starburst amacrine cell dendrites develop an asymmetric morphology, often fasciculating with their own and other starburst amacrine cell dendrites (Lefebvre et al., 2012 and see Fig 3B). Expressing just 1 of the 22 isoforms restores isoneuronal self-avoidance in starburst amacrine cell dendrites, but it also causes an increased heteroneuronal avoidance compared to wildtype. Repulsive signals caused by homophilic binding of the same γ-Pcdh isoforms mediate self-avoidance. But, the expression of a different set of isoforms in individual starburst amacrine cells is necessary to regulate heteroneuronal interactions. Thus, combinatorial factors regulate arborization patterns of retinal neurons at the single cell level, and organize arbor relationships amongst neighbors of the same type. In the future we will also need to account for factors that direct the arbor orientation of a single population of retinal ganglion cells, along a common axis, as observed in the JamB retinal ganglion cells (Kim et al., 2008). Identifying the details of the molecular control of neurite patterning in the retina is still well behind current investigations in other sensory systems (see Jan and Jan, 2010), but with the rapid advancement in mouse genetics, it is very likely that more effectors will be discovered in the near future.
It is evident that interactions with presynaptic partners and synaptic activity also influence morphogenesis of retinal neurons. For instance, in bipolar and horizontal cells, the number of dendritic branch terminals, as well as the regularity of their spacing, is dependent on the density of their presynaptic cone photoreceptors (Keeley and Reese, 2010; Raven et al., 2007; Reese et al., 2005). In developing chick retinal ganglion cells, synaptic activity largely mediated by cholinergic transmission leads to local increases in intracellular calcium that further trigger the release of calcium from intracellular stores. This calcium-induced calcium-release (CICR) acts to locally stabilize dendrites; blockade of CICR causes dendritic retraction in the retinal ganglion cells (Lohmann et al., 2002). Other forms of neurotransmission, such as that mediated by GABA, also regulate the branching patterns of retinal ganglion cells in turtle (Chabrol et al., 2012). But, not all retinal ganglion cells appear subject to dendritic regulation by neurotransmission. Although pharmacological perturbation of glutamatergic transmission disrupts the dendritic stratification of cat retinal ganglion cells (Bodnarenko and Chalupa, 1993; Bodnarenko et al., 1995; Bodnarenko et al., 1999; Deplano et al., 2004), genetic suppression of glutamate release from bipolar cells does not affect the branching patterns of mouse ON- and ON-OFF retinal ganglion cells (Kerschensteiner et al., 2009). We will further discuss the role of transmission in dendritic lamination in Section 2.4.1.. Finally, apart from synaptic interactions, contact amongst cells of the same type has been suggested to modify the size and branching pattern of the axonal and dendritic arbors of retinal neurons. The role of such cell-cell interactions in shaping the territories of neighboring retinal neurons, and their distributions, will be discussed in greater detail in the next section.
2.2. Arranging retinal cells into mosaics
A common organizational principle of the vertebrate retina is the non-random ‘mosaic-like’ distribution of cells belonging to the same type (Wässle and Riemann, 1978). Each cell type forms a mosaic independent from mosaics of other cell types such that the distributions of different cell types are not spatially correlated (Rockhill et al., 2000). Within a mosaic, the arbors of the component neurons either tile, i.e. without dendritic or axonal overlap, or their arbors overlap by a characteristic amount. Mosaic arrangements are found throughout the retinal layers and provide a uniform coverage of the visual field by each retinal neuron subtype. It is astonishing that independently arranged mosaics of processes co-exist in the IPL especially, where there is tremendous spatial intermingling of the axons and dendrites of numerous subtypes of bipolar cells, amacrine cells and ganglion cells. Mosaic-independence is preserved even between cell types that are synaptically connected (Galli-Resta, 2000; Rockhill et al., 2000). Indeed during development, mosaics of each cell type emerge before extensive synapse formation (Galli-Resta, 2002). In one study of the macaque retina, however, synaptically connected S cone and S cone bipolar cells were observed to be closer together than expected, thus raising the possibility that synaptic connections or other developmental interactions could influence the mosaic arrangements of retinal neurons (Kouyama and Marshak, 1997). Here, we discuss recent advances in our understanding of the developmental cues that organize retinal cell mosaics at the various levels of the retina – the outer and inner nuclear layers, and the ganglion cell layer.
2.2.1. Mosaics in the retinal input layer
Processing of light information begins at the photoreceptor input layer. Cone types and their ratios, however, differ across species (Fig. 2). Some cone types form mosaics, but not all (Fig. 4A). In the mouse retina, cone photoreceptors are arranged in a quasi-regular mosaic (Fei, 2003). S cones in the monkey retina, but not human retina (Roorda et al., 2001), are also organized in a non-random manner. However, L and M cones are randomly distributed both in monkey and human retina (Roorda et al., 2001). By contrast, every cone photoreceptor type in the adult zebrafish retina forms a ‘crystalline’ mosaic composed of neatly arranged rows of alternating cone types, as shown in Fig. 4A (Allison et al., 2010). This highly ordered cone arrangement in the adult zebrafish retina has prompted the search for the mechanisms underlying cone mosaic formation in this species (Fig. 4B).
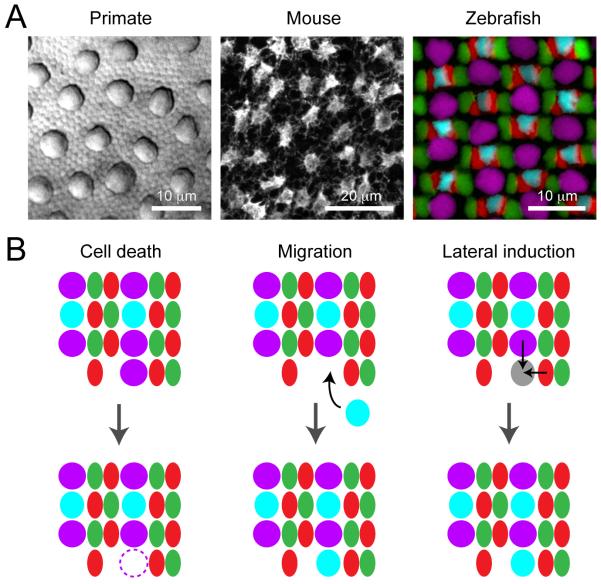
Figure 4. Mosaic arrangements of retinal photoreceptors and their formation.
(A) Cone photoreceptor distributions in primate (macaque), mouse and zebrafish retina. Macaque retina: Differential interference contrast (DIC) image of peripheral retina. Large profiles are cone photoreceptor inner segments interspersed amongst rod photoreceptors. Mouse retina: Mosaic arrangement of cone pedicles revealed by immunostaining for cone arrestin. Zebrafish: Cone mosaics in adult retina. UV cones, violet; S cones, cyan; M cones; green, L cones, red. Maximum intensity projection of a confocal image stack of a quadruple transgenic line Tg(gnat2:histone2ACFP; sws1:histone2AYFP; trβ2:tdtomato; sws2:GFP). Promoters are: sws1, UV opsin, sws2, S opsin, and trβ2, L opsin. Cones with nuclei labeled by the gnat2 promoter (labels all cones) that did not express UV, S or L opsins were identified as M cones. (Image courtesy: macaque retina, R. Sinha; mouse retina, F. A. Dunn, and zebrafish retina, S. C. Suzuki.)
(B) Possible mechanisms that could play a role in organizing the cone photoreceptor mosaic in zebrafish retina.
Because the zebrafish retina continues to grow beyond larval stages, it is possible to track how the mosaic takes shape as newly generated cones become integrated at the edge of the growing retina, the ciliary marginal zone (CMZ). How are different cone types incorporated into their proper positions as they are produced? There are at least three potential mechanisms (Fig. 4B): (i) Death of improperly incorporated cones, (ii) Active migration of cones to search for their proper positions, and (iii) Fate (lateral) induction of newly integrated cones by neighboring terminally differentiated cones (Fig. 4B). The first possibility is unlikely because cell death in the CMZ is consistently low (< 1%) from the early developmental period to adult (Biehlmaier et al., 2001). The second possibility has not yet been explored, but if new cells migrate, the distance must be locally limited because a single fluorescently-labeled retinal stem cell in the CMZ produces a continuous stripe of labeled postmitotic cells without much lateral dispersion along the annulus of the CMZ as the retina grows (Centanin et al., 2011). Theoretically, the local movement of cones, together with differential adhesion strengths between different cone types, could give rise to the row mosaic (Mochizuki, 2002). Such interactions between cone photoreceptors via adhesion molecules, such as Crumbs polarity proteins, are beginning to be understood (Zou et al., 2012). Lateral induction of cell fate is a well-known mechanism that generates an ordered array of photoreceptors within individual ommatidia in the fly compound eye (Frankfort and Mardon, 2002). Modeling studies have suggested that lateral induction of cell fate could also generate the rows of cones in zebrafish retina (Takesue et al., 1998; Tohya et al., 1999). Such mechanisms have not yet been directly demonstrated because the earliest possible method of marking different cone types has been labeling for different opsins, which are expressed only after cones are positioned in the row mosaic (Raymond and Barthel, 2004). Thus, early markers of specific cone types are needed. For example, precursors of L cones in zebrafish can be visualized in fish in which the thyroid hormone receptor β2 (Trβ2) promoter drives expression of fluorescent protein (Suzuki et al., 2013). Future in vivo time-lapse experiments that track the division, differentiation and movements of such labeled cones would be instructive. Such experiments could also reveal whether cone fate is decided before the cells are integrated into the forming mosaic, or if cones adopt their fate only after moving into position. Future experiments comparing cone mosaic development in zebrafish and mammals are also needed in order to discover mechanisms that might be conserved across species, or uncover mechanisms that are unique to organizing the cone mosaics in different animals.
2.2.2. Mosaics of retinal interneurons
Neurons in the inner nuclear layer, bipolar cells, horizontal cells and amacrine cells, also form mosaics (Fig. 5A-C). In recent years, tools that enable manipulation of gene expression in mice have greatly advanced our knowledge of the mechanisms underlying retinal mosaic formation, particularly of inner retinal neurons. As with photoreceptor mosaics, several cellular mechanisms can be conceived to contribute to the mosaic formation of inner retinal neurons. Indeed, modeling studies indicate that all three mechanisms (lateral induction, cell death and lateral dispersion) can theoretically explain mosaic formation (Eglen et al., 2000; Eglen and Willshaw, 2002), some of which have been demonstrated experimentally.
Figure 5. Mosaic arrangements of retinal cells and their development.
(A) Mouse starburst amacrine cells (SAC). Biolistic labeling of a mouse SAC (magenta) together with immunolabeling for choline acetyltransferase (green) to visualize the cell population. The image is a maximum intensity projection of confocal image planes acquired from the ganglion cell layer to the ON sublamina of the IPL in a wholemount retina.
(B) Mouse horizontal cell (HC) somata and their dendrites. A HC was intracellularly dye-filled with Alexa-555 (magenta) in the GAD1-GFP transgenic line (green), in which horizontal cells express GFP. (Adapted from Huckfeldt et al., 2009).
(C) Mouse bipolar cell axon terminals in the IPL. Individual ON-bipolar cells, including Type 7 bipolar cells, are visualized by tdtomato expression in the grm6-tdTomato transgenic line (Kerschensteiner et al., 2009). Virtually all Type 7 bipolar cells are labeled in the Gus8.4-GFP (Wong et al., 1999) line (Huang et al., 2003). A retina from a double transgenic animal shows an individual Type 7 bipolar cell (magenta-white) within the Type 7 population (green). Image adapted from Dunn and Wong, 2012.
(D) Illustration depicting the disruption of the cell body mosaic arrangement of SACs in the Megf10-deficient (Megf10−/−) mouse retina (Kay et al., 2012). The dendritic arbor of an individual SAC is provided in the background.
(E) Illustration showing perturbation of HC mosaics in Megf10/11-double knockout (Megf10−/−; Megf11−/−) animals (Kay et al., 2012). Dendritic arbor of an HC is illustrated in the background.
To date, there is no direct evidence for neighboring cells in the INL influencing each other’s fate, though it is a common mechanism in many systems, such as notch signaling between neighboring cells to control their neuronal fate choices (Louvi and Artavanis-Tsakonas, 2006). There is evidence, however, for cell death playing a role in mosaic formation in the INL, and this mechanism is employed by at least two types of amacrine cells. Like other cell types in the INL, dopaminergic amacrine cell bodies are non-randomly spaced (Eglen et al., 2003). Pairs of mouse dopaminergic amacrine cells are often found closely juxtaposed to each other upon genetic suppression of apoptosis (Raven et al., 2003). Cell death has also been suggested to contribute to the mosaic regularity of starburst amacrine cells in neonatal rodent retina (Resta et al., 2005), as suppressing cell death in these amacrine cells leads to an irregular mosaic pattern. It is not known how dopaminergic amacrine cells sense the local density of neighboring cells or how selective cell death is triggered in this population. But death of starburst amacrine cells appears to be caused by extracellular ATP via purinergic (P2X7) receptors expressed by these amacrine cells (Resta et al., 2005). Starburst amacrine cells store ATP in granules (Resta et al., 2005), raising the possibility that high concentration of ATP released by closely packed starburst amacrine cells triggers cell death to improve mosaic regularity.
Lateral dispersion of mouse dopaminergic amacrine cells during development appears to be limited (Raven et al., 2003), and thus this factor unlikely contributes to their somatal spacing. In contrast, lateral dispersion plays a role in generating the cell body mosaics of ON-starburst amacrine cells (Fig. 5A) positioned in the GCL. In rodent retina, around birth (embryonic day (E) 21- postnatal day (P) 0), ON-starburst amacrine cell somata are already arranged in a quasi-regular manner in the GCL but the newly differentiated cells migrating into the GCL do not arrive in a position optimally spaced between neighbors (Galli-Resta et al., 1997). After arriving in the proper layer, ON-starburst amacrine cells disperse tangentially to create regular spacing between each other (Galli-Resta et al., 1997), which requires the active remodeling of microtubules (Galli-Resta et al., 2002). What molecular mechanisms underlie this repulsive force between ON-starburst amacrine cell neighbors? A recent study has shown that starburst amacrine cell bodies become randomly distributed in a mouse lacking the transmembrane multiple epidermal growth factor-like domains protein 10 (Megf10) (Kay et al., 2012 and Fig. 5D). Megf10 is expressed by immature starburst amacrine cells as these cells approach their final target layers. This molecule is expressed on the cell surface, causing homophilic, repulsive interactions, which in turn generates even spacing between starburst amacrine cell bodies. The dendrites of the starburst amacrine cells, however, remain highly overlapped in the Megf10-mutant, similar to wildtype conditions. How does contact-mediated repulsive Megf10 signaling only impact the spacing of the starburst amacrine cell’s soma but not its dendrites? One possibility is that Megf10 is downregulated as starburst amacrine cell dendrites extend into each other’s territories. But, starburst amacrine cell dendrites are already fasciculated at the first postnatal week when Megf10 is still expressed (Stacy and Wong, 2003). It could be that while Megf10 is still expressed in starburst amacrine cell dendrites, the downstream signaling cascade is dismantled. Alternatively, as the dendrites of starburst amacrine cells begin to overlap, starburst amacrine cells could upregulate an adhesion factor that negates the repulsion mediated by Megf10. In summary, we do not yet have an answer for how mosaic somal spacing and overlapping dendritic territories co-exist in the same population of amacrine cell.
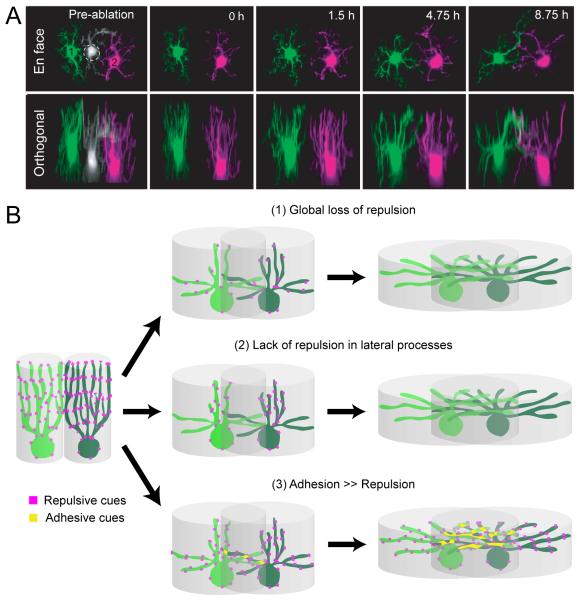
Examination of the mosaic formation of another retinal cell type, the horizontal cell, has offered a solution to the problem. Like starburst amacrine cell somas, horizontal cell bodies also form a mosaic (Fig. 5B) primarily via lateral dispersion (Raven et al., 2005), with highly overlapping dendritic fields: about 6 horizontal cell dendritic fields overlap at any given point of the mouse retina (Reese et al., 2005). How do horizontal cells attain their somal mosaic while allowing so much dendritic overlap? During late embryonic to early postnatal stages, the neurites of mouse horizontal cells are directed vertically towards the outer neuroblast layer, the future outer nuclear layer (ONL), forming a columnar arbor (Huckfeldt et al., 2009 and see Fig 6A). The arbors of neighboring immature horizontal cells form a tile-like arrangement via homotypic repulsive interactions. Multiphoton time-lapse imaging has demonstrated that upon laser-ablation of some immature horizontal cells, the processes of neighboring cells gradually fill in the vacated area. The vertical neurites of immature horizontal cells exist only transiently, giving way to lateral extensions that form mature dendritic arbors that overlap extensively. What molecular cues could dictate the repulsive interactions between vertical arbors of immature horizontal cells yet allow subsequent overlap of their dendrites at maturity? Like starburst amacrine cells, the mosaic spacing of horizontal cell bodies is also regulated by Megf10, but another isoform, Megf11, can compensate for Megf10 to regulate horizontal cell spacing (Kay et al., 2012 and see Fig. 5E). Megf10 and 11 expression in horizontal cells commences around birth, raising the possibility that these molecules underlie the repulsive interactions between vertically oriented neurites of developing horizontal cells. Dendritic overlap of mature horizontal cell could require secondary mechanisms such as loss of repulsive interactions or gain of adhesive interactions (Fig. 6B).
Figure 6. Possible mechanisms regulating retinal mosaic development.
(A) Immature horizontal cells transiently project vertical processes that form non-overlapping territories before extending lateral dendrites that overlap at maturity. Shown here are two developing horizontal cells labeled in the GAD1-GFP transgenic mouse retina, pseudocolored in green and magenta imaged with time-lapse multiphoton microscopy (h, hour). The vertical arbors of these immature cells re-tile after laser-ablation of a neighbor, suggesting that homotypic interactions regulate spacing between neighbors. Image adapted from Huckfeldt et al., Nat Neurosci., 2009.
(B) Illustrations of how potential adhesive and repulsive interactions could mediate homotypic interactions among neighboring horizontal cells that initially define their cell body mosaic arrangement, and later permit overlap of their lateral dendrites. For example repulsive cues could be downregulated either all through the horizontal cell arbor (1) or specifically from lateral processes (2) to permit dendritic overlap of mature horizontal cells. Additionally, adhesive cues (3) could facilitate dendritic overlap between neighboring horizontal cells.
For retinal neurons with arbors that do tile, one can imagine that homotypic interactions set up their mosaic. Tiling is most apparent for the dendritic and axonal arbors of bipolar cells within their respective layers in mouse retina (Fig 5C). The molecular mechanisms that regulate the tiling of bipolar cell arbors are unknown, but studies elucidating the signaling mechanisms that drosophila sensory neurons use to populate the larval body wall may be instructive (Emoto et al., 2004; Jan and Jan, 2010).
2.2.3. Mosaics in the retinal output layer
Retinal cell mosaics have long been associated with the distribution of retinal ganglion cells (Wässle et al., 1981). The cell bodies of retinal ganglion cells belonging to the same subtype are arranged in a non-random fashion. For many types, their dendritic arbors overlap by a constant amount without much local variation in their coverage or sampling of the visual field (Gauthier et al., 2009; Wässle et al., 1981). Some retinal ganglion cells such as rabbit direction-selective (DS) retinal ganglion cells that share the same preferred direction, possess dendritic arbors that do not overlap but instead tile (Vaney, 1994). In human retina, the dendritic arbors of neighboring midget ganglion cells also tile and never overlap (Dacey, 1993b). Such tiling arrangements naturally suggest a role for homotypic interactions in setting up the ganglion cell mosaic. Indeed, several observations support this notion. First, lesion-induced depletion of some ganglion cells in developing rat (Perry and Linden, 1982) and cat (Eysel et al., 1985) retinas causes surviving ganglion cells bordering the lesion to orient their dendrites toward the cell-depleted site. Also, increasing ganglion cell density in the cat retina is paralleled by a reduction in their dendritic field size (Kirby and Chalupa, 1986). Further, studies in ferret retina have shown that neighboring retinal ganglion cells of the same type can have dendrodendritic contacts, although the nature of such contacts needs to be verified at the ultrastructural level (Lohmann and Wong, 2001). Although the molecular basis of homotypic interactions between retinal ganglion cells of the same type is still unknown, there is a likelihood that retinal ganglion cells share similar strategies to those used by other retinal neurons (horizontal cells and amacrine cells) for spacing their cell bodies. In addition to dendro-dendritic interactions, cell death is also proposed to contribute to the mosaic regularity of alpha ganglion cells in the cat retina (Jeyarasasingam et al., 1998). A recent study further demonstrated the role of cell death for the mosaic formation of M1 ganglion cells in the mouse retina (Chen et al., 2013b).
Retinal ganglion cell mosaic arrangements are, however, not always influenced by homotypic interactions with neighbors of the same cell type. Dendritic arbors of ganglion cell subtypes (M1 ganglion cells and SMI-32 positive alpha-like ganglion cells) in the mouse retina both greatly overlap with neighboring cells of the same type. In mutant mice where the majority of ganglion cells are lost, surviving M1 ganglion cells and SMI-32 positive ganglion cells develop normally sized dendritic fields and maintain their mosaic arrangement (Lin et al., 2004). Thus, certain ganglion cell types can form mosaics even when dendrites of neighboring cells of the same type do not contact. It would be interesting to further explore such mechanistic differences between ganglion cell types that give rise to distinct mosaics of their dendritic fields, such as tiling via homotypic interactions, with ganglion cell types that lack apparent regulation of dendritic arbors by homotypic interactions.
2.3. Organizing retinal cell distributions for specialized tasks
Although retinal cell mosaics are found throughout the retina, cell distributions are not necessarily uniform across the retina. Often, each species has developed specialized spatial and circuit arrangements that are best suited for processing of relevant features of their visual world (Hughes, 1985; Peichl, 1991). In this section, we review some key specializations in cell distributions and discuss what is known and what is not known about the developmental mechanisms underlying their patterning. We also bring to the attention of the reader the observation that spatial distributions of cells within the retina can exhibit abrupt structural and/or functional changes, either to facilitate a specific visual task or perhaps reflecting new demands on retinal organization in the adult.
2.3.1 Central, high-acuity vision
Local peaks in cell density can be observed at specific locations in the retina of many vertebrates. For example, in cats, ganglion cells are concentrated in a small region called the ‘area centralis’, located in dorsal-temporal retina. This region of maximum ganglion cell density is responsible for high spatial resolution of images (Rapaport and Stone, 1984). In fact, all retinal cell types increase in density towards the area centralis. During development of the cat retina, the area centralis is the first part of the retina to mature (Rapaport and Stone, 1984), presumably followed by a non-uniform growth of the peripheral retina with the area centralis displaying minimal growth (Mastronarde et al., 1984). Accordingly, density of the central beta-retinal ganglion cells in the area centralis is maintained throughout eye-growth as the magnification factor decreases (Sernagor et al., 2001). The mechanisms that specify the early maturation of the area centralis in cat retina or that govern its progression into a region of peak cell density remain largely unknown.
In primate retina, a more dramatic specialization in cell distribution that is optimized for central, high acuity vision is the fovea (Latin for pit or depression). Foveal cones in macaque retina are packed at a density as high as ~200,000/mm2 (Hendrickson, 1994), which declines steeply towards the periphery, reaching less than 10,000/mm2 at the eccentricity of the optic disk (~20 degrees of visual angle). In contrast, rods are absent in the fovea (Packer et al., 1989). The spatial distribution patterns of rod and cone bipolar cell types also appear to match that of their presynaptic photoreceptor types in macaque retina. For example, the highest density of rod bipolar cells occurs at the eccentricity close to where rod density is maximum (Grunert and Martin, 1991). Despite cone density increasing towards the fovea, the ratio between S cones and S cone ON-bipolar cells is constant across macaque retina (Kouyama and Marshak, 1992; Wässle et al., 1994).
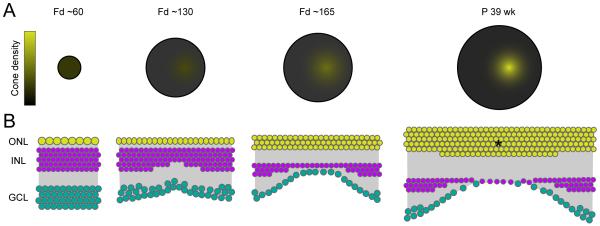
The structural development of the fovea (Fig. 7) has long fascinated investigators and has been well documented, particularly by the work of Hendrickson and colleagues. The macaque retina initially develops as a flat sheet of neuroepithelium but midway through gestation (~ fetal day (Fd) 74) (Hendrickson and Kupfer, 1976), the central fovea begins to take shape and can be seen as a small depression along the retinal sheet. Like the area centralis in cat, the distance between the fovea and the optic disk in the macaque retina remains almost constant as the retina grows (Packer et al., 1990). Expansion of the retinal area appears to explain the decrease in cone density in the retinal periphery but does not account for the increase of cone density in central retina (Hendrickson, 2006 and see Fig. 7A) as the foveal pit emerges (Hendrickson, 1992 and see Fig. 7B).
Figure 7. Formation of the foveal specialization in primate retina.
(A) Schematic illustrating how the density of cone photoreceptors increases in the primate (macaque) fovea as the retina develops (Fd: fetal day, P: Postnatal, wk: week). Retina size at each age is drawn to scale.
(B) Schematic depicting the re-arrangements of retinal cells during foveal pit formation in macaque retina. Cone photoreceptors in the outer nuclear layer (ONL) increase in density at the foveal pit (Hendrickson, 1992), whereas second order neurons in the inner nuclear layer (INL) and ganglion cell layer (GCL) are pushed aside and decrease their density concurrently. Asterisk depicts increase in cone density at the center of the foveal pit.
What cellular mechanisms underlie the initiation and formation of the foveal pit? Although this question has not been fully answered some mechanistic possibilities have been considered (Fig. 7B), such as: (i) A reduction of retinal ganglion cell density at the central fovea, (ii) An increase in the density of cone photoreceptors, and (iii) A suppression of rod photoreceptor genesis at the fovea (Hendrickson, 1992; Hendrickson and Kupfer, 1976). One possible mechanism for the reduction of retinal ganglion cell density at the fovea is by active migration of ganglion cells away from the central fovea in response to ‘repulsive’ cues, which could be triggered by a high ganglion cell density (Leventhal et al., 1989). However, retinal ganglion cells already form synapses before the pit appears and it is not common to find that differentiated neurons can actively migrate great distances together with their afferents. Alternatively, mechanical forces exerted on the developing retina may lead to the formation of the foveal pit and a ‘passive’ displacement of retinal ganglion cells (Springer, 1999; Van Essen, 1997).
The developmental mechanisms responsible for the high density of cones in the central fovea also remain largely unknown. One possibility could be movement of cones toward the center of the fovea (Hendrickson, 1994) and this process could be active or passive. One potential mechanism involves Müller glia cells. The inner segments of cones are attached tightly onto Müller cells, whose processes span vertically through the retina. Pit formation could move the inner foot of the Müller cell processes away from the central fovea. This action may lean the outer side of the Müller cell processes toward the central fovea, leading to a ‘squeezing’ of cones (Hendrickson, 1994).
Although rods are generated in the fovea first, their generation appears to be suppressed in the center of the fovea. During initial stages of fovea pit formation a sparse population of rods exists within a 1600 μm width region (Hendrickson and Kupfer, 1976; La Vail et al., 1991). As the foveal pit develops this region shrinks to a width of ~200 μm (Hendrickson, 1994). What intrinsic or extrinsic factors suppress rod genesis at the fovea, however, has not yet been detailed.
Finally, it should be realized that not all cell types within a retina alter their cell densities in parallel, which can lead to different convergence ratios of pre- and postsynaptic cells. For example, the parvocellular pathway that encodes color and spatial acuity forms a ‘private’ line of connection in the fovea where individual cone photoreceptors contact a single midget bipolar cell, which then synapses onto a single midget retinal ganglion cell (Calkins et al., 1994; Kolb and Dekorver, 1991; Wässle, 2004). Convergence along this pathway increases outside the fovea towards the periphery (Chan et al., 2001; Wässle et al., 1994). Thus, there may not be a single mechanism that organizes the spatial distributions of cell populations across the retina, but rather a set of mechanisms that coordinate the arrangements of each pre- and postsynaptic cell type to meet the changing demands of each circuit sampling different parts of the visual space.
2.3.2. Graded cell distributions
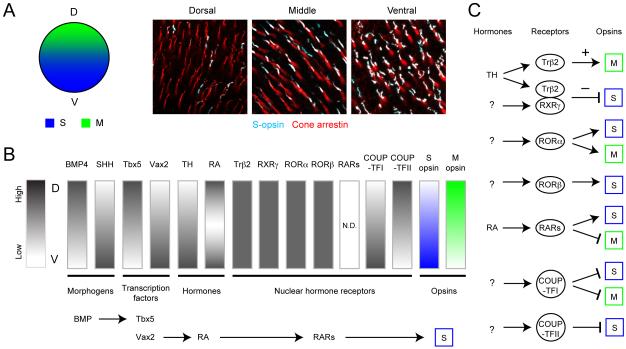
In contrast to photoreceptors in monkey and cat retina, the spatial distributions of rods and cones in mouse retina are relatively uniform, with a centro-peripheral gradient of no more than 2-fold (Jeon et al., 1998). However, the expression patterns of S and M opsins in mouse cone photoreceptors follow a dorsoventral gradient (Applebury et al., 2000; Rohlich et al., 1994; Szel et al., 1992 and see Fig. 8A). Except for ~5% of cones that exclusively express S opsins and are distributed homogeneously across the retina (Haverkamp et al., 2005) (‘pure’ S cones), mouse cones express both S and M opsins. S opsin expression dominates over M opsin expression in the ventral retina, and the opposite holds true for dorsal retina. This opsin gradient is reflected in the short wavelength-dominant responses of bipolar cells, horizontal cells and ganglion cells in ventral retina, and M opsin-dominant responses from cells in dorsal retina (Breuninger et al., 2011; Ekesten and Gouras, 2005; Yin et al., 2006, 2009).
Figure 8. Molecular interactions generating opsin expression gradients in the mouse retina.
(A) Expression of S opsin follows a dorsal (low) to ventral (high) gradient in mouse retina. Images show immunostaining for S opsin (cyan) and cone-arrestin that labels all cones of an adult mouse retina (red)(Images by F. A. Dunn). D: dorsal, V: ventral, S: S opsin, M: M opsin.
(B) Several morphogens, transcription factors, hormones and nuclear hormone receptors contribute towards generating the gradients of opsin expression. Summarized are the spatial expression patterns of known factors. N.D. : not determined.
(C) Illustration of the action of known nuclear hormone receptors that could regulate M and S opsin expression in the mouse retina. (+) Promotes, (−) suppresses.
(Schematics in B and C summarized from Alfano et al., 2011; Fujieda et al., 2009; Koshiba-Takeuchi et al., 2000; McCaffery et al., 1992; Ng et al., 2001; Peters and Cepko, 2002; Roberts et al., 2005; Roberts et al., 2006; Satoh et al., 2009; Srinivas et al., 2006; Zhang and Yang, 2001).
How are the dorsoventral gradients of short and medium wavelength cones set up during development? Transgenic approaches revealed a role for thyroid hormone and its receptor, Trβ2 for cone patterning. Trβ2 is necessary for M opsin expression and suppresses S opsin expression during development of the mouse retina (Ng et al., 2001). Thus, the spatial and temporal pattern of Trβ2 activation across the retina regulates S and M opsin expression patterns. This transcriptional control of short and medium wavelength cone identities also occurs in the zebrafish retina, although there is no gradient of cone opsin distributions (Suzuki et al., 2013).
Several studies have provided insight into how Trβ2 acts to regulate S and M opsin expression in the mouse retina (Fig. 8B-C). Trβ2 not only functions as monomers and homodimers but it also forms heterodimers with other nuclear hormone receptors such as retinoid X receptors (RXR) and chicken ovalbumin upstream promoter transcription factors (COUP-TF) that in turn can form a complex with retinoic acid receptors (RAR) (Berrodin et al., 1992). These nuclear hormone receptors can regulate the epigenetic action of Trβ2 by forming heterodimers. Their spatiotemporal expression patterns (Fig. 8B) during development could in turn control opsin expression patterns across the retina. In RXRγ KO animals, the S opsin gradient is disrupted, such that all cones express S opsin (Roberts et al., 2005; Roberts et al., 2006). The M opsin gradient, however, remains unaltered. This suggests that RXRγ forms heterodimers with Trβ2 and suppresses S opsin expression. A subset of retinoid-related orphan receptors (ROR), RORβ induces S opsin expression synergistically with another transcription factor, the cone-rod homeobox (CRX) (Srinivas et al., 2006). The spatial expression pattern of RORβ and its unknown ligand may also contribute to the S opsin expression gradient, but these expression patterns are currently not known. Retinoic acid is expressed in the dorsal and ventral retina except for an intermediate zone (McCaffery et al., 1992). The distribution pattern of RARs is not known. Another transcription factor, COUP-TFI is expressed ventrally whereas COUP-TFII is expressed dorsally during embryonic stages (Satoh et al., 2009 and see Fig. 8B). COUP-TFI expression level in the ONL decreases during the first postnatal week whereas COUP-TFII expression persists. COUP-TFI and TFII appear to suppress M opsin expression in the ventral retina, and S opsin expression in the dorsal retina, respectively (Satoh et al., 2009).
