Abstract
Multiple sclerosis (MS) is a complex multifactorial disease that results from the interplay between environmental factors and a susceptible genetic background. Experimental autoimmune encephalomyelitis (EAE) has been widely used to investigate the mechanisms underlying MS pathogenesis. Chemokines, such as C-C Chemokine Ligand 2 (CCL2), are involved in the development of EAE. We have previously shown that thiamine deficiency (TD) induced CCL2 in neurons. We hypothesized that TD may affect the pathogenesis of EAE. In this study, EAE was induced in C57BL/6J mice by the injection of myelin oligodendroglial glycoprotein (MOG) peptide 35–55 with/without TD. TD aggravated the development of EAE which was indicated by clinical scores and pathological alterations in the spinal cord. TD also accelerated the development of EAE in an adoptive transfer EAE model. TD caused microglial activation and a drastic increase (up 140%) in leukocyte infiltration in the spinal cord of the EAE mice; specifically TD increased Th-1 and Th-17 cells. TD upregulated the expression of CCL2 and its receptor CCR2 in the spinal cord of EAE mice. Cells in peripheral lymph node and spleen isolated from MOG-primed TD mice showed much stronger proliferative responses to MOG. CCL2 stimulated the proliferation and migration of T lymphocytes in vitro. Our results suggested that TD exacerbated the development of EAE through activating CCL2 and inducing pathological inflammation.
Keywords: TD, EAE, MOG, infiltration of T lymphocyte, inflammation
Introduction
Human multiple sclerosis (MS) is an autoimmune disease, in which T lymphocytes specific for myelin antigens initiate an inflammatory reaction in the central nervous system (CNS), leading to demyelination and subsequent axonal injury (1–3). The etiology of MS is unclear. Recent genome-wide association studies (GWAS) indicate that genetic factors play a critical role (4). Epidemiological studies demonstrate that environmental influences also contribute to MS pathogenesis; these influences include infection, immunisations, physical and emotional stresses, climate, diet, and occupational exposures (5–6). Mapping the distribution of MS reveals a high prevalence of the disease in high-latitude areas, suggesting a positive relationship between vitamin D deficiency and MS (7–8). Vitamin D supplements have been considered a potential therapeutic strategy for MS patients. Vitamin D3 supplementation has been found to prevent or reduce MS (9–10). A recent study showed that serum levels of vitamin B12 and foliate were lower in MS patients (11). Other studies, however, did not support an association between B12 deficiency and MS (12). Interestingly, patients receiving vitamin B12 supplementation showed remarkable improvement in clinical outcomes (13). It is necessary to conduct a prospective study to determine whether treatment with supplements at an early stage of the disease can alter biomarker levels and change the course of the disease (14).
Experimental autoimmune encephalomyelitis (EAE) is a classic model for autoimmune demyelination of the CNS and has been widely used to explore pathogenic mechanisms of MS (15). The generation of myelin protein–reactive T cells is an immunological hallmark of both EAE and MS. The disease is initiated when activated autoreactive T cells cross the blood brain barrier (BBB) to reach the CNS. Upon encountering the antigens, these cells recruit inflammatory effector cells to the CNS, resulting in devastating demyelination and axon destruction (16–17). EAE can be induced either by immunizing animals with myelin components (actively induced EAE) or by transferring encephalitogenic T cells (adoptive transfer EAE) (1, 18–19). Animals are differentially susceptible to EAE (20). C57BL/6 mice develop a monophasic disease upon myelin oligodendroglial glycoprotein (MOG) challenge, showing extensive demyelination and inflammation in the CNS and are a commonly used EAE model (21).
Chemokines or chemoattractant cytokines are crucial during inflammatory responses for the recruitment of leukocyte populations to sites of tissue damage (22). Virtually all cell types, including many tumor cells, express chemokines and chemokine receptors (23–24). Chemokines play a major role in the immune response because they regulate the migration and activation of leukocytes. C-C Chemokine Ligand 2 (CCL2) was first identified as a potent chemotaxin for monocytes in response to proinflammatory stimuli (25). CCL2 regulates the migration and activation of monocytes, T cells, NK cells and basophils (26–28). CCL2 binds solely to C-C chemokine receptor (CCR) 2, a seven-transmembrane–spanning protein that is functionally linked to downstream signaling pathways through heterotrimeric G proteins. In addition to being a receptor to CCL2, CCR2 serves as the receptor for four other ligands, MCP-2, -3, -4 and -5 (29). CCL2 and CCR2 have been implied in EAE (30–36). For example, anti-CCL2 antibody blocks relapses of adoptive transfer EAE in SJL mice (37). Mice that lack CCR2 fail to develop EAE after active immunization and are resistant to induction of EAE by the adoptive transfer of primed T cells from syngeneic wild-type mice (29, 38).