The spatial expression pattern of these nuclear hormone receptors can be further regulated by other transcription factors (Fig. 8B-C). For example, bone morphogen protein 4 (BMP4) and sonic hedgehog (SHH) antagonize and control dorsoventral expression patterns of other transcription factors, including dorsally-enriched Tbx5 (T-box 5) and ventrally-enriched Vax2 (ventral anterior homeobox 2) (Koshiba-Takeuchi et al., 2000; Peters and Cepko, 2002; Zhang and Yang, 2001). Vax2 regulates the distribution patterns of retinoic acid by controlling the ventral expression of retinoic acid synthesizing and degrading enzymes (Alfano et al., 2011). Similarly, BMP4 regulates dorsal expression of COUP-TFI and II (Satoh et al., 2009). Taken together, although the precise mechanisms that create the dorsoventral gradient of S and M opsins in mouse retina are yet to be fully understood, the asymmetric expression patterns of early transcription factors appear to determine the expression pattern of subsequent factors that eventually determine the opsin expression gradient (Fig. 8B-C). It is possible that asymmetric expression patterns of transcription factors also regulate the spatial distribution pattern of other retinal cell types.
2.3.3. Abrupt changes in distributions
Before concluding this section, it is noteworthy to mention that there are examples of retinal cell distributions for which abrupt changes in cell density and even cell composition occurs within a retina. Recently, it was demonstrated that the transition between S opsin and M opsin dominant regions occurs steeply along a narrow strip where many cones express equivalent amounts of the two opsins (Baden et al., 2013). The steep opsin expression gradient over an individual ganglion cell receptive field within this transition zone provides chromatically opponent responses to these ganglion cells without requiring specialized connectivity with upstream retinal neurons (Chang et al., 2013). Mice can discriminate UV light from visible light (Jacobs et al., 2004), and this ability may result from the presence of retinal ganglion cells in the transition zone of opsin expression (Chang et al., 2013).
As mentioned earlier (see 2.2.1.), the zebrafish retina continues to grow throughout the lifetime of the animal. New cells are consistently produced at the peripheral region of the retina called the CMZ. The larval part of the retina remains at the center and occupies a small fraction of the total retinal surface in the adult. The distribution patterns of cones in the larval ‘remnant’ and the adult retina are strikingly different. The cone mosaic is not as regular in larvae, comprising a L:M:S:UV ratio of 1.5:1.25:1:1.2 compared to a strict 2:2:1:1 ratio characteristic of the adult retina (Allison et al., 2010). Moreover, the mosaic of cone types is not as regular as that found in the adult part of the retina. As discussed in Section 2.2.1., the molecular and cellular mechanisms that orchestrate the organization of cone mosaics are not known, but several mechanisms have been proposed.
Both of these examples highlight the complexity of the developmental programs that are needed to organize the various retinal cell types and subtypes into functionally relevant distributions. As cell-type specific promoters become increasingly available for both mice and zebrafish, the prospect of determining relevant mechanisms in the not too distant future seems promising.
2.4. Emergence of the layered structure of the retina
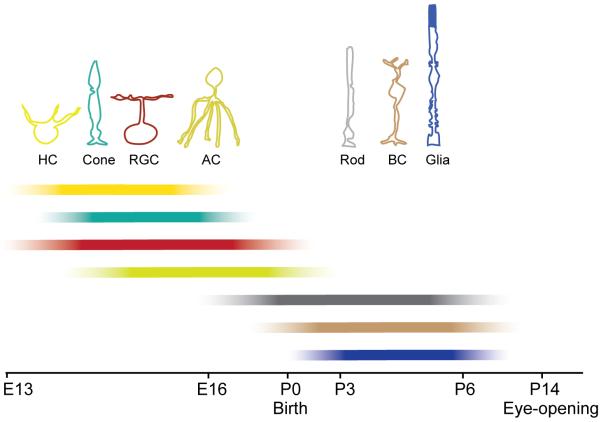
As illustrated earlier, the retina comprises three distinct cell body layers, separated by two synaptic or plexiform layers. The major classes of retinal cells forming these layers are not all generated at the same time (Fig. 9). The inner retina is established first, with the dendrites of early born ganglion cells and amacrine cells elaborating before the outer retina develops, a process that can be visualized in vivo by time-lapse microscopy (Godinho et al., 2005; Mumm et al., 2006). In the outer retina, the forming OPL first comprises processes of horizontal cells and photoreceptors. Thereafter, bipolar cells are produced and their dendrites and axons elaborate into the OPL and IPL. Because of the sequential addition of cells to the retina, one immediate question that comes to mind is which, if any, retinal cell type or types are essential for organizing the cell layers of the retina? The presence of inner and outer nuclear layers despite the lack of retinal ganglion cells in the zebrafish lakritz mutant (Kay et al., 2004) and the mouse atonal homologue Math5 KO (Brown et al., 2001) suggests that at least these neurons are not required.
Figure 9. Timeline for cell genesis in the vertebrate retina.
Sequence of cell genesis in the vertebrate retina, schematized here for the mouse. Horizontal cells (HCs), cone photoreceptors (cone) and retinal ganglion cells (RGC) are the first cells to be generated. Amacrine cell (AC) genesis follows, with their peak production occurring around embryonic day 16 (E16). Rod photoreceptors (rod) have a protracted period of genesis beginning before birth and continuing until a week after birth. Bipolar cells (BC) and Müller glial cells (Glia) are produced postnatally (P) until about a week after birth (summarized from Marquardt and Gruss, 2002; Rapaport et al., 2004; Young, 1985). Bars demonstrate the progressive increase and later decrease in neurogenesis as indicated by the intensity gradient.
What had been less apparent in the past, and has been the focus of several recent studies, is whether or not there is a particular retinal cell type that is critical for organizing synaptic sublayering in the IPL. This question has been addressed in several studies on zebrafish retina. Mislocalized retinal ganglion cells in the heart-and-soul (has) mutant zebrafish project their dendrites into the ectopic neuropil where amacrine cells also extend their processes, suggesting that one cell type may direct neurite extension of their synaptic partners (Choi et al., 2010). However, amacrine cell dendrites and bipolar cell axons can by and large stratify correctly in the absence of retinal ganglion cells (Gunhan-Agar et al., 2000; Kay et al., 2004). Amacrine cell dendrites also stratify normally in the absence of bipolar cells (Green et al., 2003). In these studies, however, one synaptic partner always remains in the circuit. Thus, a recent study in zebrafish systematically eliminated both inner retinal neurons and Müller glia cells during development. Surprisingly, bipolar cell axons can form an IPL like neuropil even in the absence of retinal ganglion cells, amacrine cells and Müller glia cells (Randlett et al., 2013). Thus, summarizing observations from the zebrafish retina, it appears that no single cell type is responsible for organizing sublamination of the IPL.
To further complicate the search for lamination cues, it was discovered that the stratification of retinal processes of the same major cell class is not directed by a single mechanism or strategy. Time-lapse imaging of retinal ganglion cell arbors in larval zebrafish and comparison of the morphology of transgenically labeled mouse retinal ganglion cell subtypes (Kim et al., 2010), revealed that some retinal ganglion cell types directly target their correct sublaminae whereas others adopt an exploratory strategy whereupon dendrites in the inappropriate sublaminae are eliminated (Kim et al., 2010; Mumm et al., 2006). There are also some retinal ganglion cell types that initially elaborate dendrites in one sublamina and later elaborate dendrites to form a separate arbor in another sublamina (Kim et al., 2010; Mumm et al., 2006).
It is clear that many different cues must be engaged separately to establish the detailed lamination patterns of the IPL. There has been excellent progress in recent years in defining some key mechanisms. These mechanisms can be broadly divided into cues requiring neurotransmission as well as interactions that are independent of neurotransmission.
2.4.1. Neurotransmission-dependent cues
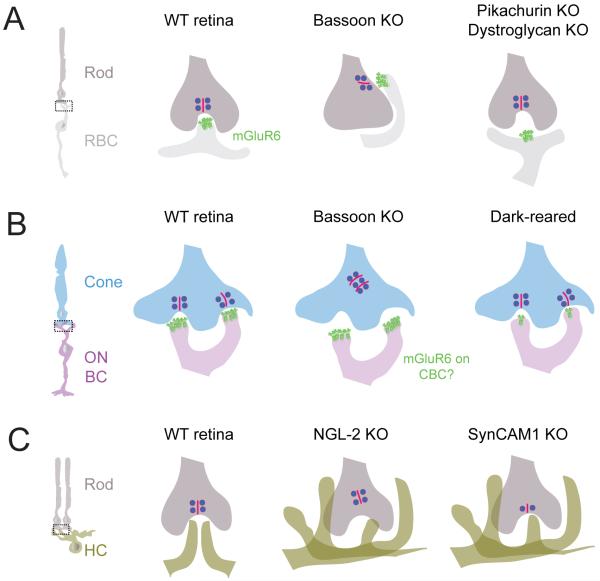
There is no doubt that neurotransmission during development can affect lamination of retinal neurons. When excitatory transmission from photoreceptor terminals is perturbed, rod bipolar cell dendrites and horizontal cell processes sprout into the ONL (Dick et al., 2003; Raven et al., 2008). Surprisingly, sprouting of bipolar cell dendrites does not occur when postsynaptic bipolar cell function is perturbed as in mice lacking the metabotropic glutamate receptor mGluR6 (Masu et al., 1995) or when the signaling cascade downstream from these receptors is disrupted (Dhingra et al., 2000; Koike et al., 2010). These findings suggest that alteration of neurotransmission between photoreceptors and their postsynaptic cells is not solely responsible for dendritic sprouting of the postsynaptic bipolar cell partners.
In the IPL, chronic blockade of ON-bipolar cell activity by intraocular injections of APB (2-amino-4-phosphonobutyric acid) causes a failure of cat retinal ganglion cells to restrict their dendritic lamination to the ON or OFF sublaminae (Bodnarenko and Chalupa, 1993). Similarly, dark-reared mice show an increased number of bistratified retinal ganglion cells (Tian and Copenhagen, 2003). Other studies, however, have reached an opposite conclusion. Retinal ganglion cell dendritic arbors stratify normally in mice lacking mGluR6 receptors, a condition that renders their presynaptic partners, the ON-bipolar cells, insensitive to light (Tagawa et al., 1999). Also, genetic suppression of vesicular transmitter release from ON-bipolar cells by expression of the light chain of tetanus toxin does not alter the stratification or morphology of mouse ON- and ON-OFF retinal ganglion cells (Kerschensteiner et al., 2009). An explanation for such disparity in findings across studies is not readily apparent, but it is unlikely to be simply due to different sites of transmission blockade because ON-bipolar cell responses should be similarly blocked by APB treatment as in the mGluR6 mutant. Importantly, these disparities, raise awareness that the outcome may be highly dependent on the nature of the transmission blockade, a problem that is not unique to studies of the retina (Bleckert and Wong, 2011).
Although not necessarily requiring transmission per se to stratify properly, the dendritic projections of retinal ganglion cells are influenced by interactions with their presynaptic bipolar cells. Genetic ablation of the major bipolar cell input type of alpha-like ON-retinal ganglion cells (see 3.4.2.), causes some of these cells’ dendrites to stray into the OFF layer, though their ON-dendritic arbor remains largely intact. The ectopic dendrites form synapses mostly with a specific type of OFF-bipolar cell, suggesting that even ON-retinal ganglion cells and OFF-bipolar cells can be ‘molecularly matched’ and form synapses, if given a chance (Okawa et al., 2014). These observations thus indicate that the presence of the primary presynaptic cell type dissuades the dendrites of the postsynaptic retinal ganglion cell from searching for new synaptic partners. In addition, these findings underscore the importance of lamination cues in preventing functional mismatching of pre- and postsynaptic cell types in the inner retina.
Our task now is to more completely understand why blockade of transmission during development disrupts synaptic lamination and connectivity in some conditions but not others, even within the same circuit (e.g. photoreceptors to bipolar cells). Attaining this knowledge for the developing retina will also be useful for deciphering the damage to circuits in retinal diseases in which cell death disrupts neurotransmission.
2.4.2. Neurotransmission-independent cues
There have been significant advances in our understanding of the transmission-independent cell-cell interactions and molecular cues that guide the lamination of processes of retinal neurons. It is now evident that a set of molecules can act either only on a specific cell type (e.g. amacrine cells) or across several but not necessarily all cell types.
In mutant mice lacking the FAT atypical cadherin3 (Fat3) the neurites of amacrine cells form an additional layer outside of the IPL (Deans et al., 2011). This ectopic layer of amacrine cell processes appears to be derived from amacrine cells that are correctly positioned in the INL, but have developed a bipolar morphology with two separate arbors. In the Fat3-mutant, there are also many more amacrine cells displaced to the GCL. The processes of AII amacrine cells that ectopically laminate in the GCL contact rod bipolar cell axons that have also mis-projected beyond the IPL. Because rod bipolar cells do not express Fat3, the alteration in their axonal projection implicates the presence of a cue directing their axons towards their usual synaptic partner, the AII amacrine cell. The misplaced neurites of amacrine cells form synapses with the bipolar cells and other amacrine cells in the ectopic plexuses, suggesting that loss of Fat3 does not disrupt synaptogenesis. The ectopic amacrine cell neuritic layers may arise because of a failure to prune mis-oriented processes during cell migration (Deans et al., 2011). Certainly, in vivo time-lapse recordings in zebrafish demonstrate that amacrine cells are multipolar during migration, and only direct their process exclusively towards the forming IPL when their cell bodies are close to their final location in the INL. Together, these observations suggest that there are molecular cues that separately organize the stratification of amacrine cell processes and their ability to form synapses, including contact with appropriate partners.
Recent studies have identified several repulsive interactions mediated by semaphorins and plexins in controlling the overall lamination of the retina (Fig. 10A, C). During embryonic and early postnatal development of the mouse retina, class 5 transmembrane semaphorins, Sema5A and 5B, are expressed by cells in the ONBL (outer neuroblastic layer or future ONL) whereas their receptors, PlexA1 and A3, are expressed by cells in the INBL (inner neuroblastic layer or future INL and GCL), which includes immature amacrine cells and ganglion cells (Matsuoka et al., 2011a and see Fig. 10A). The repulsive interactions between Sema5A/5B and PlexA1/A3 keep amacrine cell and ganglion cell dendrites away from the outer retina and in the IPL. In the absence of these proteins, several subtypes of amacrine cells and retinal ganglion cells extend their processes into the OPL and the INL, wherein they create an extra plexiform layer in addition to arborizing in the IPL (Fig. 10C). Another member of transmembrane semaphorins, Sema6A, and its receptor, PlexA4, are both expressed by horizontal cells in mouse retina (Matsuoka et al., 2012). In KO mice for either protein, horizontal cell axons fail to be constrained within the OPL (Fig. 10C). Thus, Sema6A-PlexA4 heterotypic signaling may not function as a repulsive cue, but rather, may work as an adhesive force to confine horizontal cell axons to the OPL.
Figure 10. Molecular cues guiding retinal lamination.
(A-B) Schematic showing the expression pattern of heterotypic repulsive (mouse, A) and homotypic adhesive (chick, B) molecular cues across different laminae of the retina. Expression for Sema5A/5B revealed by in situ hybridization, and expression for all other molecules was determined by immunolabeling.
(C) Illustration showing aberrant lamination of mouse retinal cell types when semaphorin (Sema)-plexin (Plex) signaling is disrupted compared to wildtype retina (WT). KO: knockout, dKO: double knockout, M1 RGC: Type 1 melanopsin positive ganglion cell, DAC: dopaminergic amacrine cell, T2 BC: Type 2 OFF-cone bipolar cells, RBC: rod bipolar cell, HC: horizontal cell and SAC: starburst amacrine cell.
(D) Schematic showing disrupted dendritic lamination of R-cadherin (R-cad+) positive ganglion cell in the Dscam knockdown (by RNAi) retina, and unusual lamination of substance P positive (SP+) amacrine cells in sidekick1 (Sdk1) over-expressing (OE) chick retina.
Summarized from Matsuoka et al., 2011a; Matsuoka et al., 2012; Matsuoka et al., 2011b; Sun et al., 2013; Yamagata and Sanes, 2008, 2012; Yamagata et al., 2002.
Repulsive interactions via semaphorin-plexin signaling also act to specify sublamination of neurites in the IPL (Fig. 10C). Dopaminergic amacrine cells and their synaptic partner, M1 retinal ganglion cells, both stratify their dendrites in the outermost layer of the IPL. In the KO mice for Sema6A or its receptor PlexA4, both cell types develop additional arbors in the inner half of the IPL, where they still contact each other (Matsuoka et al., 2011b and see Fig. 10C). Sema6A is localized in the inner half of the IPL whereas PlexA4 is present in the outer half of the IPL that includes dopaminergic amacrine cell processes but not M1 retinal ganglion cell dendrites (Fig. 10A). Thus, in the absence of repulsive Sema6A–PlexA4 signaling, dopaminergic amacrine cell dendrites fail to be repelled from the inner half of the IPL. The ectopic dendrites of M1 retinal ganglion cells are likely a secondary consequence. Similarly, Sema6A-PlexA2 signaling is necessary for the proper stratification of starburst amacrine cell dendrites (Sun et al., 2013 and see Fig. 10C). In Sema6A or PlexA2 KOs, both ON- and OFF-starburst amacrine cell dendrites fail to segregate into separate bands (Fig. 10C). PlexA2 is expressed by ON- and OFF-starburst amacrine cells whereas Sema6A is expressed by ON- but not OFF-starburst amacrine cells. Thus, repulsive interactions between PlexA2 in OFF-starburst amacrine cells and Sema6A in ON-starburst amacrine cells segregate the dendrites of the two starburst amacrine cell populations.
Repulsive interactions mediated by semaphorins and plexins are not the only molecular mechanism known to play a role in sublamination of neuronal processes within the IPL. In the chick retina, a series of immunoglobulin superfamily (IgSF) adhesion molecules including Dscam, Dscam-like (DscamL), and Sidekick1 and 2 are expressed by non-overlapping subsets of amacrine cells and ganglion cells (Yamagata and Sanes, 2008; Yamagata et al., 2002 and see Fig. 10B, D). IgSF molecules mediate homophilic adhesion of pre- and postsynaptic partners. Amacrine cells and retinal ganglion cells that express the same IgSF member stratify within the same sublamina of the IPL, which is distinct from the lamination of cells expressing a different IgSF molecule. A recent study has expanded the list of IgSF molecules that specify the lamination patterns within the IPL. Contactins (1-5) are expressed in distinct IPL sublaminae in the chick retina; loss and gain of function analyses now place them in the family of molecules that regulate laminar specificity in the IPL (Yamagata and Sanes, 2012).
In summary, the retina adopts a combinatorial code that employs adhesive and repulsive cues to ensure the precise targeting of neuronal arbors of each retinal cell type within their synaptic layers (summarized in Fig. 10). Future studies are necessary to fully decipher this code.
3. Synapse structure and connectivity of retinal neurons
Much is now known about the overall structural and functional organization of mature retinal synapses and connectivity, but there are few circuits in the vertebrate retina for which we have complete connectivity maps and defined functions. Nevertheless, the recent availability of genetic tools and transgenic lines with labeled cell types (Ivanova et al., 2010; Kim et al., 2010; Siegert et al., 2012) together with technical advancements in imaging techniques is facilitating a rapid acquisition of potential wiring diagrams of identified retinal cell types (Briggman et al., 2011; Helmstaedter et al., 2013). Here, we will provide an overview of the known synaptic organization and connectivity patterns of the adult retina, and discuss the knowledge that has accrued thus far concerning the mechanisms that underlie their construction and maintenance.
3.1. Connections at the input layer of the adult retina
3.1.1. Synapse organization in the OPL
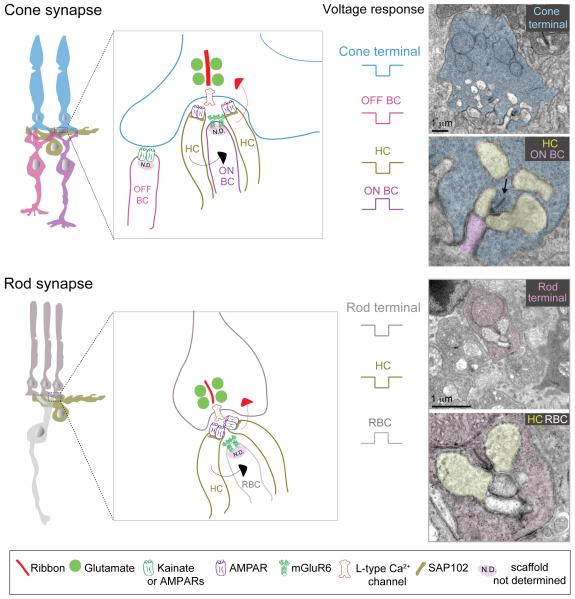
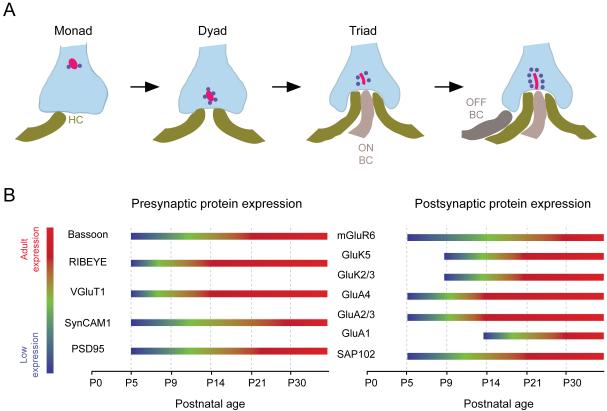
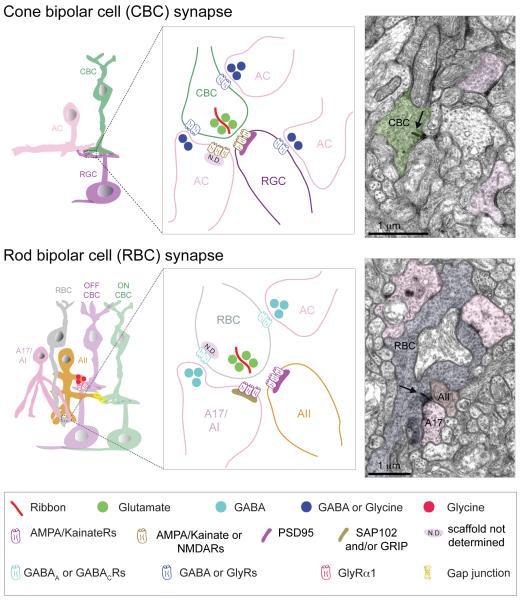
At the input layer of the retina, photoreceptor terminals synapse onto bipolar cells and horizontal cells in a ‘triad’ configuration (Fig. 11). Two horizontal cell dendritic tips invaginate the photoreceptor terminal and flank central bipolar cell dendritic tips. In general, ON-bipolar cell dendrites invaginate into photoreceptor terminals whereas OFF-bipolar cell dendritic terminals contact the base of the cone photoreceptor terminals or pedicles. At the site of the invagination is a specialized structure in the photoreceptor terminal called the ‘ribbon’, which is necessary for maintaining high rates of neurotransmitter release for sustained periods of time (Sterling and Matthews, 2005). The central component of the ribbon is a protein called RIBEYE (Magupalli et al., 2008; Schmitz, 2009). Synaptic vesicles are tethered to the ribbon near the transmitter release site (Fig. 11). Retinal ribbon synapses are also special in the type of presynaptic calcium channel (slowly inactivating L-type) they use which enables sustained neurotransmitter release, characteristic of these synapses (Heidelberger et al., 2005).
Figure 11. Synaptic connectivity at the OPL.
Schematics and ultrastructure of cone and rod photoreceptor synapses and receptor composition at each synapse type.
ON BC: ON-cone bipolar cell, OFF BC: OFF-cone bipolar cell, RBC: rod bipolar cell, HC: horizontal cell. Metabotropic glutamate receptors (mGluR6) on ON-bipolar cell dendrites mediate a hyperpolarization (sign-inverting) response to glutamate, whereas ionotropic glutamate receptors (AMPA and Kainate receptors) mediate a sign-conserving response in OFF-bipolar cells and horizontal cells. As different species or different OFF-bipolar subtypes express Kainate and/or AMPA receptor both are represented in the schematic, but OFF-bipolar cells in mouse and macaque retina primarily use Kainate receptors for signal transmission through the OPL (see text for details). Red arrow indicates negative feedback and black arrow indicates feedforward modulation. Electron micrographs of rod and cone photoreceptor terminals are from mouse retina. Arrow in electron micrograph points to a ribbon.
Rod photoreceptor axon terminals or spherules contain one ribbon, forming a single release site (Fig. 11). In contrast, cone pedicles have multiple ribbons forming separate release sites. The actual number of ribbons per pedicle differs across species. For example, zebrafish cone terminals contain 2-7 ribbons (Tarboush et al., 2012) whereas mouse cone pedicles have ~10 ribbons per pedicle (Tsukamoto et al., 2001). In macaque retina, the number of ribbon synapses per cone terminal depends on location. In central retina, each cone pedicle contains on average ~21 ribbons, whereas in peripheral retina cone terminals have ~42 ribbons per pedicle (Chun et al., 1996), indicating that peripheral cone photoreceptors have twice as many output sites compared to central cone photoreceptors. Together, these quantitative observations raise the question as to what cellular and molecular mechanisms are involved in defining the number of ribbons per cone pedicle during development, but the answer remains elusive.
On the postsynaptic side of the photoreceptor ribbon synapse, horizontal cells and OFF-bipolar cell dendrites use ionotropic glutamate receptors or iGluRs to sense glutamate release from photoreceptors (summary in Fig. 11). Their synapses are so-called ‘sign-preserving’ because like photoreceptors, horizontal cells and OFF-bipolar cells are depolarized at light offset. Mouse OFF-bipolar cell dendrites express AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and/or Kainate receptors and different proportions of AMPA (GluA1 subunit) and Kainate (GluK1 subunit) receptors have been localized on dendrites of distinct OFF-bipolar cell subtypes (Puller et al., 2013). However, recent imaging of glutamate release from OFF-bipolar cells in mouse retina and electrophysiological recordings from Type 4 OFF-bipolar cells have proposed that Kainate receptors are instrumental in mediating light-evoked responses of mouse OFF-bipolar cells (Borghuis et al., 2014). In other species like the ground squirrel, different OFF-bipolar cell types utilize AMPA or Kainate receptors. The OFF response of the Type 1 and 3 bipolar cells largely relies on Kainate receptors whereas the Type 2 cell is entirely dependent upon AMPA receptors (Lindstrom et al., 2014). AMPA receptors, however, appear to be the dominant receptors in salamander retina (Cadetti et al., 2005; Maple et al., 1999). In zebrafish, AMPA receptor subunit GluA4 is observed on OFF-bipolar dendrites innervating both rod and cone terminals (Klooster et al., 2009). At cone pedicles of the macaque retina, basal contacts of OFF-cone bipolar cells have been shown to contain GluA1-subunit containing AMPA receptors (Haverkamp et al., 2000), GluK1 and GluK2/3-subunit containing Kainate receptors and GluA2/3 and 4 subunits of AMPA receptors (Calkins, 2005; Haverkamp et al., 2000, 2001a). Recent functional recordings in macaque retina have demonstrated that the OFF-bipolar cell types that feed onto the major ganglion cell types (both midget and parasol ganglion cells) use Kainate receptors, albeit of heterogeneous composition (Puthussery et al 2014). Horizontal cell processes contain iGluRs with a predominance of AMPA (GluA2/3 and 4 subunits) receptors in macaque (Calkins, 2005; Haverkamp et al., 2000, 2001a, b). In zebrafish, immunoreactivity for the AMPA receptor GluA2 is observed in horizontal processes invaginating both rod and cone photoreceptor terminals (Klooster et al., 2009). Taken together, horizontal cell processes appear more dependent upon AMPA receptors for signal processing at the OPL, whereas OFF-bipolar dendrites (depending on species and subtype) rely with different weights on Kainate and AMPA receptors; Kainate receptors predominantly mediating transmission onto OFF-bipolar cells in mouse and macaque retina.
In contrast to OFF-bipolar cells and horizontal cells, the dendritic tips of ON-bipolar cells have metabotropic GluRs (mGluRs), predominantly mGluR6 (summary in Fig. 11). Binding of glutamate to mGluR6 triggers a signaling cascade that reverses the polarity of the signal transmitted from photoreceptors. Hyperpolarization of photoreceptors elicits a sign-inverted depolarizing ‘ON’ response from this bipolar cell type. mGluR6 receptors are present on the dendrites of both ON-cone bipolar cells and rod bipolar cells (Dunn et al., 2013; Nomura et al., 1994; Slaughter and Miller, 1981; Vardi et al., 2000). Bipolar cell dendrites in zebrafish have a more complex arrangement of glutamate receptors; with both mGluRs and NMDA (N-methyl-D-aspartate) receptors located on the dendrites of zebrafish ON-bipolar cells (Klooster et al., 2009). Similarly, in macaque retina complex interactions are involved during glutamate signaling onto ON-bipolar cells at the OPL. Pre-embedding immunogold labeling has demonstrated both AMPA (GluA2/3 and GluA4) and Kainate (GluK2/3) receptors at invaginating ON-bipolar dendrites, although at a lesser density compared to mGluRs (Calkins, 2005). Moreover, gene expression analysis by single cell PCR has shown that OFF-cone bipolar cells in macaque retina also express glutamate transporters in addition to AMPA or Kainate receptors (Hanna and Calkins, 2007). Thus, in many instances, glutamatergic transmission onto bipolar cells is mediated by a combination of receptors and/or transporter systems.
Although it is becoming increasingly apparent what types of glutamate receptors are present on specific bipolar cell and horizontal cell types, the scaffolds anchoring these receptors in place are largely unidentified. In the brain, PSD95 (postsynaptic density protein 95) is a major scaffolding protein localized to glutamatergic synapses. PSD95 is also found in mammalian OPL but it is localized presynaptically at photoreceptor terminals (Koulen et al., 1998a), and its function remains unclear. The proteins that provide a scaffold for the GluRs at bipolar cell dendrites thus remain largely unknown. However, the synaptic scaffold protein SAP102 (synapse-associated protein 102) has been found specifically at horizontal cell processes in the OPL in rat and macaque retina and may mediate clustering of iGluRs at horizontal cell processes (Haverkamp et al., 2000; Koulen et al., 1998b). Thus, compared to synapses elsewhere in the brain, much less is known with regards to the molecular composition of both the pre- and postsynaptic sites at the input layer of the retina.
Horizontal cells modulate synaptic transmission in the OPL but the exact mechanism is highly debated. There are different mechanisms by which horizontal cell activation (hyperpolarization) can in turn lead to suppression of photoreceptor activity causing them to depolarize (negative feedback) (for recent review see Thoreson and Mangel, 2012). One pathway relies on release of GABA from horizontal cells (Cueva et al., 2002; Haverkamp et al., 2000; Hirano et al., 2011; Jellali et al., 2002) that can act upon GABA receptors located on photoreceptor terminals (Pattnaik et al., 2000). This pathway can also mediate feedforward inhibition by activating GABA receptors on bipolar cell dendrites (Haverkamp et al., 2000). Other feedback pathways are: (i) non-synaptic ‘ephaptic’ route involving the presence of hemichannels (Kamermans and Fahrenfort, 2004; Vroman et al., 2013), (ii) pH changes in the synaptic cleft surrounding photoreceptor terminals (Davenport et al., 2008; Vessey et al., 2005) or (iii) the autaptic action of GABA on horizontal cell GABA receptors (Liu et al., 2013). Much work remains to be carried out to identify the mechanism or mechanisms of horizontal cell action, which may vary across species (e.g. mammals versus zebrafish).
3.1.2. Connectivity patterns of the OPL
Transmission from photoreceptors occurs along two distinct pathways. In mouse and monkey retina, rod bipolar cells largely contact rod photoreceptors, and cone bipolar cells contact cone photoreceptors. However, some mouse OFF-cone bipolar cells additionally receive input from rod photoreceptors (Soucy et al., 1998; Tsukamoto et al., 2001). Some bipolar cells, depending on the location in the retina, may only contact a specific subset of cone photoreceptors, and as mentioned earlier, can form a ‘private line’. In macaque retina, signals from L and M cones are integrated by the dendrites of ‘diffuse’ and ‘midget’ bipolar cells (Boycott and Wässle, 1999), whereas ‘S cone’ bipolar cells contact exclusively S cone photoreceptors (Dacey, 2000; Dacey et al., 2014; Kouyama and Marshak, 1992; Miyagishima et al., 2014). An S cone selective bipolar cell has also been observed in mouse retina (Haverkamp et al., 2005) and patch-clamp recordings have demonstrated blue ‘ON’ responses from this bipolar cell subtype (Breuninger et al., 2011). Bipolar cell-photoreceptor connectivity maps are even more complex in zebrafish retina where 18 different connectivity maps have been identified thus far (Li et al., 2012). In zebrafish retina, there are no bipolar cells that exclusively or predominantly receive input from rod photoreceptors (Li et al., 2012). Like monkey or mice, the functional implications of these diverse connectivity patterns between bipolar cells and photoreceptors in zebrafish are currently being investigated (Baden et al., 2011; Dreosti et al., 2011).
Like the bipolar cells, connectivity maps of horizontal cells can be disparate both across and within a species (Fig. 2B). Monkey and zebrafish retina have different subtypes of horizontal cells. In macaque retina two types of horizontal cells (HI and HII) have been observed (Dacey, 1999; Wässle et al., 1989). The HII cells show selectivity for S cones (stronger input from S compared to L and M cone contacts), whereas the HI cells avoid contact with S cones and have input only from L and M cones (Dacey et al., 1996; Goodchild et al., 1996). The ‘axon’ terminal of HI horizontal cells in macaque contact rod photoreceptors, making the HI cells responsive to both rod and cone signals (Verweij et al., 1999). Mouse retina has only one horizontal cell type – the B-type horizontal cell whose dendrites contact cone photoreceptors and axon contacts rod photoreceptors (Peichl and Gonzalez-Soriano, 1994; Trumpler et al., 2008). In contrast to horizontal cells in mice and monkey, zebrafish horizontal cells do not separately connect with rods and cones at distinct cellular compartments (‘axon’ versus ‘dendrite’). Zebrafish retina has four types of horizontal cells – three for cones (H1-3) and one horizontal cell that exclusively samples rods (Li et al., 2009). Each subtype of zebrafish cone horizontal cell displays a specific connectivity map with cone photoreceptors. H1 horizontal cells contact L, M and S cones, whereas H2 horizontal cells restrict their contact to M, S and UV cones. The H3 horizontal cell obtains input from only short-wavelength S and UV cones (Li et al., 2009). It remains to be determined how the stereotypic wiring pattern of each horizontal cell type is established during development.
In addition to understanding how the outer retinal circuitry is established during development, the diverse connectivity patterns of the OPL offer a rich variety of circuits for investigating the developmental strategies responsible for generating stereotypic connectivity maps in central nervous system circuits in general. In the next section, we will review what is currently known about how synapses in the OPL are assembled at the molecular, cellular and circuit levels.
3.2. Assembly of outer retinal circuits
3.2.1. Sequence of synapse formation in the OPL
Electron microscope (EM) studies of the mouse retina have long portrayed the sequence of synapse assembly in the OPL (Blanks et al., 1974; Olney, 1968; Rich et al., 1997; Sherry et al., 2003). Synaptogenesis begins with photoreceptor terminals making a ‘monad’ contact with a single horizontal cell process (Fig. 12A). Soon after, a ‘dyad’ synapse is formed when another horizontal cell process is recruited into the synaptic complex (Fig. 12A). At this stage, the horizontal cell processes begin to invaginate into the photoreceptor terminal. A ‘triad’ synapse emerges when the ON-bipolar cell dendrite inserts between the horizontal cell tips within the photoreceptor terminal (Fig 12A). This step completes photoreceptor triad synapse formation. OFF-bipolar cell dendrites are thereafter presumed to make synaptic contacts (see below) at the base of the photoreceptor pedicle, although this has not been confirmed by ultrastructural analysis. The sequence of photoreceptor synapse assembly is summarized in Figure 12A.
Figure 12. Assembly of OPL synapses.
(A) Schematic illustrating the formation of the photoreceptor triad at the OPL. Horizontal cells (HCs) contact photoreceptors first, followed by dendrites of ON-bipolar cells (ON BC) and later by the dendrites of OFF-bipolar cells (OFF BC). Ribbons and associated vesicles are shown in red and purple.
(B) Relative expression levels of pre- and postsynaptic proteins in the OPL at different time-points from immunohistochemistry of rodent retina (summarized from: Dick et al., 2003; Dunn et al., 2013; Guo et al., 2009; Hack et al., 2002; Johnson et al., 2003; Koulen, 1999; Nomura et al., 1994; Regus-Leidig et al., 2009; Ribic et al., 2014). The color gradients are representative of the total expression of the synaptic proteins, rather than their distribution pattern.
Structural development of the OPL is paralleled by expression of pre- and postsynaptic proteins found at the photoreceptor synapses (summarized for rodent retina in Fig 12B). In the OPL, expression of the vesicular glutamate transporter VGluT1 at photoreceptor terminals is observed before expression of the vesicular transporter for inhibitory (GABA/Glycine) neurotransmitters, vesicular inhibitory amino acid transporter or VIAAT. Immunolabeling for VGluT1 already shows expression around P3 in the mouse OPL (Johnson et al., 2003). The onset of VIAAT expression in the developing mouse OPL occurs around P5-P7, which reaches adult levels by P14 (Guo et al., 2009). VGluT1 is first detected in cone photoreceptor terminals in the mouse retina (~P2), well before it is present in rod photoreceptor terminals (~P8) (Sherry et al., 2003). These observations imply that cone excitatory pathways develop before rod pathways, and suggest that excitatory transmission precedes inhibitory transmission at the OPL (Fig. 12B).
Ribbon synapse proteins in the OPL appear after the onset of photoreceptor transmission mediated by vesicular release of glutamate (Fig. 12B). RIBEYE expression in rodent OPL is detectable between P4-P6 and reaches adult levels around P14 (Regus-Leidig et al., 2009). The ribbon anchoring protein, bassoon, is present at the rodent OPL around P4-P6 after which its expression increases rapidly to attain adult levels by three weeks after birth (Dick et al., 2003; Regus-Leidig et al., 2009). The assembly of the photoreceptor ribbon synapse in rodent retina occurs in two steps: first with the transport of the core ribbon proteins RIBEYE and Bassoon, followed by the second step, expression of the L-type calcium channel subunits (Regus-Leidig et al., 2009). At the EM level, the presence of RIBEYE containing ‘precursor spheres’ is the first sign of ribbon synapse development, followed by the appearance of immature (floating) ribbons, and ending with the presence of anchored (mature) ribbons that account for ~91% of all ribbons at maturity (Regus-Leidig et al., 2009).
The cone pedicle connections have also been extensively studied in monkey retina (Boycott and Wässle, 1999; Hopkins and Boycott, 1997). In macaque retina, the timeline for the formation of photoreceptor ribbon synapses is distinct in the fovea compared to peripheral retina. Cone photoreceptor ribbon synapses can be observed as early as Fd 60 in foveal OPL, whereas in peripheral OPL photoreceptor ribbon synapses can be observed only at Fd 105 (Hendrickson, 1996). Labeling for the synaptic vesicle protein SV2 corresponds closely to the appearance of morphological synapses in monkey retina as observed by EM (Okada et al., 1994). Accordingly, SV2 labeling can be observed around Fd 60 in foveal OPL and only around Fd119-125 in peripheral retina, with cones showing SV2 labeling before rods at the same retinal eccentricity (Okada et al., 1994). Thus similar to development in rodent retina cone photoreceptor synapses emerge before rod photoreceptor synapses in the monkey retina. In zebrafish retina, however, both rod and cone synaptic terminals develop at about the same time (Schmitt and Dowling, 1999).
On the postsynaptic side of the photoreceptor ribbon, ON-bipolar cell dendrites accrue mGluR6 at their dendritic tips with a corresponding time-course, and seem to organize their synapses at the photoreceptor triad in parallel with presynaptic ribbon synapse assembly. Onset of mGluR6 expression in the OPL occurs between P5-P8 in rodent OPL (Nomura et al., 1994). Analysis of the development of cone photoreceptor contacts onto individual mouse ON-cone bipolar cells recently demonstrated that during development, the ON-bipolar cells specifically increase their mGluR6 allocation to dendritic terminals apposed to cone photoreceptors, while reducing non-cone associated mGluR6 amounts before reaching adult levels around P30 (Dunn et al., 2013). Thus, mGluR6 localization to mouse ON-bipolar cell synapses with cones is a gradual process that appears to be finalized over the course of days to weeks. Because receptor distributions have not been mapped for OFF-bipolar cell dendrites, it remains to be determined how OFF-bipolar cells develop their synapses with photoreceptors.
Likewise, the formation of pre- and postsynaptic specializations at the horizontal cell-photoreceptor synapses in the OPL has not yet been followed in detail during development. However, it has been shown that the establishment of different iGluRs in the OPL follows a defined sequence in rodent retina. Most AMPA (GluA2/3 and 4 subunits) receptors are expressed before Kainate (GluK2/3 and GluK5 subunits) receptors (Hack et al., 2002 and see Fig. 12B). As mentioned earlier, horizontal cells form synaptic contacts at photoreceptor terminals before bipolar cell dendrites (Blanks et al., 1974). Accordingly, AMPA receptors (GluA 2/3 and 4) associated with horizontal cells are expressed earlier in the OPL compared to GluA1 AMPA receptors or Kainate receptors at OFF-cone bipolar cell dendrites. GluA1 expression in the OPL commences only after P10 in rodent retina whereas GluA2/3 and GluA4 can be detected as early as P3 (Hack et al., 2002). Thus, OFF-bipolar cell dendrites may acquire their iGluRs later compared to the timing of mGluR6 expression in ON-cone bipolar cell dendrites. Postsynaptic scaffolding proteins are present in the OPL around P5. SAP102 can be detected at P5 in horizontal cells at the outer most part of the developing rodent OPL and by P8, the expression resembles that of the adult retina (Koulen, 1999). Adult expression level of presynaptic PSD95 at photoreceptor terminals is achieved later than SAP102, at around P10 in rodent retina (Koulen, 1999).
Thus far, it is evident that rod and cone, and possibly ON and OFF synapses, are established with different time courses in the OPL. It has recently been shown that the developmental process can vary even amongst the same major bipolar cell type. An analysis of cone-bipolar cell circuits in mouse retina revealed that distinct ON-cone bipolar cell types establish synaptic connectivity with cone photoreceptors at different developmental stages and with different strategies. Formation of cone photoreceptor contacts with three different ON-cone bipolar cell types (Type 6-8) was examined in a double transgenic mouse line in which both pre- (cone) and postsynaptic (bipolar cell dendrites) partners were labeled (Dunn and Wong, 2012). Type 8 bipolar cell dendrites showed the maximum level of pruning of ‘immature’ cone contacts, whereas Type 6 bipolar cells displayed little pruning of the cone contacts established during early development. These results imply that Type 6 ON-bipolar cell dendrites use a ‘targeted’ approach to achieve their cone photoreceptor input, whereas Type 8 ON-bipolar cell dendrites rely on an ‘exploratory’ strategy (Dunn and Wong, 2012). Type 7 bipolar cells displayed an intermediate behavior. Though these three ON-bipolar cell subtypes share the same presynaptic cone partner, they each settle on their mature connectivity pattern at different developmental stages, with Type 8s altering their cone contacts well beyond P30 (Dunn and Wong, 2012). Whether the different OFF-bipolar cell types also vary in the developmental time courses over which they each establish their mature wiring patterns with cones remains to be determined. Furthermore, the developmental sequence of ON- and OFF-bipolar cell dendrites contacting the same cone photoreceptor terminal is yet to be explored. Because iGluRs appear on OFF-bipolar cell dendrites relatively late in development, ON-bipolar cells may contact the cone photoreceptor before OFF-bipolar cell dendrites elaborate and form basal contacts. Future time-lapse, live-imaging studies would help us visualize this developmental event in the OPL.
3.2.2. Developmental mechanisms that organize OPL synapse assembly
There have been major advances in identifying the molecular factors essential for the accurate establishment of the photoreceptor triad synapse (Fig. 13). One group of molecules central to the proper assembly of photoreceptor triads are ribbon-associated proteins. Loss of the ribbon-associated protein bassoon in the mouse retina leads to unanchored or ‘floating’ photoreceptor ribbons, disturbed photoreceptor output and a concomitant extension of bipolar cell and horizontal cell processes into the ONL, where they form aberrant synapses (Dick et al., 2003 and see Fig 13A-B for bipolar cell deficits). Synaptojanin plays a similar role as bassoon at zebrafish cone photoreceptor terminals (Holzhausen et al., 2009; Van Epps et al., 2004). Recent studies have also uncovered a role for adhesion proteins in the assembly of the photoreceptor triad synapse. A rod terminal-specific adhesion protein (SynCAM1) is important for the maturation of the rod ribbon synapse, because loss of SynCAM1 leads to shorter rod photoreceptor ribbons and development of fewer rod photoreceptor synapses (Ribic et al., 2014 and see Fig. 13C). Similarly, loss of the anchoring protein, dystroglycan, prevents rod bipolar cell dendrites from invaginating the rod terminal (Omori et al., 2012 and see Fig. 13A). A comparable disturbance is observed upon elimination of the extracellular matrix protein pikachurin that serves as the binding partner for dystroglycan (Sato et al., 2008). Adhesion proteins are also important for formation of horizontal cell synapses in the OPL. Eliminating the synaptic adhesion protein netrin-G ligand2 (NGL-2) localized on mouse horizontal cell axon processes leads to disrupted horizontal cell axon morphology and a reduction in the formation of synapses between horizontal cells and rod photoreceptors (Soto et al., 2013 and see Fig. 13C).
Figure 13. Mechanisms regulating synapses at the OPL.
Schematic summary of the role of synapse organizing molecules in the mouse retina that establish connectivity between: (A) rod photoreceptors (Rod) and rod bipolar cells (RBC), (B) cone photoreceptors (Cone) and ON cone bipolar cells (ON BC) and (C) rod photoreceptors and horizontal cells (HC) (summarized from: Dick et al., 2003; Dunn et al., 2013; Omori et al., 2012; Ribic et al., 2014; Sato et al., 2008; Soto et al., 2013). The presence of mGluR6 on cone bipolar cell (CBC) dendrites in the bassoon KO has not been determined and is thus represented with a question mark.
Apart from molecular cues instructing assembly of synapses in the OPL (summary from work in rodent retina in Fig. 13), photoreceptor function and transmission also contribute to the organization and maintenance of these synapses. Disruption of the photoreceptor cyclic nucleotide channel in mouse retina leads to sprouting of horizontal cell and bipolar cell processes into the ONL (Michalakis et al., 2013). Synapse maturation, but not development, appears to be dependent on expression of the L-type calcium channel by photoreceptor terminals. Loss of this calcium channel disrupts the maintenance of bassoon at the photoreceptor ribbon synapse. Additionally, altered expression of photoreceptor terminal scaffolding protein PSD95 but not vesicle-associated proteins can be observed in these retinae (Zabouri and Haverkamp, 2013). Because most of these anomalies appear in the mouse retina only after eye-opening (~P14), the action of this calcium channel seems to be needed for the maintenance but not formation of triad synapses in the OPL.
Glutamatergic neurotransmission is established in the OPL before ribbon synapses are fully organized at photoreceptor terminals. Because transmission requires glutamate to be taken up into synaptic vesicles by VGluT1 at photoreceptor terminals, it may be that synaptogenesis in the OPL is perturbed in VGluT1-deficient retina (Johnson et al., 2007). Whether VGluT1 directly impacts synaptogenesis remains undetermined, because the structural development of the photoreceptor triad synapse in the absence of VGluT1 has not yet been examined. The role of glutamate-mediated transmission has, however, been examined extensively in mouse mutants lacking mGluR6 on the dendrites of ON-bipolar cells. The loss of mGluR6 leads to an upregulation of mGluR7 in Type 7 ON-bipolar cell dendrites that are clustered opposite ectopic synaptic ribbons of rod photoreceptor terminals (Tsukamoto et al., 2007). These observations of abnormal synaptic arrangements in the OPL underscore the importance of glutamate-mediated signaling onto ON-bipolar cell dendrites for the development of the OPL synaptic circuitry.
More recently, an unexpected role for visually-driven activity in shaping cone photoreceptor to ON-cone bipolar cell connections was revealed (Dunn et al., 2013 and see Fig. 13B). Dark-reared mice exhibit a loss of mGluR6 on ON-cone bipolar cell (Type 6, 7 and 8) dendrites apposed to cone photoreceptors, which in turn leads to a reduction in the synaptic drive to these cells. Depriving the ON-cone bipolar cell dendrites of visually-driven signals from cone photoreceptors also disrupts their normal developmental program of increasing cone-associated mGluR6 and reducing non-cone associated mGluR6. Consequently, ON-cone bipolar cell dendrites from dark-reared retinas maintain immature (low) levels of non-cone associated mGluR6 (Dunn et al., 2013). Dark-rearing additionally leads to a reduction in the number of cones contacted by Type 7 and 8 cone bipolar cells, but does not alter the Type 6 bipolar cells’ cone contacts (Dunn et al., 2013). As discussed in 3.2.1., ON-cone bipolar cell subtypes establish their cone connections at different developmental ages (Dunn and Wong, 2012): Type 6 before eye-opening and Type 7 and 8 ON-cone bipolar cells weeks after eye-opening. This implies that cone bipolar cells that establish their mature synaptic connectivity late in development may need visual stimulation to attain their connectivity pattern.
The inhibitory neurotransmitter GABA has also been implicated in regulating the maturation of cone photoreceptor synaptic triads. In mice lacking the GABA-synthesizing enzyme GAD67 in their retina, cone pedicle area is increased and the level of mGluR6 associated with each pedicle also increases (Schubert et al., 2010). These results indicate that GABA might control two aspects of photoreceptor synapse development: (i) synapse structure, by affecting the size of the synaptic contact, and (ii) synapse function, by regulating the amount of receptors on bipolar cell dendrites. However, the disruptive effects of reducing GABAergic transmission on photoreceptor synapses are small compared to the effect of the loss of glutamate transmission from photoreceptors.
In summary, both activity-dependent and independent mechanisms (Fig. 13) orchestrate the development and maturation of synapses at the OPL, with different OPL circuits relying on these mechanisms to varying degrees.
3.3. Connections at the output layer of the adult retina
Excitation and inhibition are coordinated in the inner retina to generate the output of retinal ganglion cells. Synapses that provide excitatory or inhibitory drive are highly organized amongst bipolar cells, amacrine cells and ganglion cells in order to generate stereotypic connectivity patterns and function unique to each subcircuit of the inner retina. In this section, we will describe the molecular composition of excitatory and inhibitory synapses in the mature IPL, and the overall structural and functional organization of the circuits in this synaptic layer. We will then review the current knowledge of how circuits in the IPL are assembled.
3.3.1. Excitatory synaptic connections
Like photoreceptor terminals, bipolar cell axons also form ribbon synapses (Fig. 14). Bipolar ribbon synapses, however, differ in composition from photoreceptor ribbon synapses (see Heidelberger et al., 2005). Although like photoreceptors, bipolar cell ribbons contain RIBEYE (Schmitz, 2009), they do not express bassoon (Brandstatter et al., 1999). On the postsynaptic side of the bipolar cell ribbon, sign-conserving iGluRs mediate amacrine cell and retinal ganglion cell responses (summarized in Fig. 14). AMPA, Kainate and NMDA receptors have been found on both amacrine cells and retinal ganglion cells (for review see Yang, 2004). However, functional recordings have shown that only AMPA and NMDA receptors contribute to retinal ganglion cell responses (Chen and Diamond, 2002; Lukasiewicz et al., 1997). In macaque retina, immunolabeling for specific subunits of glutamate receptors revealed that ganglion cells preferentially express AMPA (GluA2/3 and 4) and NMDA receptors. In contrast, amacrine cells primarily have Kainate (GluK2/3) and orphan GluRs (with δ1/2 subunits GluD1/2) (Grunert et al., 2002). In rat retina, amacrine cells receive excitatory input from bipolar cell terminals at iGluR synapses but different amacrine cell subsets express different compositions of iGluR (Nivison-Smith et al., 2013). Recordings in mouse retina have revealed that the majority of amacrine cells show both AMPA and Kainate receptor-mediated responses, with a small fraction showing only AMPA or only Kainate-mediated responses (Dumitrescu et al., 2006).
Figure 14. Synaptic connectivity in the IPL.
Schematics showing basic organizations of cone bipolar cell (CBC) and rod bipolar cell (RBC) synapses. Neurotransmitter receptor types are shown. AC: amacrine cell, RGC: retinal ganglion cell. Only AI/A17 and AII amacrine cells are postsynaptic to RBCs. Examples of the ultrastructure of bipolar and amacrine cell synapses in mouse retina are provided in the electron micrographs. Arrow indicates ribbon.
Another feature that is different between photoreceptor and bipolar cell synapses is that the scaffolding protein PSD95 is localized to glutamatergic postsynaptic sites on retinal ganglion cells (Morgan et al., 2008, schematic in Fig. 14). Not in the schematic are MAGI proteins that have also been shown to be present at synaptic sites along with other scaffolding proteins (Yamagata and Sanes, 2010). In the rodent retina, SAP102 associates predominantly with non-NMDA iGluRs at one of the postsynaptic partners of the cone bipolar cell terminal (Koulen et al., 1998b), but proteins scaffolding other GluRs at amacrine cell processes have not yet been fully determined. It is interesting to note that at the IPL, PSD95, SAP102 and PSD93 are colocalized at synaptic clusters (Koulen et al., 1998a) but it remains to be seen whether all these scaffolds are together necessary for maintaining the IPL GluRs, or whether there is a redundancy in their function.
A synapse arrangement that is conserved across mammalian retina is that rod bipolar cells do not contact retinal ganglion cell dendrites directly but instead output onto two amacrine cell processes, forming a ‘dyad’ synapse (Fig. 14) (Bloomfield and Dacheux, 2001; Strettoi et al., 1990). This well-studied circuit comprises a glycinergic AII amacrine cell and a GABAergic A17 amacrine cell. iGluRs on AII and A17 amacrine cell processes ensure a sign-preserving transmission of signal, but distinct from cone bipolar cell synapses at the IPL, NMDA receptors have not been found to signal rod bipolar cell release at the IPL (Fletcher et al., 2000). In macaque retina, AII amacrine cells postsynaptic to rod bipolar cell terminals have been found to express AMPA receptors (GluA2/3 and 4) clustered together with PSD95 (Ghosh et al., 2001). The presence of Kainate (GluK2/3) and ionotropic δ1/2 containing GluRs at single postsynaptic sites apposed to rod bipolar cell dyads (Brandstatter et al., 1997), and localization with GluR interacting (scaffolding) protein GRIP has indicated the presence of δ1/2 GluRs together with Kainate receptors and GRIP at the postsynaptic A17 amacrine cell (Ghosh et al., 2001). In rodent retina SAP102 is localized at A17 but not at AII processes (Koulen et al., 1998b). It thus seems likely that the two amacrine cell types that are postsynaptic to rod bipolar cell terminals have distinct GluRs (summarized in Fig. 14) with unique scaffolding machineries, which could be related to the distinct functional responsibilities of these two amacrine cell types as discussed in the next section.
3.3.2. Inhibitory synaptic connections
Inhibition by diverse amacrine cell populations modulates information flow through the IPL. About half of the amacrine cells in the mammalian retina are wide-field GABAergic amacrine cells (Pourcho and Goebel, 1983; Wässle and Boycott, 1991) whereas the other half are small-field glycinergic amacrine cells (Pourcho and Goebel, 1985, 1987; Pow et al., 1995; Wässle et al., 1986). A recent study, however, revealed that there are amacrine cells that are neither GABAergic nor glycinergic (Kay et al., 2011). Within the GABAergic and glycinergic populations exist many morphological and functionally distinct amacrine cell types. AII amacrine cells are the most abundant, as they comprise about 11% of all the amacrine cells in the retina of rabbits and other mammals (Strettoi and Masland, 1996). No single amacrine cell type predominates amongst the others, and most subtypes comprise less than 5% of the total amacrine cell population (MacNeil and Masland, 1998).
Not only does the diversity of presynaptic amacrine cell types provide many possible combinations of inhibitory connections, a wide range of functionally disparate inhibitory receptors on the postsynaptic cells also adds to the complexity of inhibition provided by these interneurons. This is because inhibitory receptors with different subunit compositions confer distinct temporal characteristics on the inhibitory responses of the postsynaptic cells. Inhibition provided by amacrine cells is considered as either ‘feedforward’ i.e. amacrine cell-to-amacrine cell or amacrine cell-to-retinal ganglion cell, or as ‘feedback’ i.e. amacrine cell-to-bipolar cell axons. Amacrine cell connections can thus be broadly categorized into three types:
(i) Amacrine –> Ganglion cell inhibition
All retinal ganglion cells receive direct inhibition from amacrine cells (Wässle et al., 1998) but the exact functions of these connections are largely unknown except for a few circuits. The DS retinal ganglion cells respond to motion of a stimulus in one direction (preferred direction) but not to motion in the opposite direction (null direction) (Barlow and Hill, 1963; Vaney et al., 2012). Inhibition from starburst amacrine cells is crucial for generation of this direction-selective response (Taylor and Vaney, 2003). In particular, asymmetric GABAergic inhibition is generated in the null-direction motion to generate direction-selectivity (Briggman et al., 2011; Fried et al., 2002; Zhou and Lee, 2008). Starburst amacrine cells in the mouse retina contact dendrites of DS retinal ganglion cells at synapses containing GABAAα2 receptors (Auferkorte et al., 2012). Another class of ganglion cells called ‘approach sensitive ganglion cells’ have recently been characterized in mouse retina, and their responses rely on rapid amacrine cell-mediated inhibition that suppresses response to non-approaching objects (Munch et al., 2009). It should be noted that individual retinal ganglion cells can express GABAA receptor clusters with distinct subunits, but the functional basis for such diversity has not yet been found. One idea is that each receptor type represents contact with a specific amacrine cell type (Wässle et al., 1998), but evidence in support of this notion is still needed. The functional significance of such receptor diversity has, however, been investigated in amacrine cell -> bipolar cell connections (see below).
(ii) Amacrine –>Amacrine inhibition
Amacrine cells also synapse onto processes of other amacrine cells and modulate their activity. For example, dopaminergic amacrine cells are presynaptic to other amacrine cells in the IPL (Kolb et al., 1991; Pourcho, 1982). In particular, dopaminergic amacrine cells form GABAergic synapses (specifically at GABAAα3 receptor clusters) onto the cell bodies of AII amacrine cells (Contini and Raviola, 2003). Dopaminergic amacrine cells themselves express multiple GABA receptor subunits (Gustincich et al., 1999) at sites of amacrine cell input (Kolb et al., 1991). Electrophysiological recordings from dopaminergic amacrine cells in mouse retina revealed that different temporal phases of their light-response profile are modulated separately by glycine and GABAA- (GABAAα1 and GABAAα3 receptor synapses), but not by GABAC-mediated inhibition (Newkirk et al., 2013). Another subclass of GABAergic amacrine cell, the catecholaminergic amacrine cell, also receives robust GABAA-and glycinergic inhibitory input; blocking inhibition altogether causes oscillation in the activity of these cells (Knop et al., 2011). These examples indicate that amacrine cell-amacrine cell inhibition serves to shape the temporal response profiles of their target cells.
(iii) Amacrine –> Bipolar cell inhibition
A well-studied form of inhibition in the inner retina is amacrine cell modulation of glutamate release from the axon terminals of bipolar cells. Both rod and cone bipolar cells receive such ‘presynaptic’ inhibition. The composition of the inhibitory receptors on bipolar cell terminals, however, varies across bipolar cell types. GABAC-mediated inhibition predominantly shapes response properties of ON- (rod and cone) but not OFF-cone bipolar cells, whereas glycinergic inhibition is most pronounced on OFF-bipolar cell terminals (Eggers and Lukasiewicz, 2011; Eggers et al., 2007; Ivanova et al., 2006). In particular, glycine receptors (GlyR) containing α1-subunits (GlyRα1) are localized on rat OFF-bipolar cell terminals (Sassoe-Pognetto et al., 1994; Wässle et al., 2009a).
The best understood amacrine cell-> bipolar cell connection is that involving the rod bipolar cell and the A17 amacrine cell (see Fig. 14). The A17 amacrine cell not only forms an inhibitory feedback synapse onto the rod bipolar cell terminal (Grimes et al., 2010; Zhang et al., 2002) but it is a reciprocal synapse i.e. the A17 receives input from the rod bipolar cell at the bouton where it releases GABA. Immunolabeling studies have revealed that rod bipolar cell axon terminals contain three subsets of ionotropic GABA receptors: two distinct kinds of GABAA receptors containing α1 or α3 subunits and GABAC receptors composed of ρ-subunits (Fletcher et al., 1998; Schubert et al., 2013). These distinct GABA receptor subsets have disparate response kinetics (for example rise time, response duration) and thereby differentially modulate release from rod bipolar cell terminals (Eggers and Lukasiewicz, 2006a, b; Gingrich et al., 1995; Ortinski et al., 2004).
The AII amacrine cell receives input from rod bipolar cells and is central to providing cross-over inhibition between the ON and OFF pathways (Fig. 14). This amacrine cell type is not presynaptic to any other amacrine cell; in the OFF sublamina of the IPL the AII amacrine cell makes conventional glycinergic synapses onto the axon terminals of OFF-cone bipolar cells that transmit to OFF-retinal ganglion cells (Bloomfield and Dacheux, 2001; Strettoi et al., 1992). The AII amacrine cell makes sign-conserving gap junctions (for review on gap junctions see Volgyi et al., 2013) with the axon terminals of ON-cone bipolar cells that in turn output onto ON-retinal ganglion cells (Bloomfield and Dacheux, 2001). Accordingly, in the fovea of macaque retina where there are no rod photoreceptors, AII amacrine cells are also excluded from the circuit (Kolb et al., 2002).
What proteins anchor inhibitory synapses on bipolar cell terminals? It is likely that these scaffolding proteins differ amongst inhibitory receptor types, and perhaps bipolar cell types, but such proteins have not yet been fully identified. For GABAC receptors, the microtubule-associated protein 1B (MAP1B) has been found to interact with ρ-subunits (Hanley et al., 1999). The inhibitory receptor scaffolding proteins Gephyrin and Neuroligin2 do not colocalize with GABAA receptors on rod bipolar cell terminals (Schubert et al., 2013). Indeed, rat OFF-bipolar cell terminals express GlyRα1 but not gephyrin (Sassoe-Pognetto et al., 1994). Thus, unlike GlyRs in the rest of the central nervous system (Kirsch and Betz, 1993), GlyRα1 synapses on bipolar cell terminals might recruit a gephyrin-independent mechanism for clustering or express a splice variant of gephyrin which is not recognized by the antibody.
In summary, a myriad of inhibitory synapse types wire retinal processes into functionally discrete circuits in the IPL. Except for a handful of circuits, it remains largely unknown how diverse amacrine cell inputs act to shape the output of the neurons they contact. In order to advance knowledge in this area, it will be important to identify the amacrine cell types that provide input onto a common postsynaptic cell (convergence), as well as to ascertain each type’s multiple postsynaptic targets (divergence).
3.4. Assembly of inner retinal circuits
3.4.1. Sequence of synapse formation at the IPL
The sequence of synapse formation in the IPL seems to be conserved across species. Amacrine cells are generated and differentiate before bipolar cells (Marquardt and Gruss, 2002; Rapaport et al., 2004 and see Fig. 9), and thus amacrine cell synapses are found in the developing IPL before bipolar cell synapses (summary in Fig 15A). Electron microscopy of the developing IPL in mice suggests three phases in the establishment of amacrine cell and bipolar cell synapses: (i) An increase in the number of conventional (amacrine cell) synapses between P3-P10; ribbon synapses are not observed during this period, (ii) ribbon synapse density increases together with an increase in conventional synapse formation from P11-P15, and (iii) around eye-opening (~P15) there is a sharp reduction in the numbers of both excitatory and inhibitory synapses leading to a plateau in synapse numbers (Fisher, 1979b). It is also evident that amacrine cells form the first synapses in the IPL in the perifoveal region of the macaque retina (Nishimura and Rakic, 1987). In macaque retina, the first morphological synapses distinguishable are between amacrine cell and ganglion cells. This is followed by amacrine cell-amacrine cell synapses and ‘monad’ ribbon synapses between bipolar cell terminals and a single amacrine cell or a retinal ganglion cell process. These ‘monad’ ribbon synapses mature into ‘dyad’ contacts. Amacrine cells synapse onto bipolar cell terminals to conclude circuit assembly in the IPL (Fig. 15A). The sequence of synaptic development is different, however, for the macaque fovea compared to the peripheral retina. In the fovea of macaques, the first synapses formed are the ribbon synapses of bipolar cell axons, followed by cone photoreceptor ribbon synapses, and subsequently by amacrine cell synapses. In the rod-dominated peripheral retina, however, as discussed above, amacrine cell synapses are the first IPL synapses observed (Hendrickson, 1996). Similarly, in the rod-dominated cat retina, amacrine cell synapses are formed prior to bipolar cell synapses (Maslim and Stone, 1986). Together, observations from mice, monkeys, and cats suggest that inhibitory synapses are formed early on during IPL development (Fig. 15A) in rod-dominated retinas. By contrast, in cone-rich areas of the retina, bipolar cell ribbon synapses are established first. The overall sequence of IPL synapse assembly in mammals also seems to be adopted by the zebrafish retina: conventional synapses appear in the IPL prior to the appearance of ribbons at bipolar cell terminals and functional photoreceptor triads (Schmitt and Dowling, 1999). It should be emphasized, however, that ascertaining when bipolar cell synapses first appear has thus far largely relied on detecting ribbon-like structures in the IPL. Prior to the emergence of such ribbons, the bipolar cell synapse may appear similar to a conventional synapse, and could be mistaken for an amacrine cell synapse. Thus, other specific markers of amacrine cell and bipolar cell synapses are needed to track the early appearance and distribution patterns of these synapses in the developing IPL.