Thiamine is a cofactor of key enzymes in glucose metabolism (39). Thiamine deficiency (TD) causes mild impairment of oxidative metabolism and induces Wernicke–Korsakoff’s syndrome (WKS) in humans. TD causes regionally selective neuronal death, mitochondrial dysfunction, energy shortage and chronic oxidative stress in the brains of humans and animals (40–41). TD in animals has been used to model some neurodegenerative diseases (41). Subclinical TD is common in the elder population (42). However, it is unclear whether the status of thiamine will affect MS and EAE. Our previous studies demonstrated that TD increased CCL2 expression in the CNS (41). Here we demonstrated that TD enhanced the EAE severity by activating T cells reaction and increasing CCL2 expression in the spinal cord.
Materials and Methods
Peptides and antibodies
Rat MOG35–55 peptides were obtained from Biosynth International (Naperville, IL, USA) and purified by high performance liquid chromatography, and the purity of the peptide was >95%. The sequence of MOG35–55 was MEVGWYRSPFSRVVHLYRNGK. Purified Hamster anti-mouse CD3e, Fluorescein Isothiocyanate (FITC) rat anti-mouse CD4, APC rat Anti-mouse CD8a, APC rat anti-mouse IL-4 and PE rat anti-mouse IL-17A antibodies were purchased from BD Pharmingen (Basel, Switzerland); Goat anti-mouse ionized calcium-binding adaptor molecule-1 (IBA1) antibody was obtained from abcam (Cambridge, UK); Rat anti-mouse CD45, Rabbit anti-mouse CCL2 antibody and CCL2 (rat recombinant) was purchased from AbD Serotec (Raleigh, NC, USA)). FITC rat anti-mouse IFN-γ antibody was purchased from eBioscienc (San Diego, CA, USA); rabbit anti-mouse CCR2 antibody was purchased from Abcam (Cambridge, UK). 2-(1-benzyl-indazol-3-yl) methoxy)-2-methyl propionic acid (bindarit) was synthesized by and obtained from Angelini (Angelini Research Center-ACRAF, Italy).
Animal models
Active immunization model of EAE
C57BL/6J mice were obtained from Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China). The procedure for animal surgery was performed in accordance with the Guidelines of Animal Care and Use Committee of the Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. Induction of EAE was performed as previously described (43). Briefly, mice were subcutaneously injected at two sites with 100 μg of rat MOG peptide 35–55 (MEVGWYRSPFSRVVHLYRNGK; >95% purity; Bio-Synthesis, Naperville, IL, USA), emulsified in complete Freund’s adjuvant containing 400 μg of Mycobacterium tuberculosis (Difco Laboratories, Detroit, MI, USA). On the same day (day 0) and on day 2 p.i., mice were intravenously injected with 200 ng of pertussis toxin (Sigma-Aldrich, St Louis, MO, USA). All mice were weighed, examined and graded daily for neurological signs in a blinded manner as follows: 0, no disease; 1, decreased tail tone or slightly clumsy gait; 2, tail atony and moderately clumsy gait and/or poor righting ability; 3, limb weakness; 4, limb paralysis; and 5, moribund state. Average disease scores were assessed daily. Additionally, in the EAE model, we documented the weight changes during the disease course. Only mice with a score of at least 2 for more than two consecutive days were judged to have fully developed EAE. The maximum clinical score achieved by each animal during the 30-day-observation period was used to calculate average maximum clinical scores for each experimental group. To study the time course of disease development, average clinical scores were calculated daily for each group of mice and plotted. MOG35–55 induced monophasic EAE was monitored for 30 days. Animals were euthanized if scores were worse than grade 4.