Figure 15. Synapse assembly in the IPL.
(A) Sequential development of synapses in the IPL. Amacrine cells (ACs) first establish contact with dendrites of retinal ganglion cells (RGC) and other amacrine cells. Next, bipolar cell (BC) terminals synapse onto ganglion cells. Thereafter, presynaptic inhibition provided by amacrine cells is established at the axon terminals of bipolar cells (see text for details).
(B) Relative expression levels of excitatory and inhibitory synaptic proteins at different time-points in the IPL from immunolabeling experiments carried out in rodent retina (summarized from: Fletcher and Kalloniatis, 1997; Guo et al., 2009; Hack et al., 2002; Johnson et al., 2003; Kim et al., 2000; Koulen, 1999; Sassoe-Pognetto and Wässle, 1997; Witkovsky et al., 2005). The color gradients are representative of the total expression of the synaptic proteins, rather than their distribution pattern. Note for synaptic proteins mediating excitatory neurotransmission AMPA (GluA1-4) receptors seem to be expressed prior to Kainate (GluK2/3 and GluK5) receptors in the developing IPL. On the other hand, inhibitory neurotransmitters GABA and glycine seem to be expressed at a similar timeline. The receptors mediating inhibitory neurotransmission, however, reach adult expression levels at different time-points with GABAAα2 and GABAAα3 receptors preceding GABAAα1 and GlyRα1 receptors.
Expression of synaptic markers in the IPL (summarized from rodent retina in Fig. 15B) is largely coincident with the sequence of synapse formation that has been defined by ultrastructural analyses. VIAAT can be detected by immunolabeling as early as P0 in mouse IPL (Guo et al., 2009). VGluT1, however, can only be detected after P5 in the mouse IPL (Johnson et al., 2003). Both GABA and glycine are already localized within neurons at the time of birth in rodent retina, whereas expression of glutamate appears diffuse around birth until about a week after birth (Fletcher and Kalloniatis, 1997). GABAA-specific spontaneous postsynaptic currents can be recorded from retinal ganglion cells as early as embryonic day 17 (Unsoeld et al., 2008). Spontaneous excitatory postsynaptic currents (EPSCs), on the other hand, can be recorded from mouse retinal ganglion cells only around P7 (Johnson et al., 2003; Morgan et al., 2008). Structurally, however, excitatory postsynaptic sites can be recognized on mouse retinal ganglion cell dendrites by the clustering of PSD95, as early as P5. The expression of functional glutamate receptors on P5 mouse retinal ganglion cells is also evident from their robust response to puffs of kainic acid, a glutamate analog (Morgan et al., 2008). The appearance of EPSCs from mouse retinal ganglion cells two days later correlates with the elaboration of bipolar cell axon terminals at around P7 (Morgan et al., 2006).
Many studies have examined the expression of postsynaptic neurotransmitter receptors in the IPL during development (Fig. 15B), and across species. A common finding is that expression of the majority of GluRs in the IPL precedes onset of glutamatergic neurotransmission from bipolar cell axon terminals. In rodent retina, both AMPA and Kainate receptors are already expressed in the IPL at P0, the only exception being the Kainate receptor subunit KA2, which is first observed in rodent IPL only after P3 (Hack et al., 2002). AMPA receptors (GluA2/3 and 4 subunits) reach adult expression levels in the IPL around the time of eye-opening in rodent retina (P14), almost a week before Kainate receptors (GluK2/3 and GluK5 subunits) (Hack et al., 2002). It is possible that the late-onset Kainate receptors (Fig. 15B) rely on early expression of AMPA receptors, or the expression time-line of these two iGluRs may be totally independent with expression of each receptor type being determined separately. It is also possible that the disparity between AMPA and Kainate receptors represents differences in the time-line of synaptogenesis of different cell types in the IPL. In this regard, it would be informative to track the development of AMPA and Kainate receptors on a single amacrine cell that co-expresses (see 3.3.1.) these two iGluRs.
The early expression of most iGluRs shows a rather diffuse distribution, with iGluRs forming punctate clusters only after aggregation of receptors at synaptic sites. The AMPA receptor GluA4-subunit first shows punctate labeling around P3, but labeling for GluA2/3-subunits displays synaptic puncta only around P7 (Hack et al., 2002). Thus, iGluRs are clustered at IPL synapses around the time when vesicular release of glutamate, commences, as indicated by VGluT1 expression. The scaffold proteins at excitatory synapses, PSD95 and SAP102, are expressed in the rodent IPL already at birth albeit with a diffuse distribution that becomes punctate around the time of eye-opening (Koulen, 1999). The exact sequence of the assembly of all molecular components of glutamatergic synapses in the IPL remains to be fully determined.
Similar to iGluR expression patterns in the IPL, the first GABA receptors to appear are also diffusely expressed and as retinal development proceeds, form clustered puncta that are specifically localized at inhibitory postsynapses (Sassoe-Pognetto and Wässle, 1997). The sequence of inhibitory receptor expression (Fig. 15B) correlates with the establishment of amacrine cell type-specific circuits. As highlighted earlier, amacrine cell connections with retinal ganglion cells and other amacrine cells are established earlier than amacrine cell-bipolar cell connections in the developing IPL. Amacrine cell-bipolar cell connections develop only after the onset of glutamatergic transmission from bipolar cell terminals: spontaneous inhibitory postsynaptic currents (IPSCs) can be recorded from rodent bipolar cells only after the first postnatal week (Schubert et al., 2008). As discussed in the previous section, GABAAα2 and GABAAα3 receptors are specific to amacrine cell-retinal ganglion cell and amacrine cell-amacrine cell circuits, whereas GABAAα1 and GlyRα1 prominently mediate presynaptic inhibition on the bipolar cell axon terminals. In congruence, GABAAα2 and GABAAα3 inhibitory circuits in the IPL are established before GlyRα1 and GABAAα1 synaptic circuits. The characteristic distribution of GABAAα2 into two bands in the IPL emerges around P5 in the rodent retina (Sassoe-Pognetto and Wässle, 1997), a few days after the ON- and OFF-starburst amacrine cell plexuses appear (Kim et al., 2000). Stratification of GABAAα3 receptors follows next, at around P9 in rodent IPL. Both GABA receptor types reach adult expression levels and patterns before eye-opening (Sassoe-Pognetto and Wässle, 1997 and see Fig. 15B). GlyRα1 and GABAAα1 expression in the developing rodent IPL is relatively delayed (see Fig. 15B) and reach adult levels only around three weeks (~P21) after birth (Sassoe-Pognetto and Wässle, 1997). The inhibitory postsynaptic scaffolding protein gephyrin follows a similar expression time-line (Sassoe-Pognetto and Wässle, 1997). The expression of other inhibitory synapse-organizing molecules and their possible contribution towards the development of amacrine cell synapses has not yet been detailed.
Synaptic differentiation of the bipolar cell terminal is the last step of circuit assembly in the IPL. This process can be divided into two phases: (i) the establishment of glutamatergic release from bipolar cell terminals and (ii) the establishment of amacrine cell input onto the axonal terminals of bipolar cells. Studies from rodent retina have revealed that both these maturational processes occur over different time-lines for the functionally distinct subsets of bipolar cell terminals. Analysis of the onset of glutamatergic transmission from bipolar cell terminals in rodent retina has revealed that cone bipolar cell terminals express VGluT1 before rod bipolar cell terminals. Moreover, OFF-bipolar cell terminals express VGluT1 (P6-P8) before ON-bipolar cell terminals (around P10), followed by expression in rod bipolar cell terminals (around P12) (Sherry et al., 2003). This sequence of bipolar cell axonal maturation is opposite to the sequence of synaptic maturation of their dendrites in the OPL. As discussed in 3.2.1., mGluRs localize at the dendritic tips of ON-bipolar cells prior to expression of iGluRs on OFF-bipolar cell dendrites. Together, these observations indicate that dendrites of bipolar cells might have a different timeline for synapse formation compared to their axons. One might therefore expect that different extrinsic cues guide the establishment of synapses at these two cellular compartments.
The second step of bipolar cell axonal maturation, namely the establishment of presynaptic inhibition onto these terminals, also occurs at distinct time-points for the diverse bipolar cell subsets. Recording inhibitory input onto developing bipolar cells in rodent retina has revealed that rod bipolar cells are the first to establish inhibitory input on their axon terminals, and for the cone bipolar cells, the OFF-cone bipolar glycinergic inhibitory input is established first (Schubert et al., 2008). Thus even during establishment of inhibitory contacts onto bipolar cell terminals different bipolar cell types adopt different time-courses to establish mature patterns of connectivity.
The establishment of functional circuits in the IPL does not only rely on when pre- and postsynaptic proteins appear, but also on the accurate spatial localization of the synaptic inputs. Asymmetric inhibition by starburst amacrine cells onto the dendritic arbor of DS retinal ganglion cells is key to the generation of the direction-selectivity response at maturity (see 3.3.2.). During mouse IPL development, however, inhibition in response to the ‘null’ and ‘preferred’ directions of stimulation is equivalent. This symmetric inhibition onto the dendrites of DS retinal ganglion cells matures into the required asymmetric inhibition during the second postnatal week, just before eye-opening, as the strength of inhibitory connections increases preferentially on the null side of the ganglion cell arbor (Wei et al., 2011; Yonehara et al., 2011). Taken together, correlated synaptic protein expression establishes the first IPL circuits, with a later maturation step that refines the functional output of some identified circuits.
3.4.2. Early neurotransmission in the IPL and its role in shaping IPL circuitry
It is now well established that developing retinal ganglion cells exhibit action potential activity prior to the onset of visual stimulation. Notably, across many species, ‘waves’ of activity propagate throughout the IPL before eye-opening (Torborg and Feller, 2005; Wong, 1999). The waves act to coordinate the rhythmic bursting activity of neighboring retinal ganglion cells, generating a spatiotemporal pattern of activity that is important for the refinement of retinal projections to the brain (Torborg and Feller, 2005). Much is known about the mechanisms underlying retinal wave generation (Blankenship and Feller, 2010). Early retinal waves are mediated by excitatory cholinergic activity amongst starburst amacrine cells (Zheng et al., 2006) and connections between starburst amacrine cells and retinal ganglion cells (Blankenship and Feller, 2010). A few days before eye-opening, the spontaneous activity of starburst amacrine cells is attenuated, which leads to the disappearance of cholinergic waves and generation of glutamatergic waves that involves bipolar cells, amacrine cells, and retinal ganglion cells (Blankenship and Feller, 2010). The onset of glutamatergic waves temporally corresponds to the maturation of bipolar cell axon terminals, which are the major source of glutamate in the inner retina, and involves iGluRs expressed in the IPL (Torborg and Feller, 2005). To date, cholinergic waves, have not been found to be necessary for the structural maturation of the IPL circuitry, as large field ganglion cells and starburst amacrine cells do not demonstrate any abnormalities in the retina from mutant mice that lack the synthetic enzyme for acetylcholine (Stacy et al., 2005). Moreover, there is a homeostatic recovery of spontaneous activity in the retina from these animals, with gap-junctional connections providing a mechanism to generate patterned activity. Thus, during IPL formation, the inner retina generates functional circuits that may only serve a role during early development, and not in the processing of visual signals in the adult animal. However, work on retinal waves underscores that a single group of amacrine cells can play dual functional roles at distinct stages of the animal’s life. While cholinergic drive from starburst amacrine cells is key to the generation of early retinal waves, GABAergic transmission from these same cells is critical for shaping the direction-selective response in the DS retinal ganglion cells (Zheng et al., 2004).
Though maturation of the IPL circuitry does not require propagated activity per se, glutamatergic and GABAergic neurotransmission have been shown to play a role (Fig. 16). To delineate the role of bipolar cell-retinal ganglion cell transmission in the maturation of the IPL circuitry, numerous studies have perturbed neurotransmission using pharmacological agents (Bodnarenko and Chalupa, 1993; Bodnarenko et al., 1995; Deplano et al., 2004) or dark-rearing animals (Tian and Copenhagen, 2003). Both types of manipulations have resulted in abnormal proportions of retinal ganglion cells receiving both ON and OFF inputs. However, these global manipulations may affect retinal transmission at different levels. To directly assess whether transmission from bipolar cells to retinal ganglion cells influences their connectivity, recent studies took advantage of transgenic approaches to selectively suppress glutamate release from subsets of bipolar cells (Kerschensteiner et al., 2009). ON-bipolar cell transmission was highly reduced upon expression of the light-chain of tetanus toxin (TeNT) under the grm6 promoter (Kerschensteiner et al., 2009). TeNT cleaves VAMP2 (vesicle-associated membrane protein 2), a vesicular protein required for exocytosis. In the grm6-TeNT retina, ON-retinal ganglion cells develop fewer glutamatergic postsynaptic sites overall compared to wildtype animals (Fig 16), and this impairment results from a reduced rate of synapse formation rather than increased synapse elimination (Kerschensteiner et al., 2009). Conversely, mice deficient in the CRX gene, in which photoreceptors degenerate, show increased spontaneous release from bipolar cell terminals and consequently demonstrate enhanced synaptogenesis between bipolar cells and retinal ganglion cells (Soto et al., 2012).
Figure 16. Role of neurotransmission in regulating synaptic connectivity in the mouse IPL.
(A) Synapse number between cone bipolar cells (CBC) and ganglion cells (RGC) is regulated by neurotransmission. Type 6 bipolar cells that express tetanus toxin (TeNT) make fewer synapses with dendrites of large-field ON alpha-like ganglion cells compared to wildtype (WT) bipolar cells (Kerschensteiner et al., 2009; Morgan et al., 2011; Okawa et al., 2014).
(B) GABAergic neurotransmission is necessary for maintaining specific GABA receptor subtypes on rod bipolar cell (RBC) axon terminals. Impairing inhibitory neurotransmission from amacrine cells (ACs) in the GAD67 (GABA synthetic enzyme) deficient (GAD1 mutant) retina leads to a reduction in GABAA but not GABAC receptors on RBC axon terminals (Schubert et al., 2013).
Further studies using the grm6-TeNT mice revealed that not all ON-bipolar cell types onto an individual retinal ganglion cell are affected similarly by loss of transmission across the ON-bipolar cell population. Whereas connectivity with one type of bipolar cell providing converging input onto the retinal ganglion cell is regulated by transmission, connections with another silenced bipolar cell type is not affected. This was evident from work examining the circuitry of large field alpha-like ON-retinal ganglion cells, which receive their major (~70%) bipolar cell input from Type 6 ON-cone bipolar cells and a small fraction of their total input from Type 7 ON-cone bipolar cells (Schwartz et al., 2012). In grm6-TeNT retinas the number of synapses made by Type 6 but not Type 7 bipolar cells was reduced on the dendritic arbor of large field alpha-like ON-ganglion cells (Morgan et al., 2011; Okawa et al., 2014). The reason that neurotransmission plays a differential role on the connectivity of distinct converging input types is not known, although it raises the possibility that transmission may play a more important role in shaping synaptic contact with major inputs.
It is also now clear that neurotransmission-dependent regulation of synapse density between bipolar cells and retinal ganglion cells occurs locally, at the level of individual bipolar cell axons. In transgenic lines in which only a small fraction of ON-bipolar cells express TeNT, Type 6 bipolar cells expressing the toxin have fewer synaptic contact sites with the large-field alpha-like ON-retinal ganglion cell, compared to neighboring Type 6 terminals that do not express TeNT (Okawa et al., 2014). Thus, transmission from bipolar cells operates cell-autonomously to regulate connectivity with the target retinal ganglion cell. The impaired connectivity between the toxin-expressing Type 6 bipolar cells and the retinal ganglion cell is due to a failure of these cells’ axons to make contacts containing multiple synaptic sites. Normally, the large boutons of Type 6 bipolar cells contain more than one ribbon, and each ribbon is apposed to a separate PSD95 cluster on the retinal ganglion cell dendrite. These multisynaptic boutons are less frequently observed in TeNT-expressing bipolar cell axons (Kerschensteiner et al., 2009; Morgan et al., 2011 and see Fig. 16). Instead, fewer synapses between Type 6 bipolar cells and large-field alpha-like ON-ganglion cell dendrites can be observed in TeNT-retinas and under EM, multiple ribbons are found apposed to a single postsynaptic density, suggesting a defect in ribbon trafficking or localization in these axons.
Although converging bipolar cell axons do not engage an activity-dependent competitive mechanism to sculpt their connectivity, a recent study (Okawa et al., 2014) demonstrated that bipolar cells do influence each other’s connectivity with the target retinal ganglion cell, albeit through an activity-independent mechanism. When the majority of Type 6 bipolar cells are ablated upon transgenic expression of an attenuated form of Diphtheria toxin, the few surviving Type 6 bipolar cells increase their axon territory and connectivity with their postsynaptic partner, the large field alpha-like ON-retinal ganglion cell. Moreover, loss of Type 6 bipolar cells leads to an increase in contact with the minor input (Type 7 bipolar cell). Furthermore, in the Diphtheria toxin-expressing retina, the large field alpha-like ON-ganglion cells elaborate dendrites that stratify in the OFF sublamina of the IPL. There, the retinal ganglion cell establishes functionally mismatched, aberrant contacts with OFF-bipolar cells (Okawa et al., 2014). Thus, the presence of the major input type, but not its transmission, is important for maintaining input type specificity (i.e. ON but not OFF, and selectivity between ON-bipolar cell subtypes) of the retinal ganglion cell.
Several studies have also explored the role of neurotransmission in the development of inhibitory circuits in the inner retina. An increase in inhibitory amacrine cell synapses at the IPL has been reported in retinas from dark-reared mice (Fisher, 1979a). However, the maintenance of GABAA receptors at inhibitory synapses on rod bipolar cell terminals has been found to be dependent upon GABAergic neurotransmission (Fig. 16). Reducing presynaptic GABA release in the GAD67 (GABA synthetic enzyme) retina-specific KO causes a decrease in the density of functional GABAA but not GABAC receptors on mouse rod bipolar cell terminals. This impairment is apparent at maturity (P30) but not during development (P12) (Schubert et al., 2013). Immunolabeling for the three GABA receptors types on rod bipolar cell terminals revealed a specific reduction in GABAAα1 but not GABAAα3 or GABAC receptors on the mature rod bipolar cell axon terminals. Thus inhibitory neurotransmission is important for the selective maintenance of a subset of GABAA(α1) receptors (Schubert et al., 2013) (for summary of the role of neurotransmission in shaping IPL synapses in mouse retina see Fig. 16).
Similar to the bipolar cell-retinal ganglion cell glutamatergic circuit, not all inhibitory circuits in the IPL rely on activity for maturation. For example, blocking GABAA receptors does not prevent the generation of the direction-selective circuit in the mouse IPL (Wei et al., 2011). In fact, development of the direction-selective circuit is resistant to different forms of activity blockade including blocking action potentials or cholinergic transmission from the starburst amacrine cells (Sun et al., 2011). Visual deprivation, however, does affect the development of the starburst amacrine cells after eye-opening: 3-4 weeks of dark-rearing causes a decline in the density of starburst amacrine cells accompanied by the presence of thinner cholinergic bands at the IPL (Zhang et al., 2005). Whether or not loss of inhibitory transmission during development affects connectivity of other amacrine cell-retinal ganglion cell circuits remains an open question, and the answer will require detailed reconstructions of circuits that have not yet been as extensively characterized as the DS retinal ganglion cell circuit.
Taken together, both activity-dependent and independent mechanisms are central to the establishment of synaptic circuits in the IPL. However, unlike the OPL, molecular cues that play an instructive role in the assembly of synapses in the IPL have not yet been extensively explored. Few studies have assessed the contribution of adhesion proteins for synapse formation, maintenance and circuit organization in the IPL. Loss of the inhibitory postsynaptic adhesion molecules Neuroligin2 and Neuroligin4 leads to a loss of specific subsets of GABA (GABAAα3) and glycine (GlyRα1) receptor IPL synapses, respectively (Hoon et al., 2009; Hoon et al., 2011). But the role of adhesion proteins for the assembly and maintenance of synapses at specified IPL circuits remains to be investigated. The discovery of new transmembrane molecules that organize the synapse, together with the availability of advanced genetic tools bring forth the opportunity to rapidly fill this gap in our knowledge.
3.5. Balancing excitatory and inhibitory inputs onto individual cells
The previous sections have discussed the organization and development of excitatory and inhibitory inputs in the retina separately. The output of retinal cells, however, relies on the combination of both excitatory and inhibitory drive onto the cell. Here, we will expand on what is known about the coordinated development of these functionally distinct inputs onto retinal ganglion cells, which has been the focus of several recent studies.
Retinal ganglion cells receive both excitatory and inhibitory input on their dendrites. Fluorescent protein (FP) tagged postsynaptic markers of excitatory and inhibitory synapses have made it possible to map the spatial distribution and developmental time-course of both populations of synapses on individual retinal ganglion cells (Morgan et al., 2008). In mice, excitatory postsynaptic sites (PSD95-FP) on retinal ganglion cell dendrites show an increase in density from P5 until a month after birth, when mature densities are attained (Morgan et al., 2008). Likewise, Neuroligin2, expressed in retinal ganglion cells under the Thy1 promoter in transgenic mice, revealed the spatial distribution of inhibitory synapses on these cells across ages (Soto et al., 2011a). Comparison of the individually mapped PSD95-FP and Neuroligin2-FP on large-field ON-retinal ganglion cells suggests that excitatory and inhibitory synapses develop in a coordinated manner, giving rise to an inhibitory/excitatory (INeuroligin2/E) ratio that appears constant from P7 onwards. More recently, biolistic labeling of PSD95-FP in transgenic lines in which the Thy1 promoter drives expression of the γ2 subunit of the GABAA receptor, enabled simultaneous mapping of excitatory and inhibitory (IGABA) synapses on individual mouse retinal ganglion cells (Bleckert et al., 2013). Both separate and simultaneous mapping of the excitatory and inhibitory synapses led to the conclusion that the I/E synapse ratio for the large field ON alpha-like retinal ganglion cells is achieved soon after the onset of glutamatergic transmission. Analysis of additional retinal ganglion cell types revealed that the I/E ratio could differ amongst distinct ganglion cell types. Moreover distinct retinal ganglion cell types appear to attain their I/E ratio at different developmental time-courses. Whereas large field alpha-like ON-retinal ganglion cells and large-field alpha-like OFF-retinal ganglion cells attained their mature IGABA/E synapse ratio before eye-opening, DS retinal ganglion cells only developed their adult IGABA/E ratio three weeks after birth (Bleckert et al., 2013). Across species, the distance between excitatory (PSD95 positive) (Koizumi et al., 2011) and GABAA-inhibitory (GABAAγ2-positive) synapses on the retinal ganglion cell dendrite remains invariant across retinal ganglion cell subtypes, adopting a non-random distribution determined early in development (Bleckert et al., 2013).
Ganglion cells in monkey retina also balance the densities of excitatory and inhibitory synapses across their dendritic arbor. A study on midget ganglion cells in marmoset retina found equivalent excitatory and inhibitory synapse numbers on the ganglion cell dendrites. Ganglion cells were labeled by retrograde filling or intracellular injections and immunolabeling was carried out for AMPA receptors (GluA4 subunit) and gephyrin to determine the I/E ratio. Moreover, this I/E ratio remained comparable across different eccentricities (Abbott et al., 2012). Interestingly, the bistratified (blue-ON, yellow-OFF) ganglion cell in marmoset retina displays a characteristic pattern in arranging its excitatory synapses: bipolar cell input to the ON-arbor is about 4 times greater than input to the OFF arbor, but the ratio of the densities of bipolar cell input to both ON and OFF arbors remains the same across retinal eccentricities (Percival et al., 2009). Bipolar cell and amacrine cell inputs onto the dendrites of these bistratified cells were revealed by immunolabeling for a presynaptic ribbon marker or for GluA4 and for gephyrin, respectively (Percival et al., 2009). Almost equal numbers of bipolar cell and amacrine cell inputs were observed on bistratified ganglion cells from central retina. The developmental time-line when ganglion cells establish their ratio of amacrine cell/bipolar cell input, however, has not yet been detailed in monkey retina. It would be informative to study the development of the I/E ratio for the different ganglion cell types in monkey retina across retinal eccentricities. The functional implication of these ratios also remains currently unknown but the output of different retinal ganglion cell types could be modulated by the arrangement of excitatory and inhibitory synapses across the dendritic arbor. As the bipolar cell inputs develop earlier in the fovea compared to periphery (see 3.4.1.), one might expect different amacrine cell/bipolar cell or I/E ratios for central retinal ganglion cells compared to peripheral retinal ganglion cells early in development. It is not yet known when in development monkey ganglion cells establish their mature I/E ratio, and whether or not all ganglion cell types follow a similar developmental program.
The underlying mechanisms that set up the ratio of inhibitory and excitatory synapses on retinal ganglion cell dendrites have not yet been elucidated, but a role for neurotransmission could be expected. It would thus be informative to determine whether loss of excitatory neurotransmission affects the development of inhibitory synapses, and vice-versa. Furthermore, because different retinal ganglion cells exhibit different ratios of excitatory and inhibitory synapse densities, it is necessary to ascertain the molecular and/or functional cues that separately orchestrate these distinct synaptic arrangements to optimally drive retinal ganglion cell output.
3.6. Convergence and divergence of retinal circuits
In addition to the convergence of excitatory and inhibitory inputs onto individual retinal neurons, there is also convergence and divergence of excitatory or inhibitory connections across multiple cell types. Recently, a number of studies have applied serial EM methods (Helmstaedter et al., 2013) or fluorescence labeling of synaptic proteins (Schwartz et al., 2012) to tease apart the convergence and divergence of inputs on mouse retinal neurons.
The cone terminal itself serves as a starting point for distributing information into multiple parallel bipolar cell pathways. Apart from divergence of information into ON and OFF channels, there is additional diversity amongst ON- and OFF-bipolar cells across species (Ghosh et al., 2004). In the mouse retina, each cone pedicle is contacted by at least one member from every cone bipolar cell subtype, resulting in ~10 divergent channels for signal transmission from a single cone (Wässle et al., 2009b). Conversely, each cone bipolar cell pools information from 5 or more cone photoreceptors, with the exact number for convergence varying between cone bipolar cell subtypes (Wässle et al., 2009b). Convergence for rod bipolar cell dendrites at the mouse OPL is even more extensive with each rod bipolar cell pooling information from ~25 rod photoreceptors (Tsukamoto and Omi, 2013). Convergence and divergence of information at the OPL is also evident in zebrafish retina, where each horizontal cell and bipolar cell subtype collects information from 2 or more cone photoreceptor types, and each photoreceptor terminal outputs onto multiple horizontal cell and bipolar cell subtypes: examples shown in Figure 17 (Li et al., 2009).
Figure 17. Convergence and divergence of connectivity in the zebrafish retina.
(A-B) Convergence of photoreceptor (UV and S cone) input onto the H3 subtype of horizontal cells (HC). Cx55.5:Gal4;UAS:MFP plasmid was injected at the one-cell stage to label the H3 horizontal cells (yellow) in double transgenic (sws1:GFP;sws2:mcherry) fish with both UV (pseudocolored magenta) and S cones (pseudocolored cyan) labeled by expression of different fluorescent proteins.
(C-E) Divergence of photoreceptor (L cone, pseudocolored cyan) output onto ON (yellow) and OFF (magenta) subclasses of bipolar cells (BC). Confocal reconstructions acquired from triple transgenic Tg(trβ2:tdTomato;vsx1:MCerulean;nyx:Gal4;UAS:MYFP) fish where the various cell types expressed different fluorescent proteins. Trβ2 drives expression in L cones; vsx1 in some OFF BCs; nyx in ON BCs. (B,D,E) Arrowheads indicate dendritic tips inserting into the cone pedicles. (Image courtesy, T. Yoshimatsu.)
Not surprisingly, there is also tremendous convergence and divergence of signals in the IPL, which has been most extensively studied in monkey and mice because inner retinal cell types have been most extensively classified in these two species. Convergence of inputs from distinct bipolar cell types provides the basis for color-coding in macaque retina. The bistratified retinal ganglion cell has an antagonistic blue (S) ON-yellow (L+M) OFF response (Dacey, 1993a; Dacey and Lee, 1994). In the fovea, the ON arbor of the bistratified retinal ganglion cell receives input from 2-3 blue ON-bipolar cells that each collect information from mainly one S cone, and the OFF arbor of the bistratified retinal ganglion cell obtains input from 3-4 diffuse OFF-bipolar cells that in turn collect information from ~20 L and M cones (Calkins et al., 1998). For the midget pathway, however, there is no convergence or divergence of information at the fovea (Kolb and Dekorver, 1991). Serial section electron micrographs used to reconstruct this circuit in macaque retina have shown each cone photoreceptor to be connected to a pair of midget bipolar cells: ON- and OFF-midget bipolar cells that in turn synapse onto single ON- and OFF-midget ganglion cells (Calkins et al., 1994). In peripheral macaque retina, however, information from ≥ 3 midget bipolar cells (and hence ≥ 3 cone photoreceptors) converges onto a single midget ganglion cell arbor (Kolb and Marshak, 2003; Wässle et al., 1994). This way the midget circuitry is wired to encode for high spatial resolution. Another ganglion cell type in macaque retina, the parasol ganglion cells, displays much more convergence compared to midget ganglion cells (Jacoby et al., 2000). Serial section electron micrographic reconstructions of the synaptic connectivity of a foveal parasol retinal ganglion cell has revealed that each cell pools input from ~ 50 cones via ~ 120 bipolar cells (Calkins and Sterling, 2007). This enables the parasol cells to encode for luminance. Taken together, distinct retinal circuits utilize disparate levels of information convergence or divergence to best suit the specific functional demand of that circuit.
These examples serve not only to illustrate divergence and convergence of some key circuits in the retina, but also to emphasize that within each circuit, connectivity is stereotypic. Such stereotypic arrangements facilitate investigations of the cellular and molecular mechanisms that define the type and ratio of synapses formed between synaptic partners in both the OPL and IPL. While these mechanisms are still largely elusive, some insight has been gained from examining the development of the ON-bipolar cell to large field alpha-like ON-retinal ganglion cell circuit in mice. When the major input, the Type 6 bipolar cells (and a few Type 7 bipolar cells) are genetically ablated upon expression of Diphtheria toxin, the remaining Type 7 bipolar cells increase their connectivity with the retinal ganglion cell extensively, such that the density of bipolar cell synapses made by the Type 7 bipolar cells on the retinal ganglion cell is close to that normally made by Type 6 bipolar cells (Okawa et al., 2014). Because neurotransmission is not required for the expansion of Type 7 inputs, this finding suggest that Type 7 is restricted from making contact with the retinal ganglion cell due to physical constraints, and not due to a molecular ‘mismatch’. As discussed in section 3.4.2., neurotransmission from Type 6 bipolar cells affects the arrangement of synaptic connectivity with the dendritic arbor of the large field alpha-like ON-retinal ganglion cell. Thus, both activity-dependent and independent mechanisms are likely to act together to shape the number of synapses made by distinct input types onto a common postsynaptic cell. The interplay between the roles of activity-dependent and independent mechanisms still need to be dissected for other circuits in the mouse retina, as well as for circuits in other species. It will not be surprising, however, if we find that different circuits or similar circuits across species adopt distinct strategies to achieve their individual patterns of synaptic convergence and divergence.
3.7. Homeostasis of retinal circuits
Finally, it is important to highlight that although structural connectivity between retinal cells is well-defined, how each circuit functions also depends on physiological adjustments that do not require alterations to the wiring per se. It is clear that transmission is modulated across retinal circuits both by a balance of excitation and inhibition and also by neuromodulators such as dopamine and neuropeptides (Kolb, 1997; Vaney, 1990). But, alterations to cell-cell communication also occurs through homeostatic adjustments. Perturbations that disrupt the functional set point of the circuit can invoke cellular and network mechanisms that work together to restore function to the optimal range. For example, rod bipolar cell terminals are capable of homeostatically adjusting glutamate release when inhibitory input onto their terminals is impaired (Schubert et al., 2013). A reduction of presynaptic inhibitory input onto the terminals of rod bipolar cells initially leads to a transient increase of glutamate output from developing rod bipolar cell terminals before eye-opening. Two weeks after eye-opening, however, the release of glutamate from mutant rod bipolar cell re-adjusts to wildtype levels, indicating the existence of compensatory mechanisms at rod bipolar cell terminals. Another example of the adaptive nature of the visual system comes from experiments in mice that show that even when photoreceptor synaptic ribbons are lost, visual maps develop normally and the animal can perform visual behavioral tasks surprising well (Goetze et al., 2010). Despite the adaptive nature of retinal circuits, large-scale changes such as those that occur in disease cannot be so readily compensated for.
4. Disease: Alterations to structure and function
Pathologies of the neural retina represent some of the most common causes of visual impairment and blindness (Pascolini and Mariotti, 2012). The majority of retinal diseases can be categorized largely into those involving death of photoreceptors in the outer retina, and those directly affecting neurons of the inner retina, such as bipolar cells and ganglion cells. Table 1 lists some common retinal diseases, the associated animal models and the cell types that are the primary ‘targets’. In this section, we do not attempt to review exhaustively all the structural and functional alterations that take place (see Jones et al., 2012; Marc et al., 2007a for comprehensive reviews) but instead, we aim to convey a broad overview of circuit changes that occur in disease and discuss the challenges that remain in designing therapeutic strategies for restoring function at various levels of the retinal circuitry.
Table 1.