Adoptive transfer EAE model
To prepare encephalitogenic cells for adoptive transfer of EAE, mice were immunized with MOG35–55 as described above. Spleens and lymph nodes were collected and cell suspensions were prepared after 10 days of induction. Cells (6×106/ml) were cultured in RPMI 1640 medium (supplemented with 10% fetal bovine serum, 2mM L-glutamine, 1 mM sodium pyruvate, 100 Iuml−1 penicillin/streptomycin and 2×10−5M 2-ME (Life Technologies, Carlsbad, CA, USA)), with MOG35–55 (20 μg/ml) and IL-12 (30 ng/ml) (R&D Systems, Minneapolis, MN, USA). After 3 days in culture, the cells were harvested, washed in phosphate-buffered saline (PBS) and injected into recipient mice intravenously that were irradiated sublethally (500 rad) within 16 hours before cell injection. All mice were weighed, examined and scored daily after cell transfer (44).
TD induction
The animal TD model has been previously described. (40, 45–46). Briefly, adult male C57BL/6J mice were housed in a controlled environment (23°C and 53% humidity). The animals were fed with either a control diet or a thiamine-deficient diet (ICN Nutrition Biomedicals, Cleveland, OH, USA) ad libitum at the 0 day of EAE.
Treatment of bindarit
2-((1-benzyl-indazol-3-yl) methoxy)-2-methyl propionic acid (bindarit) is a small synthetic indazolic derivative that preferentially inhibits transcription of CCL2 (47). Bindarit has been shown some clinical efficacy in treating a broad array of experimental inflammatory, autoimmune and vascular disorders; it also had some success in recent clinical trials for diabetic nephropathy and lupus nephritis (48).
The method for bindarit treatment in animals has been previously described (48). Briefly, bindarit was prepared as a suspension in dimethyl sulfoxide (DMSO) at a concentration of 40 mg/ml. Then mice were given daily i.p. injection of bindarit (or vehicle DMSO) at 200 mg/kg for three consecutive days, beginning one day before MOG immunization (day −1), then injections every other day. This schedule was designed to minimize trauma associated with daily injections at times of peak neurologic disease and physical compromise.
Immunohistochemistry and immunofluorescence staining
For immunohistochemical (IHC) analysis of spinal cord tissues, mice were euthanized at the peak of EAE by intracardiac perfusion with ice-cold PBS, followed by 4% paraformaldehyde solution, under anesthesia. Spinal cords were rapidly dissected and sectioned at a thickness of 25 μm. The sections were rinsed in PBS, incubated with 0.3% hydrogen peroxide, blocked by the incubation with 10% bovine serum albumin at 37°C for 1 hour, then incubated overnight at 4°C with a primary antibody (rat anti-mouse CD45 antibody, 1/1,000; Goat anti-mouse IBA1 antibody, 1/1,000). The sections were then incubated with appropriate biotinylated secondary antibodies at 37°C for 1 hour and treated with diaminobenzidine. All antibodies were diluted in 1% bovine serum albumin in PBS. Negative controls were performed by the incubation of preimmune IgG. For detecting inflammatory infiltrates, the sections were stained with hematoxylin and eosin (HE).
For immunocytofluorescence staining, tissue sections or cells from lymph nodes were rinsed in PBS, blocked by incubation with 1% bovine serum albumin at 37°C for 1 hour, then incubated overnight at 4°C with primary antibodies (rabbit anti-CCL2 polyclonal antibody, 1/200; rat anti-mouse CD4 antibody, 1/50; rat anti-mouse CD8a, 1/50). The sections were incubated with appropriate FITC secondary antibodies at 37°C for 1 hour. The bright field images were taken on a BX51 Olympus microscope (Olympus Corporation, Tokyo, Japan); Immunofluorescent images were recorded using a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY, USA). For the quantification, five sections from each mouse were used for cell counting. Cells were counted using ImageJ (US National Institutes of Health) in a designated area. Data represent mean ± SD of 5 mice for each group.
T cell proliferation
To examine the proliferation of T cells, we isolated lymph nodes and spleen from MOG35–55-immunized mice and cultured T cells in a 96-well plate (1×105 per well) in the presence of MOG35–55 (0, 0.8, 4, 20 and 100 μg/ml), CCL2 (20 μg/ml) or Con A (10 μg/ml) (Sigma-Aldrich). Cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (Life Technologies), 2 mM L-glutamine, 1mM sodium pyruvate, 100 IU/ml penicillin/streptomycin and 2×10−5 M 2-ME (Life Technologies) for 72 hours. Cell proliferaton was determined using an AMR PLUS kit (Lonza Rockland, Rockland, ME, USA) according to manufacturer’s instruction. The absorbance was analyzed with a luminometer (Bio-Tek, Atlanta, GA, USA).