Animal models of common retinal diseases, primary neurons affected and associated spatial distribution of cell loss
| Disease | Animal models | Cell types affected |
Topology of cell death/ vision loss |
|
|---|---|---|---|---|
| Outer retina | Retinitis pigmentosa | Mouse: 1rd1, (rd1/rd1, rd/rd), 1rd10, 2C3H Zebrafish: 3Prpf31, 4XOPS-mCFP |
Rods, then cones | Periphery to center |
| Leber’s congenital amaurosis |
Mouse: 1rd3, 5rd12, 6,7Crx−/−, 8RetGC1/2 −/− Zebrafish: 9Gucy2d |
Rods, then cones or Rod and cone |
Periphery to center |
|
| Macular degeneration | Mouse: 10rds, Zebrafish: 11gantembein |
RPE, cones and Rods |
Center to periphery |
|
| Startgardt disease | Mouse: 12ELOVL4 mutant, 13abcr −/− |
RPE, cones and Rods |
Center to periphery |
|
| Cone-rod dystrophy | Mouse: 14GCAP1(Y99C) | Cones then rods | Peripheral | |
| Retinoschisis | Mouse: 15Rs1h−/− | Photoreceptors and bipolar cells |
Macular + Peripheral |
|
| Congenital Stationary Night Blindness |
Mouse: 16CACNA1F mutant, 17PDE6β(H258N), 18Lrit3 Zebrafish: 19Nyctalopin |
Photoreceptor to bipolar cells connection |
Presumed Uniform. |
|
| Inner retina | Glaucoma | Mouse: 20DBA/2J, 21Microbeads injection, 22laser photocoagulation, 23Episcleral vein coagulation Monkey: 24Microbeads injection, 25laser photocoagulation. Zebrafish: 26Irp2, 27wdr36 mutants |
Retinal Ganglion Cells |
Periphery to Center, Sectorial. |
| Optic nerve neuropathy | Mouse/Monkey: 28Optic nerve crush, 29Optic nerve transection. |
Retinal Ganglion Cells |
Presumed Uniform. |
Cenni et al., 1996 (see also Figure 18).
4.1. Large-scale structural alterations
Many retinal diseases are recognized by gross alterations to the structural organization of the retina (Fig. 18A). Most apparent is the thinning of the retina upon photoreceptor cell death. The loss of the photoreceptor layer is evident from optical coherence tomography scans of the retina of human patients, and it is also readily revealed in animal models by histological methods. Structural disorganization of the diseased retina is also noticeable upon the formation of rosette-like structures (Fig. 18A). Rosettes are characteristic of the retinas of patients with retinoblastoma (Tajima et al., 1994), diabetic retinopathy (Lahav et al., 1975) or retinitis pigmentosa (RP) (Tulvatana et al., 1999). These structures are also found in animals following ablation of Müller glial cells (Byrne et al., 2013), in association with defects in the outer limiting membrane (Stuck et al., 2012), in conditions where microRNA function is disrupted (Damiani et al., 2008) and when cell proliferation is abnormal (Lin et al., 2001). Gross perturbation to retinal organization is also evident in patients and animal models with retinoschisis, a condition that leads to a physical separation between cells at the level of the OPL, resulting from the loss of the protein retinoschisin (Duncan et al., 2011; Kjellstrom et al., 2007; Molday et al., 2012; Takada et al., 2008).
Figure 18. Large-scale changes in retinal structure in disease.
(A) Examples of perturbations to spatial arrangements of cell layers. GCL: ganglion cell layer, INL: inner nuclear layer, ONL: outer nuclear layer.
(B) Common spatial patterns of cell loss across the retina (large circle). Relative extent of cell loss represented by gray scale: dark regions correspond to regions of extensive cell loss (see Table 1). Small circle: optic nerve head.
Although gross abnormalities often occur across the entire retina, in some diseases, parts of the retina are more affected than others (Fig. 18B; Table 1). Notably, degeneration of neurons in the macula, a hallmark of age-related macular degeneration, leads to a loss of acuity in central vision, leaving peripheral vision relatively unaffected at the early stages of the disease. Conversely, photoreceptor degeneration in RP or ischemic diseases results in a primary loss of peripheral vision, which progressively restricts the visual field to the center, a phenomenon known as tunnel vision (Sahel et al., 2010 and see Fig. 18B). Furthermore, sectorial death of cells has been observed in conditions of glaucoma, in which ganglion cell death is more prominent in some retinal locations compared to others (Soto et al., 2011b and see Fig. 18B). It is also intriguing that the topology of degeneration of one type of retinal neuron does not necessarily reflect the spatial loss of other neuronal types. For example, Type 7 ON-bipolar cells in the rd1 mouse model of RP degenerate uniformly across the retina rather than in the center-to-peripheral gradient observed for photoreceptors (Chen et al., 2012). Perturbations of the overall architecture in retinal diseases are therefore complex, but bear features that are often hallmarks of the disease or groups of diseases.
Although large-scale changes in retinal organization generally arise because of the death of a specific retinal cell type or types, bystander cell death and secondary degeneration contribute to the massive changes manifested by disease (Jones et al., 2012). Bystander effects have been found in the mammalian outer retina, where cone photoreceptors are lost after rod photoreceptors die (Chrysostomou et al., 2009 and see Table 1). In contrast, zebrafish cones are spared when rods degenerate (Morris et al., 2005). When fish cones die in pde6cw59 mutants (Stearns et al., 2007), rod degeneration ensues, but only in larvae and not in adults. Because adult zebrafish have a higher density of rods compared to larvae, it was suspected that high rod numbers could protect these cells from bystander death when their neighboring cones die. Indeed, by genetically manipulating the number of rods in larval fish, Fadool and colleagues demonstrated that increasing rod numbers prevent rods from degenerating after cones die (Saade et al., 2013). What factors underlie bystander effects and secondary degeneration? One possibility is that there is passage of ‘apoptotic’ signals via gap-junctions amongst neighboring neurons. However, to date there is no strong evidence in support of this possibility; cones still degenerate in connexin36/rhodopsin double KO mice when rods die (Kranz et al., 2013). Instead, there is some evidence supporting a role for diffusible factors released by neighboring cells. By screening compounds released by rod photoreceptors in a mouse model for RP, it was discovered that these photoreceptors release factors that ensure cone photoreceptor survival (Mohand-Said et al., 1998). This observation may explain the protective effect of genetically increasing photoreceptor density in the zebrafish (Saade et al., 2013).
Not all retinal neurons undergo secondary degeneration when photoreceptors die. For example, retinal ganglion cells in mouse models of RP show preserved morphology (Lin and Peng, 2013; Mazzoni et al., 2008). Likewise, the degeneration of ganglion cells in glaucoma and in optic nerve injury does not trigger large-scale secondary degeneration of presynaptic neurons, thus leaving the general architecture of the retina intact (Cuenca et al., 2010). Unraveling the mechanisms that cause large-scale cell loss and alterations in retinal organization will not only require identification of factors that affect the health of the targeted cell type or types, but also necessitate a systematic investigation of the downstream effects on retinal neurons in the vicinity of and synapsing with dying cells. Understanding under which conditions secondary degenerative events occur and under which conditions damage is more limited is key to helping prevent irreversible damage.
4.2. Changes in connectivity and function
4.2.1. Outer retina
Perturbations to outer retinal function are commonly assessed clinically from electroretinogram (ERG) recordings (summarized in Fig. 19). ERG recordings have been very helpful in detecting diseased conditions before degeneration occurs fully. Abnormal ERG responses are detected before a significant degree of photoreceptor cell death takes place (Gargini et al., 2007; Jae et al., 2013). Photoreceptor loss of function is reflected by the absence or a decrease in the amplitude of the a-wave of the full field ERG (Hood and Birch, 1990). Not surprisingly, the amplitude of the rod response in patients with RP and cone-rod dystrophy is reduced compared to normal (Hood and Birch, 1994). The rod response, when present, can show altered kinetics with disease (Wen et al., 2011, 2012). In some diseases, photoreceptors are still present but their transmission is perturbed. Transmission between photoreceptors and bipolar cells is measured from the b-wave of the full-field ERG (Hood and Birch, 1992). The b-wave is perturbed in animals with mutations in key presynaptic proteins at the OPL, such as Cacna1f, the subunit of the voltage-dependent calcium channel on photoreceptors (Chang et al., 2006), or in postsynaptic proteins including nyctalopin, which is associated with congenital stationary night blindness (Bahadori et al., 2006; Gregg et al., 2007; Gregg et al., 2003; McCall and Gregg, 2008).
Figure 19. Cell death, functional alterations and secondary remodeling of the diseased retina.
(Left column) Neuronal types (red) primarily affected by retinal disease.
(Middle column) Scotopic and photopic electroretinograms (ERGs) of normal animals and of animals afflicted by disease.
(Right column) Neurons commonly undergoing remodeling (colored) after cell loss (shown in red in left column). Pr: photoreceptor, Rod: Rod photoreceptor, Cone: Cone photoreceptor, HC: horizontal cell, BC: bipolar cell, CBC, cone bipolar cell, RBC: rod bipolar cell, RGC: retinal ganglion cell.
At the cellular level, patch-clamp recordings of both rod and cone bipolar cells from the retinas of rd10 model mice for RP revealed that bipolar cells lose their response to glutamate, correlated with a loss photoreceptors and mGluR6 expression (Puthussery et al., 2009). However, the different types of bipolar cells display loss of mGluR6 currents at different rates, with ON-cone bipolar cells maintaining mGluR6-responses for a longer period of time compared to rod-bipolar cells (Puthussery et al., 2009). However, in another study, assessing glutamate-sensitivity in rod bipolar cells by patch-clamp recording did not reveal alterations in the same rd10 RP model mice, although severe morphological perturbations were evident (Barhoum et al., 2008). In vitro recordings has shown that rod bipolar cells in the rd1 mouse model of RP demonstrate an increased sensitivity to inhibitory neurotransmitters in addition to their loss of glutamate sensitivity (Varela et al., 2003). Taken together, in vitro recordings from bipolar cells suggest that many types of physiological changes occur in these cells upon loss of transmission from their presynaptic partners.
Although we have yet to fully understand how each physiological abnormality is generated, it is clear that rewiring of the surviving synaptic partners can account for some of the functional changes. An increasing number of studies at the cellular level have collectively demonstrated the potential for photoreceptors to rewire not only during development, but also in disease. Rod bipolar cells normally only contact rod photoreceptors, but these bipolar cells connect with cones in the Nrl mutant mice, in which all photoreceptors adopt the cone fate during development (Strettoi et al., 2004). Moreover, the axon of mouse horizontal cells that normally contacts rods, forms ectopic synapses with cones in the Nrl mutant. These observations suggest that connections with rods or cones can be re-specified during development. Observations in diseased retinas now indicate that rewiring with rods or cones can occur even after photoreceptor circuits are established. Notably, loss of rods in mouse models of RP causes rod bipolar cell dendrites to retract (Cuenca et al., 2004; Cuenca et al., 2005; Gargini et al., 2007; Strettoi and Pignatelli, 2000) but later form ectopic connections with cone photoreceptors (Phillips et al., 2010; Puthussery et al., 2009). Likewise, when cones are non-functional in the CNGA3 (cyclic nucleotide gated channel) deficient mouse retina, cone bipolar cells form ectopic synapses with rod photoreceptors (Haverkamp et al., 2006). When there is death of both rods and cones, changes in wiring at the OPL are often accompanied by elaboration of neuronal processes. A well-studied example is the rd/rd mouse model of RP, in which bipolar cell dendrites are found to regress but horizontal cell dendrites sprout following photoreceptor degeneration (Strettoi and Pignatelli, 2000; Strettoi et al., 2003; Strettoi et al., 2002).
Photoreceptor death can also trigger redistribution of glutamate receptors in bipolar cells, leading to functional alterations. During the period of photoreceptor degeneration, mGluR6 expression within ON-bipolar cell dendrites is reduced until the bipolar cell is largely unresponsive to glutamate (Puthussery et al., 2009). mGluR6 receptors are mis-directed to the cell body and axons of the rod bipolar cells (Gargini et al., 2007). Mislocalization of proteins to the bipolar cell body has also been observed in rd1 RP model mice for the transient receptor protein cation channel subfamily M member 1(TRPM1) channel involved in mGluR6 transduction, which normally accumulates on the dendrites of ON-bipolar cells (Krizaj et al., 2010). By contrast, iGluRs on the dendrites of OFF-bipolar cells remain at postsynaptic sites for a longer period in the absence of their presynaptic photoreceptor partners (Puthussery et al., 2009). Alongside the loss and redistribution of receptors, bipolar cells can also alter their composition of GluRs. An instance of human RP (with cone sparing) provides some evidence that rod bipolar cells could upregulate iGluRs probably at ectopic non-ribbon synapses with survivor cones (Marc et al., 2007b). Changes in glutamate receptor expression have also been observed in the mGluR6 mutant mice, in which ON-bipolar cells instead express mGluR7 at their contacts with rods and cones (Tsukamoto et al., 2007). Together, these observations suggest that receptor type and localization at bipolar cell dendritic terminals depend on maintaining contact with appropriate photoreceptors. However, the cellular and molecular signals that normally ensure proper trafficking and localization of the postsynaptic components of bipolar cell–photoreceptor synapses remain largely unknown.
4.2.2. Inner retina
Substantial changes in the connectivity and function of the inner retina is evident in conditions such as glaucoma, optic nerve injuries or retinal ischemia that lead to ganglion cell loss. Retinal ganglion cell death in these diseases is highly stereotyped, with initial injury to the retinal ganglion cell axon followed by dendritic and cell body shrinkage, and ultimately, cell death. Loss of retinal ganglion cells can be detected in vivo by changes in patterned ERGs, as shown by the retinal response to contrast-reversing checkerboxes (Nagaraju et al., 2007; Saleh et al., 2007). Full-field ERG response studies indicate that in addition to retinal ganglion cells, bipolar cell function is perturbed in multiple animal models of glaucoma (Cuenca et al., 2010; Georgiou et al., 2013). Furthermore, disruption to bipolar cell function occurs before retinal ganglion cell loss is evident (Frankfort et al., 2013). Such studies have prompted in vitro recordings of inner retinal neurons with an aim to better understand the physiological basis of diseases targeting the GCL.
Direct measurements of the light responses of retinal ganglion cells have been performed using electrophysiological approaches that measure synaptic potentials, or current or spike activity of the neurons, albeit in in vitro preparations (Weber and Harman, 2005). More recently, multielectrode arrays (MEA) have been used to record spike activity across populations of physiologically-identified retinal ganglion cells during the degenerative process in mouse models of glaucoma (Della Santina et al., 2013; Feng et al., 2013). Recordings from populations of known retinal ganglion cell types suggest that degeneration differentially affects distinct retinal ganglion cells in mice (Della Santina et al., 2013; Feng et al., 2013) in which glaucoma was stimulated by increased intraocular pressure. The dendritic arbors of macaque parasol cells in a monkey model of glaucoma shrink significantly, whereas those of midget cells do not seem to be affected within the same time frame (Weber et al., 1998). This dendritic retraction in parasol cells is associated with a functional impairment of spatial and temporal responses to patterned stimuli (Weber and Harman, 2005). Likewise, in a mouse model of glaucoma, OFF-transient ganglion cells show a reduction in their structural and functional receptive fields before ON- and OFF-sustained ganglion cells (Della Santina et al., 2013). It is unclear why some retinal ganglion cells respond more rapidly to intraocular pressure elevation compared to others. It also remains to be seen why ganglion cell death is more prominent in some regions of the retina, leading to a sectorial pattern of cell loss in DBA/2J model mice for glaucoma (Jakobs et al., 2005).
A combined analysis of the dendritic arbor morphology, synaptic connectivity and function of mouse retinal ganglion cells in a glaucoma model has recently established that synapses are lost even before dendrites retract (Della Santina et al., 2013). Mouse ON-sustained retinal ganglion cells already show altered spontaneous activity, light-responses and glutamatergic synapse density before their dendritic arbors are pruned (Della Santina et al., 2013). In parallel with the reduction of synapses between retinal ganglion cells and bipolar cells, changes in connectivity between bipolar cells and photoreceptors have also been observed in murine models of glaucoma. In particular, reduction of OPL thickness and number of photoreceptor ribbons has been reported in mice after intraocular pressure elevation (Cuenca et al., 2010). Ultrastructural alterations of photoreceptor ribbons such as the appearance of club-shaped or detached spherical ribbons have been observed in DBA/2J glaucoma model mice (Fuchs et al., 2012). These findings underscore the notion that changes in connectivity are not restricted to the GCL where cell death is occurring. Conversely, photoreceptor loss leads to changes in the inner retina (Jones and Marc, 2005). For example, in rd1 model mice for RP, the number of ribbons in cone bipolar cell axon terminals follow the decline in their photoreceptor input (Chen et al., 2012).
Even in cases in which no obvious structural alterations of specific cell types are apparent, changes to functional connectivity have been identified. Abnormal spontaneous rhythmic spiking activity is observed in retinal ganglion cells in multiple murine models of photoreceptor degeneration (Sekirnjak et al., 2011; Stasheff et al., 2011). In rd1 mice rhythmic activity is present in AII amacrine cells, and propagates amongst their gap junctionally-coupled network to neighboring ON-cone bipolar cells. There is evidence to suggest that this rhythmic activity in the inner retina is normally suppressed; pharmacological blockade of the light response in wildtype mice induces oscillatory activity in ON-bipolar cells and AII amacrine cells (Trenholm et al., 2012). Thus, while generally thought to be only a characteristic feature of the developing retina (see 3.4.2.), synchronized rhythmic activity can be reinstated in the mature retina in certain diseases, and under acute blockade of all synaptic inhibition (Toychiev et al., 2013).
In summary, it is clear that there are abnormalities in structure, synapse function and connectivity prior to cell death. It is also evident that upon cell loss, remaining synaptic partners can search for new partners, in a manner that often involves structural reorganization. Thus, even though a specific retinal cell type is destined to die in each retinal disease, the downstream effects can lead to massive structural and/or functional reorganization of the retina (Marc et al., 2007a; Marc et al., 2003). However, it should be emphasized that even diseases in which the same cell type degenerates may not share a common outcome, as in some models cell death occurs during development while in others the cell loss can be after maturity. For example, in the rd1 RP model, about half the retinal ganglion cell population develops smaller arbors (Damiani et al., 2012). But, in the rd10 mouse model for RP, retinal ganglion cell dendritic arbors appear normal (Mazzoni et al., 2008). Collectively, such observations underscore the complexity of the damage and rearrangements to retinal circuits upon death of a single cell type or in response to insult, making the task of restoring normal vision extremely challenging.
4.3. Therapeutic approaches to restore retinal cell types and connectivity
Designing an effective therapy to restore function to the diseased retina has taken on diverse approaches. One approach is to find ways to protect the neurons that appear most susceptible as well as the neurons that have not yet been damaged. This approach has been undertaken for almost all of the common retinal diseases, and it has proven to be partially effective in slowing degeneration. Supplementing the amino acid taurine has recently proven to be a viable approach for slowing retinal ganglion cell degeneration in multiple animal models (Froger et al., 2012; Froger et al., 2013), as well as improving resistance to hypoxic conditions in retinal cell cultures (Chen et al., 2009). Under ischemic conditions, modulation of adrenergic receptors either by using alpha2-agonists (Lafuente et al., 2001; Vidal-Sanz et al., 2001) or beta-2 antagonists (Chen et al., 2007) has provided neuroprotection for retinal ganglion cells. This neuroprotection has been proposed to arise from the regulation of NMDA receptor-mediated transmission onto retinal ganglion cells, despite the fact that these neurons are relatively insensitive to glutamate and NMDA excitotoxicity (Ullian et al., 2004). Preconditioning to hypoxia itself appears to provide partial neuroprotection of mouse retinas to successive hypoxic damage (Zhu et al., 2007), or to the insurgence of glaucoma in mouse models (Zhu et al., 2012). This mechanism involves activation of hypoxia-inducible factors such as HIF-1 (Grimm et al., 2002) and production of erythropoietin (Zhong et al., 2007). Neuroprotection of photoreceptors has been observed following administration of neurotrophic factors such as CNTF (ciliary neurotrophic factor). These factors normally play a regulatory role in phototransduction and regeneration of the outer segments of photoreceptors (Li et al., 2010). Behavioral paradigms such as environmental enrichment that increase in vivo levels of these neurotrophic factors, exert protective effects against mouse photoreceptor degeneration (Barone et al., 2012). The mechanism of action of CNTF as elucidated in mouse retina initially involves interaction with the cytokine receptors gp130 on Müller glia cells, since their genetic disruption abolishes CNTF neuroprotection (Rhee et al., 2013). Because photoreceptors usually die by apoptosis, neuroprotection has been induced by inhibiting the apoptotic signaling pathway mediated by the phospholipid ceramide in light-induced photoreceptor degeneration models (Chen et al., 2013a), and in the rd10 mouse model for RP (Strettoi et al., 2010). Taken together, these numerous examples indicate that there have been significant advances in minimizing cell loss in the diseased retina.
Once degeneration is advanced and there is already substantial cell death, the major goal becomes repopulating the retina with the lost population of neurons. Although many challenges persist, there has been encouraging progress in this arena. Unlike animal models like fish, amphibian and chicken that can regenerate photoreceptors (Fischer and Reh, 2001; Reh and Levine, 1998; Taylor et al., 2012), mammalian retina has limited regenerative capacity. However, recent experiments show that rod precursors transplanted into genetically blind mice can restore vision (Pearson et al., 2012) as well as reverse end-stage degeneration in rd1 model mice for RP (Singh et al., 2013). Photoreceptors derived from human embryonic stem cells are able to restore retinal function when transplanted in a mouse model of Leber Congenital Amaurosis (Lamba et al., 2009). An important advance in this area came with the recent demonstration that photoreceptors can be regenerated in mice by stimulating Müller glia cells to proliferate and produce neuron precursors (Pollak et al., 2013). In vivo stimulation of mammalian Müller glial cells to dedifferentiate, divide and produce neurons in situ would represent a major step forward.
For diseases in which non-functional proteins result from an identified genetic mutation, a logical approach is gene therapy. Viral mediated expression of phosphodiesterase has been shown to restore photoreceptor function in mice with retinal degeneration (Deng et al., 2013; Pang et al., 2011). Similarly, viral delivery of guanylate cyclase (GC) in GC1 KO mice (Boye et al., 2011) and CNGA3 in CNGA3 KO mice (Michalakis et al., 2010) restores cone function. Impressively, disruption of the retina in a mouse model of X-linked neuroschisis has been successfully prevented by delivery of the wildtype retinoschisin gene using adeno-associated viral (AAV) vectors (Park et al., 2009). Moreover, gene therapy appears to be an effective strategy to restore color vision to adult monkeys (Mancuso et al., 2009). More efficient methods for viral transfection, including targeting of specific neuronal types are rapidly becoming available in mice (Dalkara et al., 2013) and in monkeys (Vandenberghe et al., 2013). Cell-targeted therapy could allow a more efficient and directed treatment of the diseased condition, and there have been recent and significant improvements in viral delivery of genes, including into monkey retina (Dalkara et al., 2013).
In order to compensate for loss of function after massive cell death, investigators have also turned to directly stimulating the remaining retinal neurons in disease conditions. At the photoreceptor level, genetically targeting halorhodopsin (light-activated chloride pump) to photoreceptors by means of AAVs can induce light-sensitivity in cone photoreceptors and restore visual responses in mouse models of RP (Busskamp et al., 2010). At the inner retina level, channelrhodopsin-2 (light-activated cation channel) expression driven by the mGluR promoter in ON-bipolar cells restores visual function in rd1 model mice for RP (Doroudchi et al., 2011; Lagali et al., 2008), and a comparable rescue of function has also been observed after viral transfection of melanopsin in rd/rd model mice for RP (Lin et al., 2008). Recently, it has been discovered that delivering small light-sensitive molecules, such as acrylamide-azobenzene-quaternary ammonium (AAQ) or its diethylamino derivative DENAQ, confers light sensitivity to ganglion cells in mice with retinal degeneration (Polosukhina et al., 2012; Tochitsky et al., 2014). The possibility of expressing photosensitive molecules in ganglion cells represents a promising strategy to treat late stages of retinal disease when photoreceptors die or when the retinal circuitry is heavily compromised.
An ‘engineering’ approach towards restoring vision in end stages of degeneration involves electrical stimulation of retinal ganglion cells by implantable microelectrode arrays. This strategy overcomes the difficulty in re-seeding the missing photoreceptors. Direct stimulation of ganglion cells (Sekirnjak et al., 2008) has been demonstrated, even clinically, to confer light sensitivity to degenerated retinas in patients (Stronks and Dagnelie, 2014). However, strategies that bypass the conventional phototransduction cascade share a common problem in that they lead to a much-diminished light operating range for the stimulated cells. The optimal parameters for stimulating retinal ganglion cells in order to reach their native spatial and temporal resolution is being established in primate (macaque) retina (Jepson et al., 2013). Features such as color vision or a complete representation of the visual field are still distant goals, in part because of the technical limitations, but mostly because our knowledge of how the retina processes visual information is still incomplete.
5. Future goals
Although there have been tremendous advances in restoring photosensitivity to retinal neurons, much remains to be done to recapture the intricate processing of visual information normally performed by the many parallel retinal circuits. As methods to replace retinal neurons become routine, the next major challenge is to ensure that the new neurons reconnect properly. This is not a simple task because the retinal environment can change drastically even when one cell type dies. Successful reconstruction of the retinal circuitry will therefore also require neurons that survive to maintain appropriate connections. Thus, restoring sight to its fullest measure will necessitate coordination of strategies to recapitulate the original circuitry of retinal neurons that are lost, as well as of those that remain. To achieve the ultimate goal of restoring sight, two avenues of research are critical. The first is to get a comprehensive understanding of all retinal subtypes and their connectivity, and the second is to decipher the molecular and cellular cues that instruct retinal assembly during development.
The field currently lacks knowledge of the structural and functional connectivity for all retinal cell types in a given species. A key limitation is the lack of genetic markers that can be used to identify each cell type. Much work is currently underway, however, to expand the set of transgenic mouse lines in which specific cell types are either labeled, or can be targeted for manipulation (Huberman et al., 2009; Kim et al., 2008). Connectomic approaches are uncovering connections and potential connections between morphologically defined retinal cell types at the ultrastructural level (Helmstaedter et al., 2013; Marc et al., 2013). What is lacking is a direct correlation between the structural and functional organizations of a defined circuit. Experiments that will reveal the synaptic and integrative properties of the circuit, as well as demonstrate how retinal cell responses are altered by neuromodulators and varying stimulus conditions, continue to be essential to the field. Recent work has pushed forward the need to correlate structure with function by combining functional imaging with serial EM (Briggman et al., 2011). No doubt such an approach will escalate as serial EM reconstructions become more routine.
Although we have focused primarily on circuitry within the retina in this review, it should be emphasized that determining the axonal projection patterns of each ganglion cell type, and thus the central targets of these cells, is also important. Progress in this direction has been made using transgenic mice where subsets of ganglion cells are labeled and their axonal trajectories followed (Dhande et al., 2013). It is also necessary to translate approaches now routine in vertebrates amenable to genetic manipulation such as mice and zebrafish, to monkeys. As such, there have been significant advances in the use of viruses to deliver genes to monkey retinal cells in vivo (Mancuso et al., 2009; Yin et al., 2011). Moreover, recent studies have begun mapping the synaptic connectivity patterns of marmoset ganglion cells using gene-delivery approaches to visualize synapses in retinal explants (Moritoh et al., 2013; Percival et al., 2014). The application of molecular tools towards elucidating the structure, connectivity and function of monkey retinal neurons will be immensely helpful towards attaining therapies for curing blindness in humans.
In parallel with the advances made in studying the adult retina, there have also recently been significant findings in the area of retinal development. Critical cellular and molecular cues that underlie cell type-specific lamination, or those that organize retinal cells into their respective mosaics have been discovered. However, the signaling pathways downstream of the homotypic or heterotypic interactions that are involved are yet to be identified. We have also obtained a clearer view of the mechanisms that regulate synaptic connectivity, especially with regard to the key molecular players and the role of neurotransmission. A common finding is that even within a major cell class (e.g. ganglion cells, bipolar cells), different cell types adopt different developmental strategies to establish their patterns of connectivity. Identifying common principles of synaptic development across retinal circuits may therefore be challenging, but the diversity in strategies offer some hope that rewiring of circuitry may be directed in more than one way. In retinas with specialized circuits such as those found in the fovea, the identities of the cues that generate such unique circuits remains elusive. Determining the cellular and molecular interactions that create the fovea will require genetic manipulation, possible via viral transfection techniques. Finally, the molecular assembly of individual synapses in the retina remains to be fully explored, a task that is now more tenable because of the increasing knowledge of the compositions of synaptic proteins found at retinal synapses, and the availability of transgenic tools.
In summary, advancing knowledge of the structural and functional organization of the mature retina and its development will no doubt help us formulate therapies aimed at reconstructing circuits damaged by disease. As yet, we do not know whether the cellular and molecular mechanisms that are critical for the initial construction of the retina are still available for regenerative processes to take place. If so, we will need to find ways to resurrect these mechanisms to rewire new neurons into a mature environment. If not, novel genetic/molecular pathways will have to be evoked in order to reconnect retinal cells after damage. In this regard, it would be instructive to compare the genetic programs underlying neurogenesis in animals with (zebrafish) or without (mice) neuroregenerative capabilities. Thus, comparative studies of retinal organization and development across species have multiple benefits, from identifying common and unique retinal designs to retinal repair.
Acknowledgements
Supported by NIH grants (EY10699, 17101, 14358) to R.O.L.W, Knights Templar Eye Foundation grant to M.H. and a Human Frontier Science Program grant (L. Lagnado, F. Schmitz and R.O.L.W.). We would like to thank the reviewers for their helpful comments and suggestions. We would also like to thank E. Strettoi, L. Galli-Resta, R. Sinha and F.D. D’Orazi for critical reading of the manuscript and useful comments. We are grateful to R. Sinha, F.A. Dunn, S.C. Suzuki and T. Yoshimatsu for providing some of the images and E. Parker (supported by EY01730) for acquiring the electron micrographs.
List of abbreviations
- AAQ
Acrylamide-azobenzene-quaternary ammonium
- AAV
Adeno-associated virus
- AC
Amacrine cell
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- APB
2-amino-4-phosphonobutyric acid
- ATP
Adenosine triphosphate
- BC
Bipolar cell
- BDNF
Brain derived neurotrophic factor
- BMP
Bone morphogen protein
- bNOS
Brain nitric-oxide synthase
- C
Cone photoreceptor
- Cad
Cadherin
- CBC
Cone bipolar cell
- ChaT
Choline acetyltransferase
- CICR
Calcium-induced calcium-release
- CMZ
Ciliary marginal zone
- CNG
Cyclic nucleotide gated ion channel
- CNTF
Ciliary neurotrophic factor
- COUP-TF
Chicken ovalbumin upstream promoter transcription factor
- CRX
Cone-rod homeobox
- DAC
Dopaminergic amacrine cell
- dKO
Double knockout
- DS
Direction selective
- Dscam
Down-syndrome cell adhesion molecule
- Dscaml
Down-syndrome cell adhesion molecule like
- E
Embryonic day
- EM
Electron microscope
- EPSC
Excitatory postsynaptic current
- ERG
Electroretinogram
- Fat3
FAT atypical cadherin3
- Fd
Fetal day
- FP
Fluorescent protein
- GABA
γ-aminobutyric acid
- GAD
Glutamic acid decarboxylase
- GC
Guanylate cyclase
- GCL
Ganglion cell layer
- GluR
Glutamate receptor
- GlyR
Glycine receptor
- GRIP
Glutamate receptor interacting protein
- H
Hour
- Has
Heart-and-soul
- HC
Horizontal cell
- HIF
Hypoxia-inducible factor
- I/E
Inhibitory/Excitatory
- iGluR
Ionotropic glutamate receptor
- IgSF
Immunoglobulin superfamily
- INL
Inner nuclear layer
- INBL
Inner neuroblastic layer
- IPL
Inner plexiform layer
- IPSC
Inhibitory postsynaptic current
- KO
Knockout
- L
Long
- M
Medium
- MAGI
Membrane-associated guanylate kinase with inverted orientation
- MAP1B
Microtubule-associated protein 1B
- Math
Mouse atonal homologue
- MEA
Multielectrode array
- MEGF
Multiple epidermal growth factor-like domains protein
- mGluR
Metabotropic glutamate receptor
- N.D.
Not determined
- NGL-2
Netrin-G ligand2
- NMDA
N-methyl-D-aspartate
- OE
Overexpression
- ONL
Outer nuclear layer
- ONBL
Outer neuroblastic layer
- OPL
Outer plexiform layer
- P
Postnatal day
- Pcdh
Protocadherin
- Plex
Plexin
- PSD
Postsynaptic density protein
- Pr
Photoreceptor
- R
Rod photoreceptor
- RAR
Retinoic acid receptor
- RBC
Rod bipolar cell
- RGC
Retinal ganglion cell
- RNAi
RNA interference
- ROR
Retinoid-related orphan receptor
- RP
Retinitis pigmentosa
- Rs
Receptors
- RXR
Retinoid X receptor
- S
Short
- SAC
Starburst amacrine cell
- SAP102
Synapse-associated protein 102
- Sdk
Sidekick
- Sema
Semaphorin
- SHH
Sonic hedgehog
- SynCAM
Synaptic cell adhesion molecule
- Tbx
T-box 5
- TeNT
Tetanus toxin
- TH
Thyroid hormone
- TH (DAC)
Tyrosine hydroxylase
- Trβ2
Thyroid hormone receptor β2
- TRPM1
Transient receptor protein cation channel subfamily M member 1
- UV
Ultraviolet
- VAMP2
Vesicle-associated membrane protein 2
- Vax
Ventral anterior homeobox
- VGluT
Vesicular glutamate transporter
- VIAAT
Vesicular inhibitory amino acid transporter
- Wk
Week
- WT
Wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbott CJ, Percival KA, Martin PR, Grunert U. Amacrine and bipolar inputs to midget and parasol ganglion cells in marmoset retina. Vis Neurosci. 2012;29:157–168. doi: 10.1017/S095252381200017X. [DOI] [PubMed] [Google Scholar]
- Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in primate retina: a Golgi-light microscopic study of spectral connectivity. J Comp Neurol. 1994;343:387–405. doi: 10.1002/cne.903430305. [DOI] [PubMed] [Google Scholar]
- Alfano G, Conte I, Caramico T, Avellino R, Arno B, Pizzo MT, Tanimoto N, Beck SC, Huber G, Dolle P, Seeliger MW, Banfi S. Vax2 regulates retinoic acid distribution and cone opsin expression in the vertebrate eye. Development. 2011;138:261–271. doi: 10.1242/dev.051037. [DOI] [PubMed] [Google Scholar]
- Allison WT, Barthel LK, Skebo KM, Takechi M, Kawamura S, Raymond PA. Ontogeny of cone photoreceptor mosaics in zebrafish. J Comp Neurol. 2010;518:4182–4195. doi: 10.1002/cne.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Auferkorte ON, Baden T, Kaushalya SK, Zabouri N, Rudolph U, Haverkamp S, Euler T. GABA(A) receptors containing the alpha2 subunit are critical for direction-selective inhibition in the retina. PLoS One. 2012;7:e35109. doi: 10.1371/journal.pone.0035109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden T, Esposti F, Nikolaev A, Lagnado L. Spikes in retinal bipolar cells phase-lock to visual stimuli with millisecond precision. Curr Biol. 2011;21:1859–1869. doi: 10.1016/j.cub.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden T, Schubert T, Chang L, Wei T, Zaichuk M, Wissinger B, Euler T. A Tale of Two Retinal Domains: Near-Optimal Sampling of Achromatic Contrasts in Natural Scenes through Asymmetric Photoreceptor Distribution. Neuron. 2013;80:1206–1217. doi: 10.1016/j.neuron.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–8847. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadori R, Biehlmaier O, Zeitz C, Labhart T, Makhankov YV, Forster U, Gesemann M, Berger W, Neuhauss SC. Nyctalopin is essential for synaptic transmission in the cone dominated zebrafish retina. Eur J Neurosci. 2006;24:1664–1674. doi: 10.1111/j.1460-9568.2006.05053.x. [DOI] [PubMed] [Google Scholar]
- Barhoum R, Martínez-Navarrete G, Corrochano S, Germain F, Fernandez-Sanchez L, de la Rosa EJ, de la Villa P, Cuenca N. Functional and structural modifications during retinal degeneration in the rd10 mouse. Neuroscience. 2008;155:698–713. doi: 10.1016/j.neuroscience.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Hill RM. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science. 1963;139:412–414. doi: 10.1126/science.139.3553.412. [DOI] [PubMed] [Google Scholar]
- Barone I, Novelli E, Piano I, Gargini C, Strettoi E. Environmental enrichment extends photoreceptor survival and visual function in a mouse model of retinitis pigmentosa. PloS one. 2012;7:e50726. doi: 10.1371/journal.pone.0050726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. Spectral sensitivity of cones of the monkey Macaca fascicularis. J Physiol. 1987;390:145–160. doi: 10.1113/jphysiol.1987.sp016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrodin TJ, Marks MS, Ozato K, Linney E, Lazar MA. Heterodimerization among thyroid hormone receptor, retinoic acid receptor, retinoid X receptor, chicken ovalbumin upstream promoter transcription factor, and an endogenous liver protein. Molecular endocrinology. 1992;6:1468–1478. doi: 10.1210/mend.6.9.1331778. [DOI] [PubMed] [Google Scholar]
- Biehlmaier O, Neuhauss SC, Kohler K. Onset and time course of apoptosis in the developing zebrafish retina. Cell Tissue Res. 2001;306:199–207. doi: 10.1007/s004410100447. [DOI] [PubMed] [Google Scholar]
- Biehlmaier O, Neuhauss SC, Kohler K. Double cone dystrophy and RPE degeneration in the retina of the zebrafish gnn mutant. Invest Ophthalmol Vis Sci. 2003;44:1287–1298. doi: 10.1167/iovs.02-0363. [DOI] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanks JC, Adinolfi AM, Lolley RN. Synaptogenesis in the photoreceptor terminal of the mouse retina. J Comp Neurol. 1974;156:81–93. doi: 10.1002/cne.901560107. [DOI] [PubMed] [Google Scholar]
- Bleckert A, Parker ED, Kang Y, Pancaroglu R, Soto F, Lewis R, Craig AM, Wong RO. Spatial relationships between GABAergic and glutamatergic synapses on the dendrites of distinct types of mouse retinal ganglion cells across development. PLoS One. 2013;8:e69612. doi: 10.1371/journal.pone.0069612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckert A, Wong RO. Identifying roles for neurotransmission in circuit assembly: insights gained from multiple model systems and experimental approaches. Bioessays. 2011;33:61–72. doi: 10.1002/bies.201000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–384. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Bodnarenko SR, Chalupa LM. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993;364:144–146. doi: 10.1038/364144a0. [DOI] [PubMed] [Google Scholar]
- Bodnarenko SR, Jeyarasasingam G, Chalupa LM. Development and regulation of dendritic stratification in retinal ganglion cells by glutamate-mediated afferent activity. J Neurosci. 1995;15:7037–7045. doi: 10.1523/JNEUROSCI.15-11-07037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnarenko SR, Yeung G, Thomas L, McCarthy M. The development of retinal ganglion cell dendritic stratification in ferrets. Neuroreport. 1999;10:2955–2959. doi: 10.1097/00001756-199909290-00015. [DOI] [PubMed] [Google Scholar]
- Borghuis BG, Looger LL, Tomita S, Demb JB. Kainate Receptors Mediate Signaling in Both Transient and Sustained OFF Bipolar Cell Pathways in Mouse Retina. J Neurosci. 2014;34:6128–6139. doi: 10.1523/JNEUROSCI.4941-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott B, Wässle H. Parallel processing in the mammalian retina: the Proctor Lecture. Invest Ophthalmol Vis Sci. 1999;40:1313–1327. [PubMed] [Google Scholar]
- Boye SL, Conlon T, Erger K, Ryals R, Neeley A, Cossette T, Pang J, Dyka FM, Hauswirth WW, Boye SE. Long-term preservation of cone photoreceptors and restoration of cone function by gene therapy in the guanylate cyclase-1 knockout (GC1KO) mouse. Investigative ophthalmology & visual science. 2011;52:7098–7108. doi: 10.1167/iovs.11-7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstatter JH, Fletcher EL, Garner CC, Gundelfinger ED, Wässle H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur J Neurosci. 1999;11:3683–3693. doi: 10.1046/j.1460-9568.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- Brandstatter JH, Koulen P, Wässle H. Selective synaptic distribution of kainate receptor subunits in the two plexiform layers of the rat retina. J Neurosci. 1997;17:9298–9307. doi: 10.1523/JNEUROSCI.17-23-09298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuninger T, Puller C, Haverkamp S, Euler T. Chromatic bipolar cell pathways in the mouse retina. J Neurosci. 2011;31:6504–6517. doi: 10.1523/JNEUROSCI.0616-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188. doi: 10.1038/nature09818. [DOI] [PubMed] [Google Scholar]
- Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busskamp V, Duebel J, Balya D, Fradot M, Viney TJ, Siegert S, Groner AC, Cabuy E, Forster V, Seeliger M, Biel M, Humphries P, Paques M, Mohand-Said S, Trono D, Deisseroth K, Sahel JA, Picaud S, Roska B. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science. 2010;329:413–417. doi: 10.1126/science.1190897. [DOI] [PubMed] [Google Scholar]
- Byrne LC, Khalid F, Lee T, Zin EA, Greenberg KP, Visel M, Schaffer DV, Flannery JG. AAV-mediated, optogenetic ablation of Müller Glia leads to structural and functional changes in the mouse retina. PloS one. 2013;8:e76075. doi: 10.1371/journal.pone.0076075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadetti L, Tranchina D, Thoreson WB. A comparison of release kinetics and glutamate receptor properties in shaping rod-cone differences in EPSC kinetics in the salamander retina. J Physiol. 2005;569:773–788. doi: 10.1113/jphysiol.2005.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ. Localization of ionotropic glutamate receptors to invaginating dendrites at the cone synapse in primate retina. Vis Neurosci. 2005;22:469–477. doi: 10.1017/S0952523805224082. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Schein SJ, Tsukamoto Y, Sterling P. M and L cones in macaque fovea connect to midget ganglion cells by different numbers of excitatory synapses. Nature. 1994;371:70–72. doi: 10.1038/371070a0. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Sterling P. Microcircuitry for two types of achromatic ganglion cell in primate fovea. J Neurosci. 2007;27:2646–2653. doi: 10.1523/JNEUROSCI.4739-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ, Tsukamoto Y, Sterling P. Microcircuitry and mosaic of a blue-yellow ganglion cell in the primate retina. J Neurosci. 1998;18:3373–3385. doi: 10.1523/JNEUROSCI.18-09-03373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DA. Mapping absorbance spectra, cone fractions, and neuronal mechanisms to photopic spectral sensitivity in the zebrafish. Vis Neurosci. 2002;19:365–372. doi: 10.1017/s0952523802192121. [DOI] [PubMed] [Google Scholar]
- Cenni MC, Bonfanti L, Martinou JC, Ratto GM, Strettoi E, Maffei L. Long-term survival of retinal ganglion cells following optic nerve section in adult bcl-2 transgenic mice. Eur J Neurosci. 1996;8:1735–1745. doi: 10.1111/j.1460-9568.1996.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Centanin L, Hoeckendorf B, Wittbrodt J. Fate restriction and multipotency in retinal stem cells. Cell stem cell. 2011;9:553–562. doi: 10.1016/j.stem.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Chabrol FP, Eglen SJ, Sernagor E. GABAergic control of retinal ganglion cell dendritic development. Neuroscience. 2012;227:30–43. doi: 10.1016/j.neuroscience.2012.09.040. [DOI] [PubMed] [Google Scholar]
- Chan TL, Martin PR, Clunas N, Grunert U. Bipolar cell diversity in the primate retina: morphologic and immunocytochemical analysis of a new world monkey, the marmoset Callithrix jacchus. J Comp Neurol. 2001;437:219–239. doi: 10.1002/cne.1280. [DOI] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision research. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- Chang B, Heckenlively JR, Bayley PR, Brecha NC, Davisson MT, Hawes NL, Hirano AA, Hurd RE, Ikeda A, Johnson BA, McCall MA, Morgans CW, Nusinowitz S, Peachey NS, Rice DS, Vessey KA, Gregg RG. The nob2 mouse, a null mutation in Cacna1f: anatomical and functional abnormalities in the outer retina and their consequences on ganglion cell visual responses. Vis Neurosci. 2006;23:11–24. doi: 10.1017/S095252380623102X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Breuninger T, Euler T. Chromatic coding from cone-type unselective circuits in the mouse retina. Neuron. 2013;77:559–571. doi: 10.1016/j.neuron.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Chen H, Tran J-TA, Eckerd A, Huynh T-P, Elliott MH, Brush RS, Mandal NA. Inhibition of de novo ceramide biosynthesis by FTY720 protects rat retina from light-induced degeneration. Journal of lipid research. 2013a;54:1616–1629. doi: 10.1194/jlr.M035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Zhang Q, Wang J, Liu F, Mi M, Xu H, Chen F, Zeng K. Taurine protects transformed rat retinal ganglion cells from hypoxia-induced apoptosis by preventing mitochondrial dysfunction. Brain research. 2009;1279:131–138. doi: 10.1016/j.brainres.2009.04.054. [DOI] [PubMed] [Google Scholar]
- Chen M, Wang K, Lin B. Development and degeneration of cone bipolar cells are independent of cone photoreceptors in a mouse model of retinitis pigmentosa. PloS one. 2012;7:e44036. doi: 10.1371/journal.pone.0044036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci. 2002;22:2165–2173. doi: 10.1523/JNEUROSCI.22-06-02165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Chew KS, McNeill DS, Keeley PW, Ecker JL, Mao BQ, Pahlberg J, Kim B, Lee SC, Fox MA, Guido W, Wong KY, Sampath AP, Reese BE, Kuruvilla R, Hattar S. Apoptosis regulates ipRGC spacing necessary for rods and cones to drive circadian photoentrainment. Neuron. 2013b;77:503–515. doi: 10.1016/j.neuron.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-N, Yamada H, Mao W, Matsuyama S, Aihara M, Araie M. Hypoxia-induced retinal ganglion cell death and the neuroprotective effects of beta-adrenergic antagonists. Brain research. 2007;1148:28–37. doi: 10.1016/j.brainres.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Chinen A, Hamaoka T, Yamada Y, Kawamura S. Gene duplication and spectral diversification of cone visual pigments of zebrafish. Genetics. 2003;163:663–675. doi: 10.1093/genetics/163.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Law MY, Chien CB, Link BA, Wong RO. In vivo development of dendritic orientation in wild-type and mislocalized retinal ganglion cells. Neural Dev. 2010;5:29. doi: 10.1186/1749-8104-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysostomou V, Valter K, Stone J. Cone-rod dependence in the rat retina: variation with the rate of rod damage. Invest Ophthalmol Vis Sci. 2009;50:3017–3023. doi: 10.1167/iovs.08-3004. [DOI] [PubMed] [Google Scholar]
- Chun MH, Grunert U, Martin PR, Wässle H. The synaptic complex of cones in the fovea and in the periphery of the macaque monkey retina. Vision Res. 1996;36:3383–3395. doi: 10.1016/0042-6989(95)00334-7. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Lom B. Neurotrophic regulation of retinal ganglion cell synaptic connectivity: from axons and dendrites to synapses. Int J Dev Biol. 2004;48:947–956. doi: 10.1387/ijdb.041883sc. [DOI] [PubMed] [Google Scholar]
- Contini M, Raviola E. GABAergic synapses made by a retinal dopaminergic neuron. Proc Natl Acad Sci U S A. 2003;100:1358–1363. doi: 10.1073/pnas.0337681100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca N, Pinilla I, Fernandez-Sanchez L, Salinas-Navarro M, Alarcon-Martinez L, Aviles-Trigueros M, de la Villa P, Miralles de Imperial J, Villegas-Perez MP, Vidal-Sanz M. Changes in the inner and outer retinal layers after acute increase of the intraocular pressure in adult albino Swiss mice. Exp Eye Res. 2010;91:273–285. doi: 10.1016/j.exer.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Cuenca N, Pinilla I, Sauve Y, Lu B, Wang S, Lund RD. Regressive and reactive changes in the connectivity patterns of rod and cone pathways of P23H transgenic rat retina. Neuroscience. 2004;127:301–317. doi: 10.1016/j.neuroscience.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Cuenca N, Pinilla I, Sauve Y, Lund R. Early changes in synaptic connectivity following progressive photoreceptor degeneration in RCS rats. Eur J Neurosci. 2005;22:1057–1072. doi: 10.1111/j.1460-9568.2005.04300.x. [DOI] [PubMed] [Google Scholar]
- Cueva JG, Haverkamp S, Reimer RJ, Edwards R, Wässle H, Brecha NC. Vesicular gamma-aminobutyric acid transporter expression in amacrine and horizontal cells. J Comp Neurol. 2002;445:227–237. doi: 10.1002/cne.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. Morphology of a small-field bistratified ganglion cell type in the macaque and human retina. Vis Neurosci. 1993a;10:1081–1098. doi: 10.1017/s0952523800010191. [DOI] [PubMed] [Google Scholar]
- Dacey DM. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993b;13:5334–5355. doi: 10.1523/JNEUROSCI.13-12-05334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. Primate retina: cell types, circuits and color opponency. Prog Retin Eye Res. 1999;18:737–763. doi: 10.1016/s1350-9462(98)00013-5. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Parallel pathways for spectral coding in primate retina. Annu Rev Neurosci. 2000;23:743–775. doi: 10.1146/annurev.neuro.23.1.743. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Crook JD, Packer OS. Distinct synaptic mechanisms create parallel S-ON and S-OFF color opponent pathways in the primate retina. Vis Neurosci. 2014;31:139–151. doi: 10.1017/S0952523813000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Lee BB. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB, Stafford DK, Pokorny J, Smith VC. Horizontal cells of the primate retina: cone specificity without spectral opponency. Science. 1996;271:656–659. doi: 10.1126/science.271.5249.656. [DOI] [PubMed] [Google Scholar]
- Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5:189ra176. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- Damiani D, Alexander JJ, O'Rourke JR, McManus M, Jadhav AP, Cepko CL, Hauswirth WW, Harfe BD, Strettoi E. Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J Neurosci. 2008;28:4878–4887. doi: 10.1523/JNEUROSCI.0828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani D, Novelli E, Mazzoni F, Strettoi E. Undersized dendritic arborizations in retinal ganglion cells of the rd1 mutant mouse: a paradigm of early onset photoreceptor degeneration. J Comp Neurol. 2012;520:1406–1423. doi: 10.1002/cne.22802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport CM, Detwiler PB, Dacey DM. Effects of pH buffering on horizontal and ganglion cell light responses in primate retina: evidence for the proton hypothesis of surround formation. J Neurosci. 2008;28:456–464. doi: 10.1523/JNEUROSCI.2735-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Krol A, Abraira VE, Copley CO, Tucker AF, Goodrich LV. Control of neuronal morphology by the atypical cadherin Fat3. Neuron. 2011;71:820–832. doi: 10.1016/j.neuron.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Santina L, Inman DM, Lupien CB, Horner PJ, Wong ROL. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J Neurosci. 2013;33:17444–17457. doi: 10.1523/JNEUROSCI.5461-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W-T, Sakurai K, Kolandaivelu S, Kolesnikov AV, Dinculescu A, Li J, Zhu P, Liu X, Pang J, Chiodo VA, Boye SL, Chang B, Ramamurthy V, Kefalov VJ, Hauswirth WW. Cone phosphodiesterase-6α' restores rod function and confers distinct physiological properties in the rod phosphodiesterase-6β-deficient rd10 mouse. J Neurosci. 2013;33:11745–11753. doi: 10.1523/JNEUROSCI.1536-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplano S, Gargini C, Maccarone R, Chalupa LM, Bisti S. Long-term treatment of the developing retina with the metabotropic glutamate agonist APB induces long-term changes in the stratification of retinal ganglion cell dendrites. Dev Neurosci. 2004;26:396–405. doi: 10.1159/000082282. [DOI] [PubMed] [Google Scholar]
- Dhande OS, Estevez ME, Quattrochi LE, El-Danaf RN, Nguyen PL, Berson DM, Huberman AD. Genetic dissection of retinal inputs to brainstem nuclei controlling image stabilization. J Neurosci. 2013;33:17797–17813. doi: 10.1523/JNEUROSCI.2778-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Lyubarsky A, Jiang M, Pugh EN, Jr., Birnbaumer L, Sterling P, Vardi N. The light response of ON bipolar neurons requires G[alpha]o. J Neurosci. 2000;20:9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, Gundelfinger ED, Brandstatter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA, Arman AC, Janani R, Boye SE, Boye SL, Gordon GM, Matteo BC, Sampath AP, Hauswirth WW, Horsager A. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol Ther. 2011;19:1220–1229. doi: 10.1038/mt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreosti E, Esposti F, Baden T, Lagnado L. In vivo evidence that retinal bipolar cells generate spikes modulated by light. Nat Neurosci. 2011;14:951–952. doi: 10.1038/nn.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu ON, Protti DA, Majumdar S, Zeilhofer HU, Wässle H. Ionotropic glutamate receptors of amacrine cells of the mouse retina. Vis Neurosci. 2006;23:79–90. doi: 10.1017/S0952523806231079. [DOI] [PubMed] [Google Scholar]
- Duncan JL, Ratnam K, Birch DG, Sundquist SM, Lucero AS, Zhang Y, Meltzer M, Smaoui N, Roorda A. Abnormal cone structure in foveal schisis cavities in X-linked retinoschisis from mutations in exon 6 of the RS1 gene. Investigative ophthalmology & visual science. 2011;52:9614–9623. doi: 10.1167/iovs.11-8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Della Santina L, Parker ED, Wong RO. Sensory Experience Shapes the Development of the Visual System’s First Synapse. Neuron. 2013;80:1159–1166. doi: 10.1016/j.neuron.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Wong RO. Diverse strategies engaged in establishing stereotypic wiring patterns among neurons sharing a common input at the visual system’s first synapse. J Neurosci. 2012;32:10306–10317. doi: 10.1523/JNEUROSCI.1581-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol. 2006a;572:215–225. doi: 10.1113/jphysiol.2005.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci. 2006b;26:9413–9425. doi: 10.1523/JNEUROSCI.2591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis Neurosci. 2011;28:95–108. doi: 10.1017/S0952523810000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol. 2007;582:569–582. doi: 10.1113/jphysiol.2007.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen SJ, Raven MA, Tamrazian E, Reese BE. Dopaminergic amacrine cells in the inner nuclear layer and ganglion cell layer comprise a single functional retinal mosaic. J Comp Neurol. 2003;466:343–355. doi: 10.1002/cne.10891. [DOI] [PubMed] [Google Scholar]
- Eglen SJ, van Ooyen A, Willshaw DJ. Lateral cell movement driven by dendritic interactions is sufficient to form retinal mosaics. Network. 2000;11:103–118. [PubMed] [Google Scholar]
- Eglen SJ, Willshaw DJ. Influence of cell fate mechanisms upon retinal mosaic formation: a modelling study. Development. 2002;129:5399–5408. doi: 10.1242/dev.00118. [DOI] [PubMed] [Google Scholar]
- Ekesten B, Gouras P. Cone and rod inputs to murine retinal ganglion cells: evidence of cone opsin specific channels. Vis Neurosci. 2005;22:893–903. doi: 10.1017/S0952523805226172. [DOI] [PubMed] [Google Scholar]
- Emoto K, He Y, Ye B, Grueber WB, Adler PN, Jan LY, Jan YN. Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell. 2004;119:245–256. doi: 10.1016/j.cell.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Eysel UT, Peichl L, Wässle H. Dendritic plasticity in the early postnatal feline retina: quantitative characteristics and sensitive period. J Comp Neurol. 1985;242:134–145. doi: 10.1002/cne.902420109. [DOI] [PubMed] [Google Scholar]
- Fei Y. Development of the cone photoreceptor mosaic in the mouse retina revealed by fluorescent cones in transgenic mice. Mol Vis. 2003;9:31–42. [PubMed] [Google Scholar]
- Feng L, Zhao Y, Yoshida M, Chen H, Yang JF, Kim TS, Cang J, Troy JB, Liu X. Sustained ocular hypertension induces dendritic degeneration of mouse retinal ganglion cells that depends on cell type and location. Investigative ophthalmology & visual science. 2013;54:1106–1117. doi: 10.1167/iovs.12-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Fisher LJ. Development of retinal synaptic arrays in the inner plexiform layer of dark-reared mice. J Embryol Exp Morphol. 1979a;54:219–227. [PubMed] [Google Scholar]
- Fisher LJ. Development of synaptic arrays in the inner plexiform layer of neonatal mouse retina. J Comp Neurol. 1979b;187:359–372. doi: 10.1002/cne.901870207. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Hack I, Brandstatter JH, Wässle H. Synaptic localization of NMDA receptor subunits in the rat retina. J Comp Neurol. 2000;420:98–112. [PubMed] [Google Scholar]
- Fletcher EL, Kalloniatis M. Localisation of amino acid neurotransmitters during postnatal development of the rat retina. J Comp Neurol. 1997;380:449–471. doi: 10.1002/(sici)1096-9861(19970421)380:4<449::aid-cne3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Koulen P, Wässle H. GABAA and GABAC receptors on mammalian rod bipolar cells. J Comp Neurol. 1998;396:351–365. doi: 10.1002/(sici)1096-9861(19980706)396:3<351::aid-cne6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Frankfort BJ, Khan AK, Tse DY, Chung I, Pang JJ, Yang Z, Gross RL, Wu SM. Elevated intraocular pressure causes inner retinal dysfunction before cell loss in a mouse model of experimental glaucoma. Invest Ophthalmol Vis Sci. 2013;54:762–770. doi: 10.1167/iovs.12-10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfort BJ, Mardon G. R8 development in the Drosophila eye: a paradigm for neural selection and differentiation. Development. 2002;129:1295–1306. doi: 10.1242/dev.129.6.1295. [DOI] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- Froger N, Cadetti L, Lorach H, Martins J, Bemelmans AP, Dubus E, Degardin J, Pain D, Forster V, Chicaud L, Ivkovic I, Simonutti M, Fouquet S, Jammoul F, Leveillard T, Benosman R, Sahel JA, Picaud S. Taurine Provides Neuroprotection against Retinal Ganglion Cell Degeneration. PloS one. 2012;7:e42017. doi: 10.1371/journal.pone.0042017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froger N, Jammoul F, Gaucher D, Cadetti L, Lorach H, Degardin J, Pain D, Dubus E, Forster V, Ivkovic I, Simonutti M, Sahel J-A, Picaud S. Taurine is a crucial factor to preserve retinal ganglion cell survival. Advances in experimental medicine and biology. 2013;775:69–83. doi: 10.1007/978-1-4614-6130-2_6. [DOI] [PubMed] [Google Scholar]
- Fu CT, Sretavan D. Laser-induced ocular hypertension in albino CD-1 mice. Invest Ophthalmol Vis Sci. 2010;51:980–990. doi: 10.1167/iovs.09-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs M, Scholz M, Sendelbeck A, Atorf J, Schlegel C, Enz R, Brandstatter JH. Rod photoreceptor ribbon synapses in DBA/2J mice show progressive age-related structural changes. PloS one. 2012;7:e44645. doi: 10.1371/journal.pone.0044645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Bruce F, Tian M, Wei W, Elstrott J, Feller MB, Erskine L, Singer JH, Burgess RW. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron. 2009;64:484–497. doi: 10.1016/j.neuron.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451:470–474. doi: 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujieda H, Bremner R, Mears AJ, Sasaki H. Retinoic acid receptor-related orphan receptor alpha regulates a subset of cone genes during mouse retinal development. J Neurochem. 2009;108:91–101. doi: 10.1111/j.1471-4159.2008.05739.x. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nature genetics. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L. Local, possibly contact-mediated signalling restricted to homotypic neurons controls the regular spacing of cells within the cholinergic arrays in the developing rodent retina. Development. 2000;127:1509–1516. doi: 10.1242/dev.127.7.1509. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L. Putting neurons in the right places: local interactions in the genesis of retinal architecture. Trends Neurosci. 2002;25:638–643. doi: 10.1016/s0166-2236(02)02279-8. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Novelli E, Viegi A. Dynamic microtubule-dependent interactions position homotypic neurones in regular monolayered arrays during retinal development. Development. 2002;129:3803–3814. doi: 10.1242/dev.129.16.3803. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Resta G, Tan SS, Reese BE. Mosaics of islet-1-expressing amacrine cells assembled by short-range cellular interactions. J Neurosci. 1997;17:7831–7838. doi: 10.1523/JNEUROSCI.17-20-07831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Valenzuela E, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. The Journal of comparative neurology. 2007;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier JL, Field GD, Sher A, Greschner M, Shlens J, Litke AM, Chichilnisky EJ. Receptive fields in primate retina are coordinated to sample visual space more uniformly. PLoS Biol. 2009;7:e1000063. doi: 10.1371/journal.pbio.1000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou AL, Francesca Cordeiro LGM, Salt TE. Electroretinogram and Visual-Evoked Potential Assessment of Retinal and Central Visual Function in a Rat Ocular Hypertension Model of Glaucoma. Current eye research. 2013 doi: 10.3109/02713683.2013.848902. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Haverkamp S, Wässle H. Glutamate receptors in the rod pathway of the mammalian retina. J Neurosci. 2001;21:8636–8647. doi: 10.1523/JNEUROSCI.21-21-08636.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489(Pt 2):529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho L, Mumm JS, Williams PR, Schroeter EH, Koerber A, Park SW, Leach SD, Wong RO. Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development. 2005;132:5069–5079. doi: 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- Goetze B, Schmidt KF, Lehmann K, Altrock WD, Gundelfinger ED, Lowel S. Vision and visual cortical maps in mice with a photoreceptor synaptopathy: reduced but robust visual capabilities in the absence of synaptic ribbons. Neuroimage. 2010;49:1622–1631. doi: 10.1016/j.neuroimage.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Goodchild AK, Chan TL, Grunert U. Horizontal cell connections with short-wavelength-sensitive cones in macaque monkey retina. Vis Neurosci. 1996;13:833–845. doi: 10.1017/s0952523800009093. [DOI] [PubMed] [Google Scholar]
- Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. In search of the visual pigment template. Vis Neurosci. 2000;17:509–528. doi: 10.1017/s0952523800174036. [DOI] [PubMed] [Google Scholar]
- Green ES, Stubbs JL, Levine EM. Genetic rescue of cell number in a mouse model of microphthalmia: interactions between Chx10 and G1-phase cell cycle regulators. Development. 2003;130:539–552. doi: 10.1242/dev.00275. [DOI] [PubMed] [Google Scholar]
- Gregg RG, Kamermans M, Klooster J, Lukasiewicz PD, Peachey NS, Vessey KA, McCall MA. Nyctalopin expression in retinal bipolar cells restores visual function in a mouse model of complete X-linked congenital stationary night blindness. J Neurophysiol. 2007;98:3023–3033. doi: 10.1152/jn.00608.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RG, Mukhopadhyay S, Candille SI, Ball SL, Pardue MT, McCall MA, Peachey NS. Identification of the gene and the mutation responsible for the mouse nob phenotype. Invest Ophthalmol Vis Sci. 2003;44:378–384. doi: 10.1167/iovs.02-0501. [DOI] [PubMed] [Google Scholar]
- Grimes WN, Zhang J, Graydon CW, Kachar B, Diamond JS. Retinal parallel processors: more than 100 independent microcircuits operate within a single interneuron. Neuron. 2010;65:873–885. doi: 10.1016/j.neuron.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Remé CE. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nature medicine. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Sagasti A. Self-avoidance and tiling: Mechanisms of dendrite and axon spacing. Cold Spring Harb Perspect Biol. 2010;2:a001750. doi: 10.1101/cshperspect.a001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunert U, Haverkamp S, Fletcher EL, Wässle H. Synaptic distribution of ionotropic glutamate receptors in the inner plexiform layer of the primate retina. J Comp Neurol. 2002;447:138–151. doi: 10.1002/cne.10220. [DOI] [PubMed] [Google Scholar]
- Grunert U, Martin PR. Rod bipolar cells in the macaque monkey retina: immunoreactivity and connectivity. J Neurosci. 1991;11:2742–2758. doi: 10.1523/JNEUROSCI.11-09-02742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunhan-Agar E, Kahn D, Chalupa LM. Segregation of on and off bipolar cell axonal arbors in the absence of retinal ganglion cells. J Neurosci. 2000;20:306–314. doi: 10.1523/JNEUROSCI.20-01-00306.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Stella SL, Jr., Hirano AA, Brecha NC. Plasmalemmal and vesicular gamma-aminobutyric acid transporter expression in the developing mouse retina. J Comp Neurol. 2009;512:6–26. doi: 10.1002/cne.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustincich S, Feigenspan A, Sieghart W, Raviola E. Composition of the GABA(A) receptors of retinal dopaminergic neurons. J Neurosci. 1999;19:7812–7822. doi: 10.1523/JNEUROSCI.19-18-07812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack I, Koulen P, Peichl L, Brandstatter JH. Development of glutamatergic synapses in the rat retina: the postnatal expression of ionotropic glutamate receptor subunits. Vis Neurosci. 2002;19:1–13. doi: 10.1017/s0952523801191017. [DOI] [PubMed] [Google Scholar]
- Hanley JG, Koulen P, Bedford F, Gordon-Weeks PR, Moss SJ. The protein MAP-1B links GABA(C) receptors to the cytoskeleton at retinal synapses. Nature. 1999;397:66–69. doi: 10.1038/16258. [DOI] [PubMed] [Google Scholar]
- Hanna MC, Calkins DJ. Expression of genes encoding glutamate receptors and transporters in rod and cone bipolar cells of the primate retina determined by single-cell polymerase chain reaction. Mol Vis. 2007;13:2194–2208. [PubMed] [Google Scholar]