Flow cytometry
T cells (1× 106/ml) obtained from lymph nodes were washed and resuspended in PBS. Cells were stained for surface markers with specific primary antibodies and appropriate fluorescein isothiocyanate-conjugated (FITC) secondary antibodies in fluorescence-activated cell sorting (FACS) buffer at 4°C for 40 min. Cells were washed twice and resuspended in the 200–400 μl of PBS for flow cytometry analysis as previously described (49–50). The cell sorting was performed with a FACSCalibur (BD Biosciences, San Diego, CA, USA) equipped with CellQuest software (BD Biosciences). Data were analyzed with FlowJo software (Tree Star, San Carlos, CA, USA). For intracellular staining, cells were maintained in a 6-well plate (2×106 per well) and treated with MOG35–55 (20 μg/ml) for 72 hours. A inhibitor of protein transport Brefeldin A (1: 10 dilution; BD Biosciences, San Jose, CA, USA) was added to the cultures during the last 4–5 hours., then cells were collected, fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences, San Jose, CA, USA), followed by the staining using fluorescence labeled rat anti-mouse antibodies directed against IFN-γ, IL-4 or IL-17. Cells were then analyzed with FACSCalibur. Isotype-matched antibodies were used as controls. For the CCL2 binding experiments, draining lymph node cells were incubated with IgG, CCL2 or CCL2 plus anti-CCL2 antibody for 60 min. The cells were then incubated with appropriate FITC antibodies and analyzed with FACSCalibur.
Transwell migration assay
Chemotaxis assays were performed with purified lymph node T cells using uncoated 8-μm transwell filters (Corning Costar, Corning, NY) as previously described (51). Briefly, T cells (2×105/ml) suspended in RPMI 1640 containing 1% FBS were added to the upper chamber; CCL2 (5 μg/ml) as a T cell chemokine was added to the lower chamber. Cells were allowed to migrate for 4 hours at 37°C in 5% CO2 atmosphere. The transwell filters were collected and fixed with methanol for 10 min. then stained with Diff-Quick (Baxter Diagnostics, Inc., Deerfield, IL) for 20 min. The migrated T cells were counted under a microscope at ×400 magnification (Axiovert 200M; Carl Zeiss). Data are expressed as mean ± SD for duplicate filters from four independent experiments.
Statistical analysis
Statistical analysis was assessed by ANOVA followed by Student–Newman–Keuls analyses. An unpaired t test was used for the analysis of quantitative data of cell counting. Data were presented as means ± SD. Difference in which p < 0.05 was considered statistically significant.
Results
TD exacerbates EAE in active immunization model
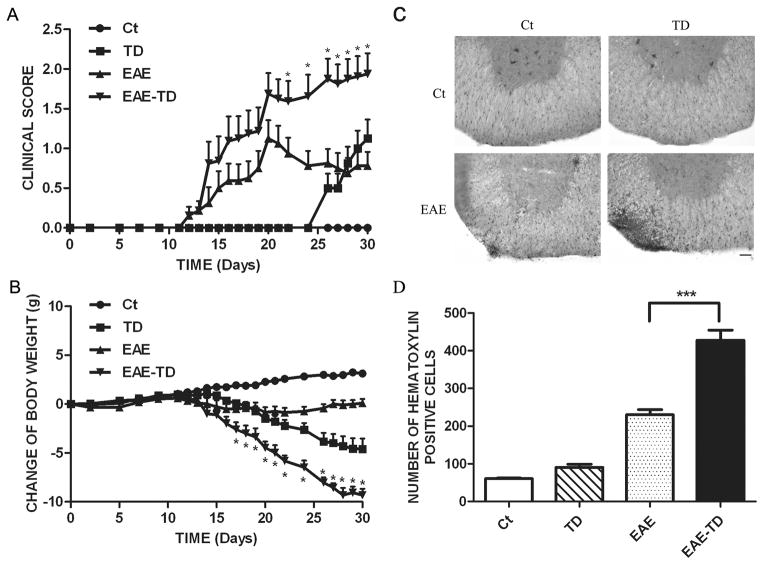
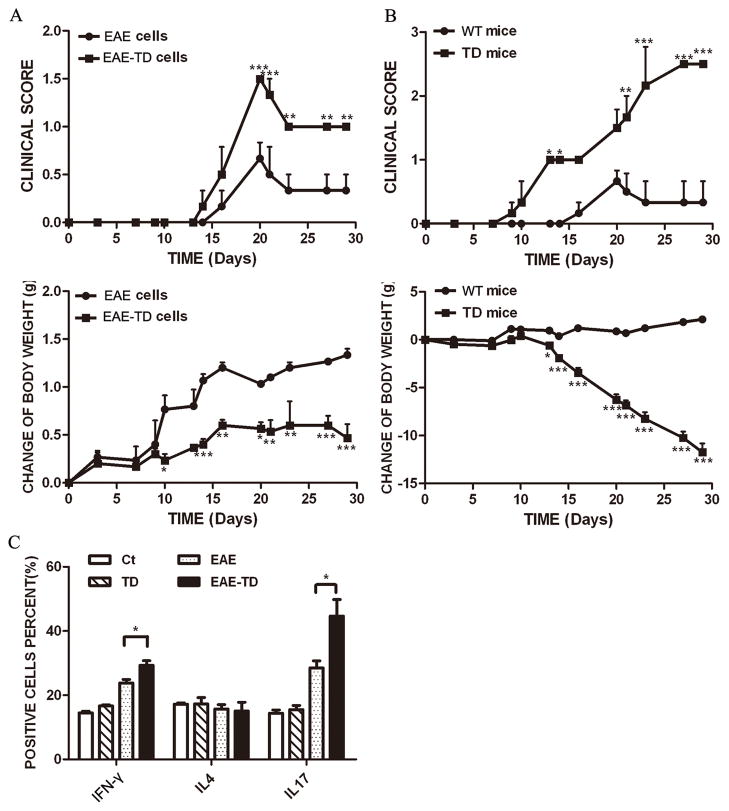