- Haverkamp S, Grunert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Grunert U, Wässle H. Localization of kainate receptors at the cone pedicles of the primate retina. J Comp Neurol. 2001a;436:471–486. doi: 10.1002/cne.1081. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Grunert U, Wässle H. The synaptic architecture of AMPA receptors at the cone pedicle of the primate retina. J Neurosci. 2001b;21:2488–2500. doi: 10.1523/JNEUROSCI.21-07-02488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Michalakis S, Claes E, Seeliger MW, Humphries P, Biel M, Feigenspan A. Synaptic plasticity in CNGA3(−/−) mice: cone bipolar cells react on the missing cone input and form ectopic synapses with rods. J Neurosci. 2006;26:5248–5255. doi: 10.1523/JNEUROSCI.4483-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Thoreson WB, Witkovsky P. Synaptic transmission at retinal ribbon synapses. Prog Retin Eye Res. 2005;24:682–720. doi: 10.1016/j.preteyeres.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, Denk W. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature. 2013;500:168–174. doi: 10.1038/nature12346. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. A morphological comparison of foveal development in man and monkey. Eye (Lond) 1992;6(Pt 2):136–144. doi: 10.1038/eye.1992.29. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Kupfer C. The histogenesis of the fovea in the macaque monkey. Invest Ophthalmol Vis Sci. 1976;15:746–756. [PubMed] [Google Scholar]
- Hendrickson A.a.P., J . Book chapter in ‘Retinal Development’. Cambridge: 2006. Comparison of development of the primate fovea centralis with peripheral retina. [Google Scholar]
- Hendrickson AE. Primate foveal development: a microcosm of current questions in neurobiology. Invest Ophthalmol Vis Sci. 1994;35:3129–3133. [PubMed] [Google Scholar]
- Hendrickson AE. Synaptic development in macaque monkey retina and its implications for other developmental sequences. Perspect Dev Neurobiol. 1996;3:195–201. [PubMed] [Google Scholar]
- Hennig AK, Peng GH, Chen S. Regulation of photoreceptor gene expression by Crx-associated transcription factor network. Brain Res. 2008;1192:114–133. doi: 10.1016/j.brainres.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Brandstatter JH, Morgans CW, Brecha NC. SNAP25 expression in mammalian retinal horizontal cells. J Comp Neurol. 2011;519:972–988. doi: 10.1002/cne.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzhausen LC, Lewis AA, Cheong KK, Brockerhoff SE. Differential role for synaptojanin 1 in rod and cone photoreceptors. J Comp Neurol. 2009;517:633–644. doi: 10.1002/cne.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DC, Birch DG. The A-wave of the human electroretinogram and rod receptor function. Investigative ophthalmology & visual science. 1990;31:2070–2081. [PubMed] [Google Scholar]
- Hood DC, Birch DG. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis Neurosci. 1992;8:107–126. doi: 10.1017/s0952523800009275. [DOI] [PubMed] [Google Scholar]
- Hood DC, Birch DG. Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Investigative ophthalmology & visual science. 1994;35:2948–2961. [PubMed] [Google Scholar]
- Hoon M, Bauer G, Fritschy JM, Moser T, Falkenburger BH, Varoqueaux F. Neuroligin 2 controls the maturation of GABAergic synapses and information processing in the retina. J Neurosci. 2009;29:8039–8050. doi: 10.1523/JNEUROSCI.0534-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon M, Soykan T, Falkenburger B, Hammer M, Patrizi A, Schmidt KF, Sassoe-Pognetto M, Lowel S, Moser T, Taschenberger H, Brose N, Varoqueaux F. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc Natl Acad Sci U S A. 2011;108:3053–3058. doi: 10.1073/pnas.1006946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins JM, Boycott BB. The cone synapses of cone bipolar cells of primate retina. J Neurocytol. 1997;26:313–325. doi: 10.1023/a:1018504718282. [DOI] [PubMed] [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T. G protein subunit G gamma 13 is coexpressed with G alpha o, G beta 3, and G beta 4 in retinal ON bipolar cells. J Comp Neurol. 2003;455:1–10. doi: 10.1002/cne.10396. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–334. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckfeldt RM, Schubert T, Morgan JL, Godinho L, Di Cristo G, Huang ZJ, Wong RO. Transient neurites of retinal horizontal cells exhibit columnar tiling via homotypic interactions. Nat Neurosci. 2009;12:35–43. doi: 10.1038/nn.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. New perspectives in Retinal Organisation. Progress in Retinal Research. 1985;4:243–313. [Google Scholar]
- Imai H, Kefalov V, Sakurai K, Chisaka O, Ueda Y, Onishi A, Morizumi T, Fu Y, Ichikawa K, Nakatani K, Honda Y, Chen J, Yau KW, Shichida Y. Molecular properties of rhodopsin and rod function. J Biol Chem. 2007;282:6677–6684. doi: 10.1074/jbc.M610086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Hwang GS, Pan ZH. Characterization of transgenic mouse lines expressing Cre recombinase in the retina. Neuroscience. 2010;165:233–243. doi: 10.1016/j.neuroscience.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Muller U, Wässle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci. 2006;23:350–364. doi: 10.1111/j.1460-9568.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Williams GA, Fenwick JA. Influence of cone pigment coexpression on spectral sensitivity and color vision in the mouse. Vision Res. 2004;44:1615–1622. doi: 10.1016/j.visres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Jacoby RA, Wiechmann AF, Amara SG, Leighton BH, Marshak DW. Diffuse bipolar cells provide input to OFF parasol ganglion cells in the macaque retina. J Comp Neurol. 2000;416:6–18. doi: 10.1002/(sici)1096-9861(20000103)416:1<6::aid-cne2>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jae SA, Ahn KN, Kim JY, Seo JH, Kim HK, Goo YS. Electrophysiological and Histologic Evaluation of the Time Course of Retinal Degeneration in the rd10 Mouse Model of Retinitis Pigmentosa. The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2013;17:229–235. doi: 10.4196/kjpp.2013.17.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–325. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellali A, Stussi-Garaud C, Gasnier B, Rendon A, Sahel JA, Dreyfus H, Picaud S. Cellular localization of the vesicular inhibitory amino acid transporter in the mouse and human retina. J Comp Neurol. 2002;449:76–87. doi: 10.1002/cne.10272. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson LH, Hottowy P, Mathieson K, Gunning DE, Dabrowski W, Litke AM, Chichilnisky EJ. Focal electrical stimulation of major ganglion cell types in the primate retina for the design of visual prostheses. J Neurosci. 2013;33:7194–7205. doi: 10.1523/JNEUROSCI.4967-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyarasasingam G, Snider CJ, Ratto GM, Chalupa LM. Activity-regulated cell death contributes to the formation of ON and OFF alpha ganglion cell mosaics. J Comp Neurol. 1998;394:335–343. [PubMed] [Google Scholar]
- John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- Johnson J, Fremeau RT, Jr., Duncan JL, Renteria RC, Yang H, Hua Z, Liu X, LaVail MM, Edwards RH, Copenhagen DR. Vesicular glutamate transporter 1 is required for photoreceptor synaptic signaling but not for intrinsic visual functions. J Neurosci. 2007;27:7245–7255. doi: 10.1523/JNEUROSCI.0815-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Tian N, Caywood MS, Reimer RJ, Edwards RH, Copenhagen DR. Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J Neurosci. 2003;23:518–529. doi: 10.1523/JNEUROSCI.23-02-00518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Kondo M, Terasaki H, Lin Y, McCall M, Marc RE. Retinal remodeling. Jpn J Ophthalmol. 2012;56:289–306. doi: 10.1007/s10384-012-0147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Marc RE. Retinal remodeling during retinal degeneration. Exp Eye Res. 2005;81:123–137. doi: 10.1016/j.exer.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I. Ephaptic interactions within a chemical synapse: hemichannel-mediated ephaptic inhibition in the retina. Curr Opin Neurobiol. 2004;14:531–541. doi: 10.1016/j.conb.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Karan G, Lillo C, Yang Z, Cameron DJ, Locke KG, Zhao Y, Thirumalaichary S, Li C, Birch DG, Vollmer-Snarr HR, Williams DS, Zhang K. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc Natl Acad Sci U S A. 2005;102:4164–4169. doi: 10.1073/pnas.0407698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, Chu MW, Sanes JR. MEGF10 and MEGF11 mediate homotypic interactions required for mosaic spacing of retinal neurons. Nature. 2012;483:465–469. doi: 10.1038/nature10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, Roeser T, Mumm JS, Godinho L, Mrejeru A, Wong RO, Baier H. Transient requirement for ganglion cells during assembly of retinal synaptic layers. Development. 2004;131:1331–1342. doi: 10.1242/dev.01040. [DOI] [PubMed] [Google Scholar]
- Kay JN, Voinescu PE, Chu MW, Sanes JR. Neurod6 expression defines new retinal amacrine cell subtypes and regulates their fate. Nat Neurosci. 2011;14:965–972. doi: 10.1038/nn.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley PW, Reese BE. Role of afferents in the differentiation of bipolar cells in the mouse retina. J Neurosci. 2010;30:1677–1685. doi: 10.1523/JNEUROSCI.5153-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley PW, Sliff BJ, Lee SC, Fuerst PG, Burgess RW, Eglen SJ, Reese BE. Neuronal clustering and fasciculation phenotype in Dscam- and Bax-deficient mouse retinas. J Comp Neurol. 2012;520:1349–1364. doi: 10.1002/cne.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong RO. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–1020. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IB, Lee EJ, Kim MK, Park DK, Chun MH. Choline acetyltransferase-immunoreactive neurons in the developing rat retina. J Comp Neurol. 2000;427:604–616. doi: 10.1002/1096-9861(20001127)427:4<604::aid-cne8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci. 2010;30:1452–1462. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- Kirby MA, Chalupa LM. Retinal crowding alters the morphology of alpha ganglion cells. J Comp Neurol. 1986;251:532–541. doi: 10.1002/cne.902510408. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Widespread expression of gephyrin, a putative glycine receptor-tubulin linker protein, in rat brain. Brain Res. 1993;621:301–310. doi: 10.1016/0006-8993(93)90120-c. [DOI] [PubMed] [Google Scholar]
- Kjellstrom S, Bush RA, Zeng Y, Takada Y, Sieving PA. Retinoschisin gene therapy and natural history in the Rs1h-KO mouse: long-term rescue from retinal degeneration. Investigative ophthalmology & visual science. 2007;48:3837–3845. doi: 10.1167/iovs.07-0203. [DOI] [PubMed] [Google Scholar]
- Klooster J, Yazulla S, Kamermans M. Ultrastructural analysis of the glutamatergic system in the outer plexiform layer of zebrafish retina. J Chem Neuroanat. 2009;37:254–265. doi: 10.1016/j.jchemneu.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Knop GC, Feigenspan A, Weiler R, Dedek K. Inputs underlying the ON-OFF light responses of type 2 wide-field amacrine cells in TH::GFP mice. J Neurosci. 2011;31:4780–4791. doi: 10.1523/JNEUROSCI.6235-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M, Furukawa T. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi A, Jakobs TC, Masland RH. Regular mosaic of synaptic contacts among three retinal neurons. J Comp Neurol. 2011;519:341–357. doi: 10.1002/cne.22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. Organization of the outer plexiform layer of the primate retina: electron microscopy of Golgi-impregnated cells. Philos Trans R Soc Lond B Biol Sci. 1970;258:261–283. doi: 10.1098/rstb.1970.0036. [DOI] [PubMed] [Google Scholar]
- Kolb H. The connections between horizontal cells and photoreceptors in the retina of the cat: electron microscopy of Golgi preparations. J Comp Neurol. 1974;155:1–14. doi: 10.1002/cne.901550102. [DOI] [PubMed] [Google Scholar]
- Kolb H. Amacrine cells of the mammalian retina: neurocircuitry and functional roles. Eye (Lond) 1997;11(Pt 6):904–923. doi: 10.1038/eye.1997.230. [DOI] [PubMed] [Google Scholar]
- Kolb H, Cuenca N, Dekorver L. Postembedding immunocytochemistry for GABA and glycine reveals the synaptic relationships of the dopaminergic amacrine cell of the cat retina. J Comp Neurol. 1991;310:267–284. doi: 10.1002/cne.903100210. [DOI] [PubMed] [Google Scholar]
- Kolb H, Dekorver L. Midget ganglion cells of the parafovea of the human retina: a study by electron microscopy and serial section reconstructions. J Comp Neurol. 1991;303:617–636. doi: 10.1002/cne.903030408. [DOI] [PubMed] [Google Scholar]
- Kolb H, Marshak D. The midget pathways of the primate retina. Doc Ophthalmol. 2003;106:67–81. doi: 10.1023/a:1022469002511. [DOI] [PubMed] [Google Scholar]
- Kolb H, Zhang L, Dekorver L, Cuenca N. A new look at calretinin-immunoreactive amacrine cell types in the monkey retina. J Comp Neurol. 2002;453:168–184. doi: 10.1002/cne.10405. [DOI] [PubMed] [Google Scholar]
- Koshiba-Takeuchi K, Takeuchi JK, Matsumoto K, Momose T, Uno K, Hoepker V, Ogura K, Takahashi N, Nakamura H, Yasuda K, Ogura T. Tbx5 and the retinotectum projection. Science. 2000;287:134–137. doi: 10.1126/science.287.5450.134. [DOI] [PubMed] [Google Scholar]
- Koulen P. Localization of synapse-associated proteins during postnatal development of the rat retina. Eur J Neurosci. 1999;11:2007–2018. doi: 10.1046/j.1460-9568.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- Koulen P, Fletcher EL, Craven SE, Bredt DS, Wässle H. Immunocytochemical localization of the postsynaptic density protein PSD-95 in the mammalian retina. J Neurosci. 1998a;18:10136–10149. doi: 10.1523/JNEUROSCI.18-23-10136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Garner CC, Wässle H. Immunocytochemical localization of the synapse-associated protein SAP102 in the rat retina. J Comp Neurol. 1998b;397:326–336. [PubMed] [Google Scholar]
- Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the macaque retina. J Neurosci. 1992;12:1233–1252. doi: 10.1523/JNEUROSCI.12-04-01233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyama N, Marshak DW. The topographical relationship between two neuronal mosaics in the short wavelength-sensitive system of the primate retina. Vis Neurosci. 1997;14:159–167. doi: 10.1017/s0952523800008841. [DOI] [PubMed] [Google Scholar]
- Kranz K, Paquet-Durand F, Weiler R, Janssen-Bienhold U, Dedek K. Testing for a gap junction-mediated bystander effect in retinitis pigmentosa: secondary cone death is not altered by deletion of connexin36 from cones. PloS one. 2013;8:e57163. doi: 10.1371/journal.pone.0057163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Huang W, Furukawa T, Punzo C, Xing W. Plasticity of TRPM1 expression and localization in the wild type and degenerating mouse retina. Vision research. 2010;50:2460–2465. doi: 10.1016/j.visres.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Vail MM, Rapaport DH, Rakic P. Cytogenesis in the monkey retina. J Comp Neurol. 1991;309:86–114. doi: 10.1002/cne.903090107. [DOI] [PubMed] [Google Scholar]
- Lafuente MP, Villegas-Perez MP, Sobrado-Calvo P, García-Avilés A, Miralles de Imperial J, Vidal-Sanz M. Neuroprotective effects of alpha(2)-selective adrenergic agonists against ischemia-induced retinal ganglion cell death. Investigative ophthalmology & visual science. 2001;42:2074–2084. [PubMed] [Google Scholar]
- Lagali PS, Balya D, Awatramani GB, Münch TA, Kim DS, Busskamp V, Cepko CL, Roska B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- Lahav M, Albert DM, Craft JL. Light and electron microscopic study of dysplastic rosette-like structures occurring in the disorganized mature retina. Albrecht von Graefes Archiv für klinische und experimentelle Ophthalmologie. Albrecht von Graefe's archive for clinical and experimental ophthalmology. 1975;195:57–68. doi: 10.1007/BF02390031. [DOI] [PubMed] [Google Scholar]
- Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell stem cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre JL, Kostadinov D, Chen WV, Maniatis T, Sanes JR. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature. 2012;488:517–521. doi: 10.1038/nature11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre JL, Zhang Y, Meister M, Wang X, Sanes JR. gamma-Protocadherins regulate neuronal survival but are dispensable for circuit formation in retina. Development. 2008;135:4141–4151. doi: 10.1242/dev.027912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AG, Ault SJ, Vitek DJ, Shou T. Extrinsic determinants of retinal ganglion cell development in primates. J Comp Neurol. 1989;286:170–189. doi: 10.1002/cne.902860204. [DOI] [PubMed] [Google Scholar]
- Li Y, Schlamp CL, Nickells RW. Experimental induction of retinal ganglion cell death in adult mice. Invest Ophthalmol Vis Sci. 1999;40:1004–1008. [PubMed] [Google Scholar]
- Li Y, Tao W, Luo L, Huang D, Kauper K, Stabila P, Lavail MM, Laties AM, Wen R. CNTF induces regeneration of cone outer segments in a rat model of retinal degeneration. PloS one. 2010;5:e9495. doi: 10.1371/journal.pone.0009495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YN, Matsui JI, Dowling JE. Specificity of the horizontal cell-photoreceptor connections in the zebrafish (Danio rerio) retina. J Comp Neurol. 2009;516:442–453. doi: 10.1002/cne.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YN, Tsujimura T, Kawamura S, Dowling JE. Bipolar cell-photoreceptor connectivity in the zebrafish (Danio rerio) retina. J Comp Neurol. 2012;520:3786–3802. doi: 10.1002/cne.23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci U S A. 2008;105:16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Peng EB. Retinal ganglion cells are resistant to photoreceptor loss in retinal degeneration. PloS one. 2013;8:e68084. doi: 10.1371/journal.pone.0068084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Wang SW, Masland RH. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron. 2004;43:475–485. doi: 10.1016/j.neuron.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Lin SC, Skapek SX, Papermaster DS, Hankin M, Lee EY. The proliferative and apoptotic activities of E2F1 in the mouse retina. Oncogene. 2001;20:7073–7084. doi: 10.1038/sj.onc.1204932. [DOI] [PubMed] [Google Scholar]
- Linder B, Dill H, Hirmer A, Brocher J, Lee GP, Mathavan S, Bolz HJ, Winkler C, Laggerbauer B, Fischer U. Systemic splicing factor deficiency causes tissue-specific defects: a zebrafish model for retinitis pigmentosa. Hum Mol Genet. 2011;20:368–377. doi: 10.1093/hmg/ddq473. [DOI] [PubMed] [Google Scholar]
- Lindstrom SH, Ryan DG, Shi J, DeVries SH. Kainate receptor subunit diversity underlying response diversity in retinal Off bipolar cells. J Physiol. 2014;592:1457–1477. doi: 10.1113/jphysiol.2013.265033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hirano AA, Sun X, Brecha NC, Barnes S. Calcium channels in rat horizontal cells regulate feedback inhibition of photoreceptors through an unconventional GABA- and pH-sensitive mechanism. J Physiol. 2013;591:3309–3324. doi: 10.1113/jphysiol.2012.248179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann C, Myhr KL, Wong RO. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418:177–181. doi: 10.1038/nature00850. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Wong RO. Cell-type specific dendritic contacts between retinal ganglion cells during development. J Neurobiol. 2001;48:150–162. [PubMed] [Google Scholar]
- Lolley RN, Schmidt SY, Farber DB. Alterations in cyclic AMP metabolism associated with photoreceptor cell degeneration in the C3H mouse. J Neurochem. 1974;22:701–707. doi: 10.1111/j.1471-4159.1974.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Wilson JA, Lawrence JE. AMPA-preferring receptors mediate excitatory synaptic inputs to retinal ganglion cells. J Neurophysiol. 1997;77:57–64. doi: 10.1152/jn.1997.77.1.57. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- Magupalli VG, Schwarz K, Alpadi K, Natarajan S, Seigel GM, Schmitz F. Multiple RIBEYE-RIBEYE interactions create a dynamic scaffold for the formation of synaptic ribbons. J Neurosci. 2008;28:7954–7967. doi: 10.1523/JNEUROSCI.1964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso K, Hauswirth WW, Li Q, Connor TB, Kuchenbecker JA, Mauck MC, Neitz J, Neitz M. Gene therapy for red-green colour blindness in adult primates. Nature. 2009;461:784–787. doi: 10.1038/nature08401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- Maple BR, Gao F, Wu SM. Glutamate receptors differ in rod- and cone-dominated off-center bipolar cells. Neuroreport. 1999;10:3605–3610. doi: 10.1097/00001756-199911260-00026. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH, Wensel T, Lucas RJ. Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci. 2007a;48:3364–3371. doi: 10.1167/iovs.07-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH, Wensel T, Lucas RJ. Neural reprogramming in retinal degeneration. Investigative ophthalmology & visual science. 2007b;48:3364–3371. doi: 10.1167/iovs.07-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Anderson JR, Sigulinsky C, Lauritzen S. Retinal connectomics: towards complete, accurate networks. Prog Retin Eye Res. 2013;37:141–162. doi: 10.1016/j.preteyeres.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Gruss P. Generating neuronal diversity in the retina: one for nearly all. Trends Neurosci. 2002;25:32–38. doi: 10.1016/s0166-2236(00)02028-2. [DOI] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Maslim J, Stone J. Synaptogenesis in the retina of the cat. Brain Res. 1986;373:35–48. doi: 10.1016/0006-8993(86)90313-6. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN, Thibeault MA, Dubin MW. Non-uniform postnatal growth of the cat retina. J Comp Neurol. 1984;228:598–608. doi: 10.1002/cne.902280410. [DOI] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, Chedotal A, Peachey NS, Nathans J, Yoshida Y, Giger RJ, Kolodkin AL. Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron. 2011a;71:460–473. doi: 10.1016/j.neuron.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka RL, Jiang Z, Samuels IS, Nguyen-Ba-Charvet KT, Sun LO, Peachey NS, Chedotal A, Yau KW, Kolodkin AL. Guidance-cue control of horizontal cell morphology, lamination, and synapse formation in the mammalian outer retina. J Neurosci. 2012;32:6859–6868. doi: 10.1523/JNEUROSCI.0267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011b;470:259–263. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni F, Novelli E, Strettoi E. Retinal ganglion cells survive and maintain normal dendritic morphology in a mouse model of inherited photoreceptor degeneration. J Neurosci. 2008;28:14282–14292. doi: 10.1523/JNEUROSCI.4968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery P, Lee MO, Wagner MA, Sladek NE, Drager UC. Asymmetrical retinoic acid synthesis in the dorsoventral axis of the retina. Development. 1992;115:371–382. doi: 10.1242/dev.115.2.371. [DOI] [PubMed] [Google Scholar]
- McCall MA, Gregg RG. Comparisons of structural and functional abnormalities in mouse b-wave mutants. J Physiol. 2008;586:4385–4392. doi: 10.1113/jphysiol.2008.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis S, Muhlfriedel R, Tanimoto N, Krishnamoorthy V, Koch S, Fischer MD, Becirovic E, Bai L, Huber G, Beck SC, Fahl E, Buning H, Paquet-Durand F, Zong X, Gollisch T, Biel M, Seeliger MW. Restoration of cone vision in the CNGA3−/− mouse model of congenital complete lack of cone photoreceptor function. Mol Ther. 2010;18:2057–2063. doi: 10.1038/mt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis S, Schaferhoff K, Spiwoks-Becker I, Zabouri N, Koch S, Koch F, Bonin M, Biel M, Haverkamp S. Characterization of neurite outgrowth and ectopic synaptogenesis in response to photoreceptor dysfunction. Cell Mol Life Sci. 2013;70:1831–1847. doi: 10.1007/s00018-012-1230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishima KJ, Grunert U, Li W. Processing of S-cone signals in the inner plexiform layer of the mammalian retina. Vis Neurosci. 2014;31:153–163. doi: 10.1017/S0952523813000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki A. Pattern formation of the cone mosaic in the zebrafish retina: a cell rearrangement model. J Theor Biol. 2002;215:345–361. doi: 10.1006/jtbi.2001.2508. [DOI] [PubMed] [Google Scholar]
- Mohand-Said S, Deudon-Combe A, Hicks D, Simonutti M, Forster V, Fintz AC, Leveillard T, Dreyfus H, Sahel JA. Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc Natl Acad Sci U S A. 1998;95:8357–8362. doi: 10.1073/pnas.95.14.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, Kellner U, Weber BH. X-linked juvenile retinoschisis: clinical diagnosis, genetic analysis, and molecular mechanisms. Prog Retin Eye Res. 2012;31:195–212. doi: 10.1016/j.preteyeres.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Friedlander MJ. Expression of an intrinsic growth strategy by mammalian retinal neurons. Proc Natl Acad Sci U S A. 1989;86:7223–7227. doi: 10.1073/pnas.86.18.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Friedlander MJ. Morphogenesis and territorial coverage by isolated mammalian retinal ganglion cells. J Neurosci. 1991;11:1440–1457. doi: 10.1523/JNEUROSCI.11-05-01440.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, Dhingra A, Vardi N, Wong RO. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci. 2006;9:85–92. doi: 10.1038/nn1615. [DOI] [PubMed] [Google Scholar]
- Morgan JL, Schubert T, Wong RO. Developmental patterning of glutamatergic synapses onto retinal ganglion cells. Neural Dev. 2008;3:8. doi: 10.1186/1749-8104-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, Soto F, Wong RO, Kerschensteiner D. Development of cell type-specific connectivity patterns of converging excitatory axons in the retina. Neuron. 2011;71:1014–1021. doi: 10.1016/j.neuron.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritoh S, Komatsu Y, Yamamori T, Koizumi A. Diversity of retinal ganglion cells identified by transient GFP transfection in organotypic tissue culture of adult marmoset monkey retina. PLoS One. 2013;8:e54667. doi: 10.1371/journal.pone.0054667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC, Schroeter EH, Bilotta J, Wong RO, Fadool JM. Cone survival despite rod degeneration in XOPS-mCFP transgenic zebrafish. Invest Ophthalmol Vis Sci. 2005;46:4762–4771. doi: 10.1167/iovs.05-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JS, Williams PR, Godinho L, Koerber A, Pittman AJ, Roeser T, Chien CB, Baier H, Wong RO. In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells. Neuron. 2006;52:609–621. doi: 10.1016/j.neuron.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci. 2009;12:1308–1316. doi: 10.1038/nn.2389. [DOI] [PubMed] [Google Scholar]
- Nagaraju M, Saleh M, Porciatti V. IOP-dependent retinal ganglion cell dysfunction in glaucomatous DBA/2J mice. Investigative ophthalmology & visual science. 2007;48:4573–4579. doi: 10.1167/iovs.07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuille M, El Shamieh S, Orhan E, Michiels C, Antonio A, Lancelot ME, Condroyer C, Bujakowska K, Poch O, Sahel JA, Audo I, Zeitz C. Lrit3 Deficient Mouse (nob6): A Novel Model of Complete Congenital Stationary Night Blindness (cCSNB) PLoS One. 2014;9:e90342. doi: 10.1371/journal.pone.0090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newkirk GS, Hoon M, Wong RO, Detwiler PB. Inhibitory inputs tune the light response properties of dopaminergic amacrine cells in mouse retina. J Neurophysiol. 2013;110:536–552. doi: 10.1152/jn.00118.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nature genetics. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr. Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127:359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir I, Kedzierski W, Chen J, Travis GH. Expression of Bcl-2 protects against photoreceptor degeneration in retinal degeneration slow (rds) mice. J Neurosci. 2000;20:2150–2154. doi: 10.1523/JNEUROSCI.20-06-02150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Rakic P. Development of the rhesus monkey retina: II. A three-dimensional analysis of the sequences of synaptic combinations in the inner plexiform layer. J Comp Neurol. 1987;262:290–313. doi: 10.1002/cne.902620209. [DOI] [PubMed] [Google Scholar]
- Nivison-Smith L, Sun D, Fletcher EL, Marc RE, Kalloniatis M. Mapping kainate activation of inner neurons in the rat retina. J Comp Neurol. 2013;521:2416–2438. doi: 10.1002/cne.23305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Okada M, Erickson A, Hendrickson A. Light and electron microscopic analysis of synaptic development in Macaca monkey retina as detected by immunocytochemical labeling for the synaptic vesicle protein, SV2. J Comp Neurol. 1994;339:535–558. doi: 10.1002/cne.903390406. [DOI] [PubMed] [Google Scholar]
- Okawa H, Della Santina L, Schwartz GW, Rieke F, Wong RO. Interplay of cell-autonomous and nonautonomous mechanisms tailors synaptic connectivity of converging axons in vivo. Neuron. 2014;82:125–137. doi: 10.1016/j.neuron.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW. An electron microscopic study of synapse formation, receptor outer segment development, and other aspects of developing mouse retina. Invest Ophthalmol. 1968;7:250–268. [PubMed] [Google Scholar]
- Olshevskaya EV, Calvert PD, Woodruff ML, Peshenko IV, Savchenko AB, Makino CL, Ho YS, Fain GL, Dizhoor AM. The Y99C mutation in guanylyl cyclase-activating protein 1 increases intracellular Ca2+ and causes photoreceptor degeneration in transgenic mice. J Neurosci. 2004;24:6078–6085. doi: 10.1523/JNEUROSCI.0963-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, Araki F, Chaya T, Kajimura N, Irie S, Terada K, Muranishi Y, Tsujii T, Ueno S, Koyasu T, Tamaki Y, Kondo M, Amano S, Furukawa T. Presynaptic dystroglycan-pikachurin complex regulates the proper synaptic connection between retinal photoreceptor and bipolar cells. J Neurosci. 2012;32:6126–6137. doi: 10.1523/JNEUROSCI.0322-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Lu C, Takagaki K, Fu Z, Vicini S. Expression of distinct alpha subunits of GABAA receptor regulates inhibitory synaptic strength. J Neurophysiol. 2004;92:1718–1727. doi: 10.1152/jn.00243.2004. [DOI] [PubMed] [Google Scholar]
- Packer O, Hendrickson AE, Curcio CA. Photoreceptor topography of the retina in the adult pigtail macaque (Macaca nemestrina) J Comp Neurol. 1989;288:165–183. doi: 10.1002/cne.902880113. [DOI] [PubMed] [Google Scholar]
- Packer O, Hendrickson AE, Curcio CA. Development redistribution of photoreceptors across the Macaca nemestrina (pigtail macaque) retina. J Comp Neurol. 1990;298:472–493. doi: 10.1002/cne.902980408. [DOI] [PubMed] [Google Scholar]
- Pang J-J, Chang B, Hawes NL, Hurd RE, Davisson MT, Li J, Noorwez SM, Malhotra R, McDowell JH, Kaushal S, Hauswirth WW, Nusinowitz S, Thompson DA, Heckenlively JR. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA) Molecular vision. 2005;11:152–162. [PubMed] [Google Scholar]
- Pang J-J, Dai X, Boye SE, Barone I, Boye SL, Mao S, Everhart D, Dinculescu A, Liu L, Umino Y, Lei B, Chang B, Barlow R, Strettoi E, Hauswirth WW. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:234–242. doi: 10.1038/mt.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TK, Wu Z, Kjellstrom S, Zeng Y, Bush RA, Sieving PA, Colosi P. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene therapy. 2009;16:916–926. doi: 10.1038/gt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- Pattnaik B, Jellali A, Sahel J, Dreyfus H, Picaud S. GABAC receptors are localized with microtubule-associated protein 1B in mammalian cone photoreceptors. J Neurosci. 2000;20:6789–6796. doi: 10.1523/JNEUROSCI.20-18-06789.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, Duran Y, Smith AJ, Chuang JZ, Azam SA, Luhmann UFO, Benucci A, Sung CH, Bainbridge JW, Carandini M, Yau K-W, Sowden JC, Ali RR. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L. Alpha ganglion cells in mammalian retinae: common properties, species differences, and some comments on other ganglion cells. Vis Neurosci. 1991;7:155–169. doi: 10.1017/s0952523800011020. [DOI] [PubMed] [Google Scholar]
- Peichl L, Gonzalez-Soriano J. Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci. 1994;11:501–517. doi: 10.1017/s095252380000242x. [DOI] [PubMed] [Google Scholar]
- Percival KA, Jusuf PR, Martin PR, Grunert U. Synaptic inputs onto small bistratified (blue-ON/yellow-OFF) ganglion cells in marmoset retina. J Comp Neurol. 2009;517:655–669. doi: 10.1002/cne.22183. [DOI] [PubMed] [Google Scholar]
- Percival KA, Koizumi A, Masri RA, Buzas P, Martin PR, Grunert U. Identification of a pathway from the retina to koniocellular layer k1 in the lateral geniculate nucleus of marmoset. J Neurosci. 2014;34:3821–3825. doi: 10.1523/JNEUROSCI.4491-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Linden R. Evidence for dendritic competition in the developing retina. Nature. 1982;297:683–685. doi: 10.1038/297683a0. [DOI] [PubMed] [Google Scholar]
- Peters MA, Cepko CL. The dorsal-ventral axis of the neural retina is divided into multiple domains of restricted gene expression which exhibit features of lineage compartments. Dev Biol. 2002;251:59–73. doi: 10.1006/dbio.2002.0791. [DOI] [PubMed] [Google Scholar]
- Phillips MJ, Otteson DC, Sherry DM. Progression of neuronal and synaptic remodeling in the rd10 mouse model of retinitis pigmentosa. The Journal of comparative neurology. 2010;518:2071–2089. doi: 10.1002/cne.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak J, Wilken MS, Ueki Y, Cox KE, Sullivan JM, Taylor RJ, Levine EM, Reh TA. ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development (Cambridge, England) 2013;140:2619–2631. doi: 10.1242/dev.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosukhina A, Litt J, Tochitsky I, Nemargut J, Sychev Y, De Kouchkovsky I, Huang T, Borges K, Trauner D, Van Gelder RN, Kramer RH. Photochemical restoration of visual responses in blind mice. Neuron. 2012;75:271–282. doi: 10.1016/j.neuron.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcho RG. Dopaminergic amacrine cells in the cat retina. Brain Res. 1982;252:101–109. doi: 10.1016/0006-8993(82)90982-9. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. Neuronal subpopulations in cat retina which accumulate the GABA agonist, (3H)muscimol: a combined Golgi and autoradiographic study. J Comp Neurol. 1983;219:25–35. doi: 10.1002/cne.902190104. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. A combined Golgi and autoradiographic study of (3H)glycine-accumulating amacrine cells in the cat retina. J Comp Neurol. 1985;233:473–480. doi: 10.1002/cne.902330406. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. A combined Golgi and autoradiographic study of 3H-glycine-accumulating cone bipolar cells in the cat retina. J Neurosci. 1987;7:1178–1188. doi: 10.1523/JNEUROSCI.07-04-01178.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow DV, Wright LL, Vaney DI. The immunocytochemical detection of amino-acid neurotransmitters in paraformaldehyde-fixed tissues. J Neurosci Methods. 1995;56:115–123. doi: 10.1016/0165-0270(94)00113-u. [DOI] [PubMed] [Google Scholar]
- Puller C, Ivanova E, Euler T, Haverkamp S, Schubert T. OFF bipolar cells express distinct types of dendritic glutamate receptors in the mouse retina. Neuroscience. 2013;243:136–148. doi: 10.1016/j.neuroscience.2013.03.054. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Gayet-Primo J, Pandey S, Duvoisin RM, Taylor WR. Differential loss and preservation of glutamate receptor function in bipolar cells in the rd10 mouse model of retinitis pigmentosa. The European journal of neuroscience. 2009;29:1533–1542. doi: 10.1111/j.1460-9568.2009.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthussery T, Percival KA, Venkataramani S, Gayet-Primo J, Grunert U, Taylor WR. Kainate Receptors Mediate Synaptic Input to Transient and Sustained OFF Visual Pathways in Primate Retina. J Neurosci. 2014;34:7611–7621. doi: 10.1523/JNEUROSCI.4855-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randlett O, MacDonald RB, Yoshimatsu T, Almeida AD, Suzuki SC, Wong RO, Harris WA. Cellular requirements for building a retinal neuropil. Cell Rep. 2013;3:282–290. doi: 10.1016/j.celrep.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH, Stone J. The area centralis of the retina in the cat and other mammals: focal point for function and development of the visual system. Neuroscience. 1984;11:289–301. doi: 10.1016/0306-4522(84)90024-1. [DOI] [PubMed] [Google Scholar]
- Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- Raven MA, Eglen SJ, Ohab JJ, Reese BE. Determinants of the exclusion zone in dopaminergic amacrine cell mosaics. J Comp Neurol. 2003;461:123–136. doi: 10.1002/cne.10693. [DOI] [PubMed] [Google Scholar]
- Raven MA, Oh EC, Swaroop A, Reese BE. Afferent control of horizontal cell morphology revealed by genetic respecification of rods and cones. J Neurosci. 2007;27:3540–3547. doi: 10.1523/JNEUROSCI.0372-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven MA, Orton NC, Nassar H, Williams GA, Stell WK, Jacobs GH, Bech-Hansen NT, Reese BE. Early afferent signaling in the outer plexiform layer regulates development of horizontal cell morphology. J Comp Neurol. 2008;506:745–758. doi: 10.1002/cne.21526. [DOI] [PubMed] [Google Scholar]
- Raven MA, Stagg SB, Nassar H, Reese BE. Developmental improvement in the regularity and packing of mouse horizontal cells: implications for mechanisms underlying mosaic pattern formation. Vis Neurosci. 2005;22:569–573. doi: 10.1017/S095252380522504X. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK. A moving wave patterns the cone photoreceptor mosaic array in the zebrafish retina. Int J Dev Biol. 2004;48:935–945. doi: 10.1387/ijdb.041873pr. [DOI] [PubMed] [Google Scholar]
- Reese BE, Raven MA, Stagg SB. Afferents and homotypic neighbors regulate horizontal cell morphology, connectivity, and retinal coverage. J Neurosci. 2005;25:2167–2175. doi: 10.1523/JNEUROSCI.4876-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regus-Leidig H, Tom Dieck S, Specht D, Meyer L, Brandstatter JH. Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: the involvement of precursor spheres. J Comp Neurol. 2009;512:814–824. doi: 10.1002/cne.21915. [DOI] [PubMed] [Google Scholar]
- Reh TA, Levine EM. Multipotential stem cells and progenitors in the vertebrate retina. Journal of neurobiology. 1998;36:206–220. [PubMed] [Google Scholar]
- Resta V, Novelli E, Di Virgilio F, Galli-Resta L. Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development. 2005;132:2873–2882. doi: 10.1242/dev.01855. [DOI] [PubMed] [Google Scholar]
- Rhee KD, Nusinowitz S, Chao K, Yu F, Bok D, Yang X-J. CNTF-mediated protection of photoreceptors requires initial activation of the cytokine receptor gp130 in Müller glial cells. Proc Natl Acad Sci U S A. 2013;110:E4520–4529. doi: 10.1073/pnas.1303604110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribic A, Liu X, Crair MC, Biederer T. Structural organization and function of mouse photoreceptor ribbon synapses involve the immunoglobulin protein synaptic cell adhesion molecule 1. J Comp Neurol. 2014;522:900–920. doi: 10.1002/cne.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich KA, Zhan Y, Blanks JC. Migration and synaptogenesis of cone photoreceptors in the developing mouse retina. J Comp Neurol. 1997;388:47–63. [PubMed] [Google Scholar]
- Rieke F. Mechanisms of single-photon detection in rod photoreceptors. Methods Enzymol. 2000;316:186–202. doi: 10.1016/s0076-6879(00)16724-2. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Hendrickson A, McGuire CR, Reh TA. Retinoid X receptor (gamma) is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Investigative ophthalmology & visual science. 2005;46:2897–2904. doi: 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6218–6223. doi: 10.1073/pnas.0509981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Schmitt EA, Harosi FI, Reece RJ, Dowling JE. Zebrafish ultraviolet visual pigment: absorption spectrum, sequence, and localization. Proc Natl Acad Sci U S A. 1993;90:6009–6012. doi: 10.1073/pnas.90.13.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhill RL, Euler T, Masland RH. Spatial order within but not between types of retinal neurons. Proc Natl Acad Sci U S A. 2000;97:2303–2307. doi: 10.1073/pnas.030413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlich P, van Veen T, Szel A. Two different visual pigments in one retinal cone cell. Neuron. 1994;13:1159–1166. doi: 10.1016/0896-6273(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Roorda A, Metha AB, Lennie P, Williams DR. Packing arrangement of the three cone classes in primate retina. Vision Res. 2001;41:1291–1306. doi: 10.1016/s0042-6989(01)00043-8. [DOI] [PubMed] [Google Scholar]
- Saade CJ, Alvarez-Delfin K, Fadool JM. Rod photoreceptors protect from cone degeneration-induced retinal remodeling and restore visual responses in zebrafish. J Neurosci. 2013;33:1804–1814. doi: 10.1523/JNEUROSCI.2910-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahel J, Bonnel S, Mrejen S, Paques M. Retinitis pigmentosa and other dystrophies. Dev Ophthalmol. 2010;47:160–167. doi: 10.1159/000320079. [DOI] [PubMed] [Google Scholar]
- Saleh M, Nagaraju M, Porciatti V. Longitudinal evaluation of retinal ganglion cell function and IOP in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2007;48:4564–4572. doi: 10.1167/iovs.07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath AP, Rieke F. Selective transmission of single photon responses by saturation at the rod to-rod bipolar synapse. Neuron. 2004;41:431–443. doi: 10.1016/s0896-6273(04)00005-4. [DOI] [PubMed] [Google Scholar]
- Sappington RM, Carlson BJ, Crish SD, Calkins DJ. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci. 2010;51:207–216. doi: 10.1167/iovs.09-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Wässle H. Synaptogenesis in the rat retina: subcellular localization of glycine receptors, GABA(A) receptors, and the anchoring protein gephyrin. J Comp Neurol. 1997;381:158–174. doi: 10.1002/(sici)1096-9861(19970505)381:2<158::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Wässle H, Grunert U. Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the alpha 1 subunit of the glycine receptor. J Neurosci. 1994;14:5131–5146. doi: 10.1523/JNEUROSCI.14-08-05131.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, Sawai H, Kobayashi K, Tani A, Toda T, Usukura J, Tano Y, Fujikado T, Furukawa T. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 2008;11:923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- Satoh S, Tang K, Iida A, Inoue M, Kodama T, Tsai SY, Tsai MJ, Furuta Y, Watanabe S. The spatial patterning of mouse cone opsin expression is regulated by bone morphogenetic protein signaling through downstream effector COUP-TF nuclear receptors. J Neurosci. 2009;29:12401–12411. doi: 10.1523/JNEUROSCI.0951-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Early retinal development in the zebrafish, Danio rerio: light and electron microscopic analyses. J Comp Neurol. 1999;404:515–536. [PubMed] [Google Scholar]
- Schmitz F. The making of synaptic ribbons: how they are built and what they do. Neuroscientist. 2009;15:611–624. doi: 10.1177/1073858409340253. [DOI] [PubMed] [Google Scholar]
- Schubert T, Hoon M, Euler T, Lukasiewicz PD, Wong RO. Developmental regulation and activity-dependent maintenance of GABAergic presynaptic inhibition onto rod bipolar cell axonal terminals. Neuron. 2013;78:124–137. doi: 10.1016/j.neuron.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T, Huckfeldt RM, Parker E, Campbell JE, Wong RO. Assembly of the outer retina in the absence of GABA synthesis in horizontal cells. Neural Dev. 2010;5:15. doi: 10.1186/1749-8104-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T, Kerschensteiner D, Eggers ED, Misgeld T, Kerschensteiner M, Lichtman JW, Lukasiewicz PD, Wong RO. Development of presynaptic inhibition onto retinal bipolar cell axon terminals is subclass-specific. J Neurophysiol. 2008;100:304–316. doi: 10.1152/jn.90202.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GW, Okawa H, Dunn FA, Morgan JL, Kerschensteiner D, Wong RO, Rieke F. The spatial structure of a nonlinear receptive field. Nat Neurosci. 2012;15:1572–1580. doi: 10.1038/nn.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirnjak C, Hottowy P, Sher A, Dabrowski W, Litke AM, Chichilnisky EJ. High-resolution electrical stimulation of primate retina for epiretinal implant design. J Neurosci. 2008;28:4446–4456. doi: 10.1523/JNEUROSCI.5138-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirnjak C, Jepson LH, Hottowy P, Sher A, Dabrowski W, Litke AM, Chichilnisky EJ. Changes in physiological properties of rat ganglion cells during retinal degeneration. J Neurophysiol. 2011;105:2560–2571. doi: 10.1152/jn.01061.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Eglen SJ, Wong RO. Development of retinal ganglion cell structure and function. Prog Retin Eye Res. 2001;20:139–174. doi: 10.1016/s1350-9462(00)00024-0. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Wang MM, Bates J, Frishman LJ. Expression of vesicular glutamate transporter 1 in the mouse retina reveals temporal ordering in development of rod vs. cone and ON vs. OFF circuits. J Comp Neurol. 2003;465:480–498. doi: 10.1002/cne.10838. [DOI] [PubMed] [Google Scholar]
- Siegert S, Cabuy E, Scherf BG, Kohler H, Panda S, Le YZ, Fehling HJ, Gaidatzis D, Stadler MB, Roska B. Transcriptional code and disease map for adult retinal cell types. Nat Neurosci. 2012;15:487–495. S481–482. doi: 10.1038/nn.3032. [DOI] [PubMed] [Google Scholar]
- Singh MS, Charbel Issa P, Butler R, Martin C, Lipinski DM, Sekaran S, Barnard AR, MacLaren RE. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc Natl Acad Sci U S A. 2013;110:1101–1106. doi: 10.1073/pnas.1119416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarie JM, Link BA. The primary open-angle glaucoma gene WDR36 functions in ribosomal RNA processing and interacts with the p53 stress-response pathway. Hum Mol Genet. 2008;17:2474–2485. doi: 10.1093/hmg/ddn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Soto F, Bleckert A, Lewis R, Kang Y, Kerschensteiner D, Craig AM, Wong RO. Coordinated increase in inhibitory and excitatory synapses onto retinal ganglion cells during development. Neural Dev. 2011a;6:31. doi: 10.1186/1749-8104-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Ma X, Cecil JL, Vo BQ, Culican SM, Kerschensteiner D. Spontaneous activity promotes synapse formation in a cell-type-dependent manner in the developing retina. J Neurosci. 2012;32:5426–5439. doi: 10.1523/JNEUROSCI.0194-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Watkins KL, Johnson RE, Schottler F, Kerschensteiner D. NGL-2 regulates pathway-specific neurite growth and lamination, synapse formation, and signal transmission in the retina. J Neurosci. 2013;33:11949–11959. doi: 10.1523/JNEUROSCI.1521-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto I, Pease ME, Son JL, Shi X, Quigley HA, Marsh-Armstrong N. Retinal ganglion cell loss in a rat ocular hypertension model is sectorial and involves early optic nerve axon loss. Investigative ophthalmology & visual science. 2011b;52:434–441. doi: 10.1167/iovs.10-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Springer AD. New role for the primate fovea: a retinal excavation determines photoreceptor deployment and shape. Visual neuroscience. 1999;16:629–636. doi: 10.1017/s0952523899164034. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Ng L, Liu H, Jia L, Forrest D. Activation of the blue opsin gene in cone photoreceptor development by retinoid-related orphan receptor beta. Molecular endocrinology. 2006;20:1728–1741. doi: 10.1210/me.2005-0505. [DOI] [PubMed] [Google Scholar]
- Stacy RC, Demas J, Burgess RW, Sanes JR, Wong RO. Disruption and recovery of patterned retinal activity in the absence of acetylcholine. J Neurosci. 2005;25:9347–9357. doi: 10.1523/JNEUROSCI.1800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy RC, Wong RO. Developmental relationship between cholinergic amacrine cell processes and ganglion cell dendrites of the mouse retina. J Comp Neurol. 2003;456:154–166. doi: 10.1002/cne.10509. [DOI] [PubMed] [Google Scholar]
- Stasheff SF, Shankar M, Andrews MP. Developmental time course distinguishes changes in spontaneous and light-evoked retinal ganglion cell activity in rd1 and rd10 mice. J Neurophysiol. 2011;105:3002–3009. doi: 10.1152/jn.00704.2010. [DOI] [PubMed] [Google Scholar]
- Stearns G, Evangelista M, Fadool JM, Brockerhoff SE. A mutation in the cone-specific pde6 gene causes rapid cone photoreceptor degeneration in zebrafish. J Neurosci. 2007;27:13866–13874. doi: 10.1523/JNEUROSCI.3136-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Stiebel-Kalish H, Reich E, Rainy N, Vatine G, Nisgav Y, Tovar A, Gothilf Y, Bach M. Gucy2f zebrafish knockdown--a model for Gucy2d-related leber congenital amaurosis. Eur J Hum Genet. 2012;20:884–889. doi: 10.1038/ejhg.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Synaptic connections of rod bipolar cells in the inner plexiform layer of the rabbit retina. J Comp Neurol. 1990;295:449–466. doi: 10.1002/cne.902950309. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Gargini C, Novelli E, Sala G, Piano I, Gasco P, Ghidoni R. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2010;107:18706–18711. doi: 10.1073/pnas.1007644107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Masland RH. The number of unidentified amacrine cells in the mammalian retina. Proc Natl Acad Sci U S A. 1996;93:14906–14911. doi: 10.1073/pnas.93.25.14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Mears AJ, Swaroop A. Recruitment of the rod pathway by cones in the absence of rods. J Neurosci. 2004;24:7576–7582. doi: 10.1523/JNEUROSCI.2245-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Pignatelli V. Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2000;97:11020–11025. doi: 10.1073/pnas.190291097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Pignatelli V, Rossi C, Porciatti V, Falsini B. Remodeling of second-order neurons in the retina of rd/rd mutant mice. Vision Res. 2003;43:867–877. doi: 10.1016/s0042-6989(02)00594-1. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Porciatti V, Falsini B, Pignatelli V, Rossi C. Morphological and functional abnormalities in the inner retina of the rd/rd mouse. J Neurosci. 2002;22:5492–5504. doi: 10.1523/JNEUROSCI.22-13-05492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol. 1992;325:152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- Stronks HC, Dagnelie G. The functional performance of the Argus II retinal prosthesis. Expert review of medical devices. 2014;11:23–30. doi: 10.1586/17434440.2014.862494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuck MW, Conley SM, Naash MI. Defects in the outer limiting membrane are associated with rosette development in the Nrl−/− retina. PloS one. 2012;7:e32484. doi: 10.1371/journal.pone.0032484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Han X, He S. Direction-selective circuitry in rat retina develops independently of GABAergic, cholinergic and action potential activity. PLoS One. 2011;6:e19477. doi: 10.1371/journal.pone.0019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LO, Jiang Z, Rivlin-Etzion M, Hand R, Brady CM, Matsuoka RL, Yau KW, Feller MB, Kolodkin AL. On and off retinal circuit assembly by divergent molecular mechanisms. Science. 2013;342:1241974. doi: 10.1126/science.1241974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki SC, Bleckert A, Williams PR, Takechi M, Kawamura S, Wong RO. Cone photoreceptor types in zebrafish are generated by symmetric terminal divisions of dedicated precursors. Proc Natl Acad Sci U S A. 2013;110:15109–15114. doi: 10.1073/pnas.1303551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szel A, Rohlich P, Caffe AR, Juliusson B, Aguirre G, Van Veen T. Unique topographic separation of two spectral classes of cones in the mouse retina. J Comp Neurol. 1992;325:327–342. doi: 10.1002/cne.903250302. [DOI] [PubMed] [Google Scholar]
- Tagawa Y, Sawai H, Ueda Y, Tauchi M, Nakanishi S. Immunohistological studies of metabotropic glutamate receptor subtype 6-deficient mice show no abnormality of retinal cell organization and ganglion cell maturation. J Neurosci. 1999;19:2568–2579. doi: 10.1523/JNEUROSCI.19-07-02568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima Y, Munakata S, Ishida Y, Nakajima T, Sugano I, Nagao K, Minoda K, Kondo Y. Photoreceptor differentiation of retinoblastoma: an electron microscopic study of 29 retinoblastomas. Pathology international. 1994;44:837–843. doi: 10.1111/j.1440-1827.1994.tb01681.x. [DOI] [PubMed] [Google Scholar]
- Takada Y, Vijayasarathy C, Zeng Y, Kjellstrom S, Bush RA, Sieving PA. Synaptic pathology in retinoschisis knockout (Rs1-/y) mouse retina and modification by rAAV-Rs1 gene delivery. Invest Ophthalmol Vis Sci. 2008;49:3677–3686. doi: 10.1167/iovs.07-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesue A, Mochizuki A, Iwasa Y. Cell-differentiation rules that generate regular mosaic patterns: modelling motivated by cone mosaic formation in fish retina. J Theor Biol. 1998;194:575–586. doi: 10.1006/jtbi.1998.0777. [DOI] [PubMed] [Google Scholar]
- Tarboush R, Chapman GB, Connaughton VP. Ultrastructure of the distal retina of the adult zebrafish, Danio rerio. Tissue Cell. 2012;44:264–279. doi: 10.1016/j.tice.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Taylor S, Chen J, Luo J, Hitchcock P. Light-induced photoreceptor degeneration in the retina of the zebrafish. Methods in molecular biology (Clifton, N.J.) 2012;884:247–254. doi: 10.1007/978-1-61779-848-1_17. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. New directions in retinal research. Trends Neurosci. 2003;26:379–385. doi: 10.1016/S0166-2236(03)00167-X. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retin Eye Res. 2012;31:407–441. doi: 10.1016/j.preteyeres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- Tochitsky I, Polosukhina A, Degtyar VE, Gallerani N, Smith CM, Friedman A, Van Gelder RN, Trauner D, Kaufer D, Kramer RH. Restoring Visual Function to Blind Mice with a Photoswitch that Exploits Electrophysiological Remodeling of Retinal Ganglion Cells. Neuron. 2014;81:800–813. doi: 10.1016/j.neuron.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohya S, Mochizuki A, Iwasa Y. Formation of cone mosaic of zebrafish retina. J Theor Biol. 1999;200:231–244. doi: 10.1006/jtbi.1999.0990. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Toychiev AH, Yee CW, Sagdullaev BT. Correlated spontaneous activity persists in adult retina and is suppressed by inhibitory inputs. PLoS One. 2013;8:e77658. doi: 10.1371/journal.pone.0077658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, Borowska J, Zhang J, Hoggarth A, Johnson K, Barnes S, Lewis TJ, Awatramani GB. Intrinsic oscillatory activity arising within the electrically coupled AII amacrine-ON cone bipolar cell network is driven by voltage-gated Na+ channels. The Journal of physiology. 2012;590:2501–2517. doi: 10.1113/jphysiol.2011.225060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpler J, Dedek K, Schubert T, de Sevilla Muller LP, Seeliger M, Humphries P, Biel M, Weiler R. Rod and cone contributions to horizontal cell light responses in the mouse retina. J Neurosci. 2008;28:6818–6825. doi: 10.1523/JNEUROSCI.1564-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang SH, Woodruff ML, Jun L, Mahajan V, Yamashita CK, Pedersen R, Lin CS, Goff SP, Rosenberg T, Larsen M, Farber DB, Nusinowitz S. Transgenic mice carrying the H258N mutation in the gene encoding the beta-subunit of phosphodiesterase-6 (PDE6B) provide a model for human congenital stationary night blindness. Hum Mutat. 2007;28:243–254. doi: 10.1002/humu.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ishii M, Takao M, Iwatsuki K, Nakanishi S, Fukuda Y. A novel connection between rods and ON cone bipolar cells revealed by ectopic metabotropic glutamate receptor 7 (mGluR7) in mGluR6-deficient mouse retinas. J Neurosci. 2007;27:6261–6267. doi: 10.1523/JNEUROSCI.5646-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Omi N. Functional allocation of synaptic contacts in microcircuits from rods via rod bipolar to AII amacrine cells in the mouse retina. J Comp Neurol. 2013;521:3541–3555. doi: 10.1002/cne.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulvatana W, Adamian M, Berson EL, Dryja TP. Photoreceptor rosettes in autosomal dominant retinitis pigmentosa with reduced penetrance. Archives of ophthalmology. 1999;117:399–402. doi: 10.1001/archopht.117.3.399. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Barkis WB, Chen S, Diamond JS, Barres BA. Invulnerability of retinal ganglion cells to NMDA excitotoxicity. Molecular and cellular neurosciences. 2004;26:544–557. doi: 10.1016/j.mcn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Unsoeld T, Stradomska AM, Wang R, Rathjen FG, Juttner R. Early maturation of GABAergic synapses in mouse retinal ganglion cells. Int J Dev Neurosci. 2008;26:233–238. doi: 10.1016/j.ijdevneu.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Van Epps HA, Hayashi M, Lucast L, Stearns GW, Hurley JB, De Camilli P, Brockerhoff SE. The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. J Neurosci. 2004;24:8641–8650. doi: 10.1523/JNEUROSCI.2892-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Vandenberghe LH, Bell P, Maguire AM, Xiao R, Hopkins TB, Grant R, Bennett J, Wilson JM. AAV9 targets cone photoreceptors in the nonhuman primate retina. PloS one. 2013;8:e53463. doi: 10.1371/journal.pone.0053463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI. The Mosaic of Amacrine Cells in the Mammalian Retina. Prog Retinal Res. 1990;9:49–100. [Google Scholar]
- Vaney DI. Territorial organization of direction-selective ganglion cells in rabbit retina. J Neurosci. 1994;14:6301–6316. doi: 10.1523/JNEUROSCI.14-11-06301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, Sivyer B, Taylor WR. Direction selectivity in the retina: symmetry and asymmetry in structure and function. Nat Rev Neurosci. 2012;13:194–208. doi: 10.1038/nrn3165. [DOI] [PubMed] [Google Scholar]
- Vardi N, Duvoisin R, Wu G, Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol. 2000;423:402–412. doi: 10.1002/1096-9861(20000731)423:3<402::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Varela C, Igartua I, De la Rosa EJ, De la Villa P. Functional modifications in rod bipolar cells in a mouse model of retinitis pigmentosa. Vision research. 2003;43:879–885. doi: 10.1016/s0042-6989(02)00493-5. [DOI] [PubMed] [Google Scholar]
- Verweij J, Dacey DM, Peterson BB, Buck SL. Sensitivity and dynamics of rod signals in H1 horizontal cells of the macaque monkey retina. Vision Res. 1999;39:3662–3672. doi: 10.1016/s0042-6989(99)00093-0. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;25:4108–4117. doi: 10.1523/JNEUROSCI.5253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veth KN, Willer JR, Collery RF, Gray MP, Willer GB, Wagner DS, Mullins MC, Udvadia AJ, Smith RS, John SW, Gregg RG, Link BA. Mutations in zebrafish lrp2 result in adult-onset ocular pathogenesis that models myopia and other risk factors for glaucoma. PLoS Genet. 2011;7:e1001310. doi: 10.1371/journal.pgen.1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Sanz M, Lafuente MP, Mayor S, de Imperial JM, Villegas-Perez MP. Retinal ganglion cell death induced by retinal ischemia. neuroprotective effects of two alpha-2 agonists. Survey of ophthalmology. 2001;45(Suppl 3):S261–267. doi: 10.1016/s0039-6257(01)00205-3. discussion S273-266. [DOI] [PubMed] [Google Scholar]
- Volgyi B, Kovacs-Oller T, Atlasz T, Wilhelm M, Gabriel R. Gap junctional coupling in the vertebrate retina: variations on one theme? Prog Retin Eye Res. 2013;34:1–18. doi: 10.1016/j.preteyeres.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Vroman R, Klaassen LJ, Kamermans M. Ephaptic communication in the vertebrate retina. Front Hum Neurosci. 2013;7:612. doi: 10.3389/fnhum.2013.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YV, Weick M, Demb JB. Spectral and temporal sensitivity of cone-mediated responses in mouse retinal ganglion cells. J Neurosci. 2011;31:7670–7681. doi: 10.1523/JNEUROSCI.0629-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Wässle H, Boycott BB, Rohrenbeck J. Horizontal Cells in the Monkey Retina: Cone connections and dendritic network. Eur J Neurosci. 1989;1:421–435. doi: 10.1111/j.1460-9568.1989.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grunert U, Martin PR, Boycott BB. Immunocytochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina. Vision Res. 1994;34:561–579. doi: 10.1016/0042-6989(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Wässle H, Heinze L, Ivanova E, Majumdar S, Weiss J, Harvey RJ, Haverkamp S. Glycinergic transmission in the Mammalian retina. Front Mol Neurosci. 2009a;2:6. doi: 10.3389/neuro.02.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Koulen P, Brandstatter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998;38:1411–1430. doi: 10.1016/s0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- Wässle H, Peichl L, Boycott BB. Dendritic territories of cat retinal ganglion cells. Nature. 1981;292:344–345. doi: 10.1038/292344a0. [DOI] [PubMed] [Google Scholar]
- Wässle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009b;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Riemann HJ. The mosaic of nerve cells in the mammalian retina. Proc R Soc Lond B Biol Sci. 1978;200:441–461. doi: 10.1098/rspb.1978.0026. [DOI] [PubMed] [Google Scholar]
- Wässle H, Schafer-Trenkler I, Voigt T. Analysis of a glycinergic inhibitory pathway in the cat retina. J Neurosci. 1986;6:594–604. doi: 10.1523/JNEUROSCI.06-02-00594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AJ, Harman CD. Structure-function relations of parasol cells in the normal and glaucomatous primate retina. Invest Ophthalmol Vis Sci. 2005;46:3197–3207. doi: 10.1167/iovs.04-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AJ, Kaufman PL, Hubbard WC. Morphology of single ganglion cells in the glaucomatous primate retina. Invest Ophthalmol Vis Sci. 1998;39:2304–2320. [PubMed] [Google Scholar]
- Weber AJ, Zelenak D. Experimental glaucoma in the primate induced by latex microspheres. J Neurosci Methods. 2001;111:39–48. doi: 10.1016/s0165-0270(01)00443-5. [DOI] [PubMed] [Google Scholar]
- Weber BH, Schrewe H, Molday LL, Gehrig A, White KL, Seeliger MW, Jaissle GB, Friedburg C, Tamm E, Molday RS. Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proc Natl Acad Sci U S A. 2002;99:6222–6227. doi: 10.1073/pnas.092528599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Hamby AM, Zhou K, Feller MB. Development of asymmetric inhibition underlying direction selectivity in the retina. Nature. 2011;469:402–406. doi: 10.1038/nature09600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Locke KG, Hood DC, Birch DG. Rod photoreceptor temporal properties in retinitis pigmentosa. Exp Eye Res. 2011;92:202–208. doi: 10.1016/j.exer.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Locke KG, Hood DC, Birch DG. Rod photoreceptor temporal properties in retinal degenerative diseases. Advances in experimental medicine and biology. 2012;723:495–502. doi: 10.1007/978-1-4614-0631-0_62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Arango-Gonzalez B, Haycock JW, Kohler K. Rat retinal dopaminergic neurons: differential maturation of somatodendritic and axonal compartments. J Comp Neurol. 2005;481:352–362. doi: 10.1002/cne.20389. [DOI] [PubMed] [Google Scholar]
- Wong GT, Ruiz-Avila L, Margolskee RF. Directing gene expression to gustducin-positive taste receptor cells. J Neurosci. 1999;19:5802–5809. doi: 10.1523/JNEUROSCI.19-14-05802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY, Dowling JE. Retinal bipolar cell input mechanisms in giant danio. III. ON-OFF bipolar cells and their color-opponent mechanisms. J Neurophysiol. 2005;94:265–272. doi: 10.1152/jn.00271.2004. [DOI] [PubMed] [Google Scholar]
- Wong RO. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- Wu SM, Gao F, Maple BR. Functional architecture of synapses in the inner retina: segregation of visual signals by stratification of bipolar cell axon terminals. J Neurosci. 2000;20:4462–4470. doi: 10.1523/JNEUROSCI.20-12-04462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Synaptic localization and function of Sidekick recognition molecules require MAGI scaffolding proteins. J Neurosci. 2010;30:3579–3588. doi: 10.1523/JNEUROSCI.6319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Expanding the Ig superfamily code for laminar specificity in retina: expression and role of contactins. J Neurosci. 2012;32:14402–14414. doi: 10.1523/JNEUROSCI.3193-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Yang XL. Characterization of receptors for glutamate and GABA in retinal neurons. Prog Neurobiol. 2004;73:127–150. doi: 10.1016/j.pneurobio.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Yin L, Greenberg K, Hunter JJ, Dalkara D, Kolstad KD, Masella BD, Wolfe R, Visel M, Stone D, Libby RT, Diloreto D, Jr., Schaffer D, Flannery J, Williams DR, Merigan WH. Intravitreal injection of AAV2 transduces macaque inner retina. Invest Ophthalmol Vis Sci. 2011;52:2775–2783. doi: 10.1167/iovs.10-6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Smith RG, Sterling P, Brainard DH. Chromatic properties of horizontal and ganglion cell responses follow a dual gradient in cone opsin expression. J Neurosci. 2006;26:12351–12361. doi: 10.1523/JNEUROSCI.1071-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Smith RG, Sterling P, Brainard DH. Physiology and morphology of color-opponent ganglion cells in a retina expressing a dual gradient of S and M opsins. J Neurosci. 2009;29:2706–2724. doi: 10.1523/JNEUROSCI.5471-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara K, Balint K, Noda M, Nagel G, Bamberg E, Roska B. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature. 2011;469:407–410. doi: 10.1038/nature09711. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Zabouri N, Haverkamp S. Calcium channel-dependent molecular maturation of photoreceptor synapses. PLoS One. 2013;8:e63853. doi: 10.1371/journal.pone.0063853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li W, Trexler EB, Massey SC. Confocal analysis of reciprocal feedback at rod bipolar terminals in the rabbit retina. J Neurosci. 2002;22:10871–10882. doi: 10.1523/JNEUROSCI.22-24-10871.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang Z, Wu SM. Development of cholinergic amacrine cells is visual activity-dependent in the postnatal mouse retina. J Comp Neurol. 2005;484:331–343. doi: 10.1002/cne.20470. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Yang XJ. Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev Biol. 2001;233:271–290. doi: 10.1006/dbio.2000.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Toris CB, Liu Y, Ye W, Gong H. Morphological and hydrodynamic correlates in monkey eyes with laser induced glaucoma. Exp Eye Res. 2009;89:748–756. doi: 10.1016/j.exer.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci. 2006;9:363–371. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]
- Zheng JJ, Lee S, Zhou ZJ. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron. 2004;44:851–864. doi: 10.1016/j.neuron.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Zhong L, Bradley J, Schubert W, Ahmed E, Adamis AP, Shima DT, Robinson GS, Ng Y-S. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Investigative ophthalmology & visual science. 2007;48:1212–1218. doi: 10.1167/iovs.06-0757. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ, Lee S. Synaptic physiology of direction selectivity in the retina. J Physiol. 2008;586:4371–4376. doi: 10.1113/jphysiol.2008.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Schmidt JF, Gidday JM. Glaucoma-induced degeneration of retinal ganglion cells prevented by hypoxic preconditioning: a model of glaucoma tolerance. Molecular medicine (Cambridge, Mass.) 2012;18:697–706. doi: 10.2119/molmed.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang Y, Ojwang BA, Brantley MA, Gidday JM. Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF-1alpha and heme oxygenase-1. Investigative ophthalmology & visual science. 2007;48:1735–1743. doi: 10.1167/iovs.06-1037. [DOI] [PubMed] [Google Scholar]
- Zou J, Wang X, Wei X. Crb apical polarity proteins maintain zebrafish retinal cone mosaics via intercellular binding of their extracellular domains. Dev Cell. 2012;22:1261–1274. doi: 10.1016/j.devcel.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]