We investigated the effects of TD on EAE using an active immunization model of EAE. The EAE mice developed a monophasic disease characterized by ascending paralysis 10–18 days after immunization, and EAE mice with TD showed markedly more severe neurologic dysfunction. Table 1 summarizes the neurobehavioral features of MOG35–55-(MOG) induced EAE mice with/without TD. In our EAE mouse model (MOG injection), 11 out of 16 (69%) mice developed fatal EAE, whereas in EAE plus TD model, 13 out of 16 (81%) mice developed fatal EAE (Table 1). The onset of EAE was earlier in EAE plus TD model (13 days) compared to EAE mouse model (16 days). The neurological signs of EAE in mice were quantified and expressed as clinical scores and presented in Fig. 1A. Mice with TD alone revealed neurological alterations, such as clumsy gait or poor righting ability after 25 days. The clinical score for EAE model peaked at day 20 and then slightly declined; however, in EAE plus TD model, the clinical score was significantly higher and kept increasing after day 20 (Fig. 1A). Additionally, in EAE plus TD model, mice had a more severe loss of body weight (Fig. 1B).
Table 1.
EAE in wild-type and TD mice
| Mouse genotype | Incidence | Days of EAE onset (mean ± S.D.) | Maximum clinical score (mean ± S.D.) |
|---|---|---|---|
| Wild type | 11 of 16 (69%) | 16.09 (±2.91) | 1.55 (±0.55) |
| TD | 13 of 16 (81%) | 13.61 (±3.50) | 2.38 (±0.46)* |
Results are cumulative data from three different experiments,
P<0.05, as compared to corresponding wild-type controls.
Figure 1. Effect of TD on the progression of EAE.
EAE was induced in C57BL/6 mice by injection of MOG35-55 with or without TD as described under the Materials and Methods. The clinical score (A) and body weight (B) was determined in these mice as described under the Materials and Methods. Data were presented as mean ± SEM; *p < 0.05, compared with EAE mice; n = 20 for each group. (C) The infiltration of inflammatory cells in the spinal cord was detected by hematoxylin and eosin staining on the peak of EAE. Bars = 50 μm. (D) The number of hematoxylin positive cells in the spinal cord was quantified using ImageJ software in a designated area. Data were presented as mean ± SD; ***p<0.001, compared with EAE mice; n = 5 for each group.
We next performed histopathological analysis on spinal cords of EAE mice with or without TD. Inflammatory cell infiltration in lumbosacral enlargement was examined by hematoxylin and eosin staining (Fig. 1C) and the infiltrated cells were quantified (Fig. 1D). Compared to controls, the number of infiltrated cells increased by 283% and 613% in EAE mice and EAE plus TD mice, respectively (Fig 1D).
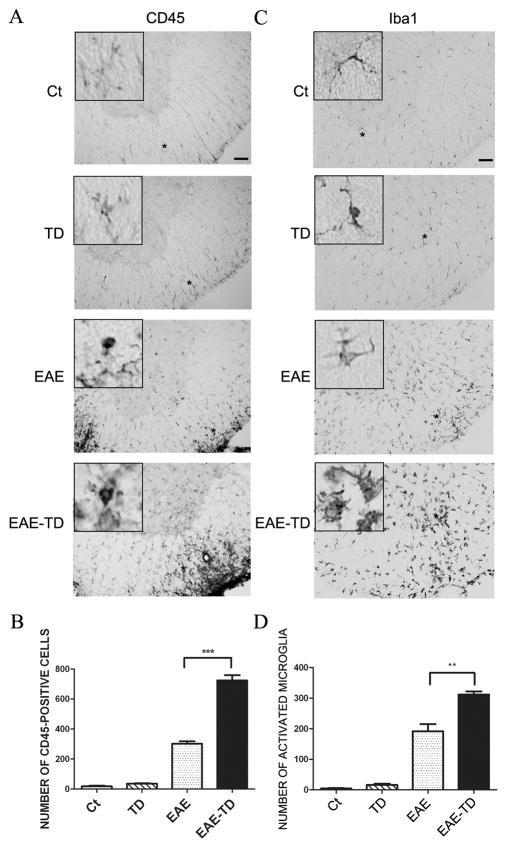
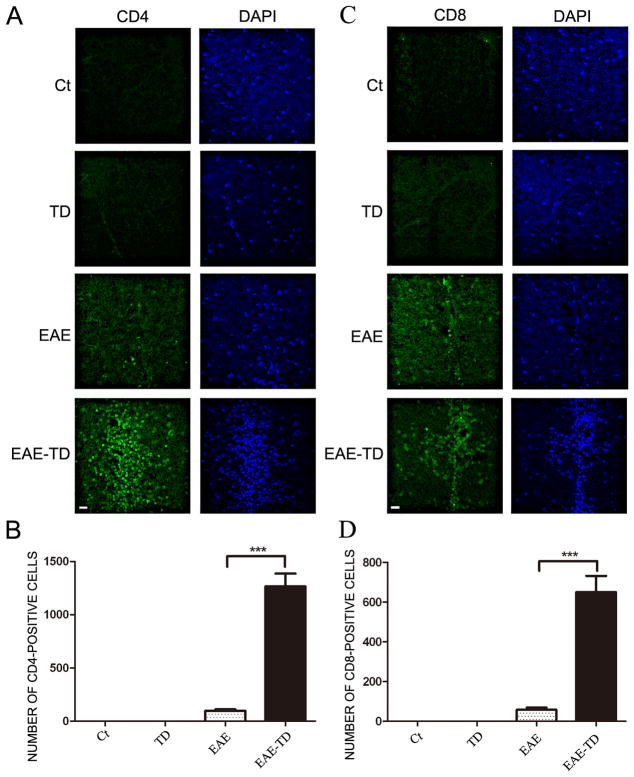
To determine the identity of inflammatory cells, we performed immunohistochemical analysis of CD45 and Iba1 on the spinal cord (Fig. 2). CD45 is a marker for the mononuclear cells from the circulation, while Iba1 is a marker for microglia in the CNS. In the EAE plus TD model, there were much more CD45+ cells (139% increase) compared to the EAE model. EAE activated microglia in the spinal cord; the active microglia displayed a larger cell body and thicker processes. TD significantly increased the number of active microglia in EAE mice (Figs 2C and D). We further examined the infiltrated lymphocytes in the spinal cord by IHC of CD4 and CD8. CD4+ and CD8+ cells were only observed in EAE mice and there was no CD4+ and CD8+ T lymphocytes in the spinal cord of wild-type mice and TD mice. TD drastically increased CD4+ and CD8+ cells in EAE mice (Fig. 3). We have also performed FACS to tested the proportion of CD4 and CD8 cells obtained from spleen and PLN in different group mice on the onset and peak of EAE. The results showed there were not obvious difference between different group (Supplementary Fig. 1).
Figure 2. Effect of TD on microglial activation in EAE mice.
EAE in C57BL/6 mice was induced by injection of MOG35-55 with or without TD. The activation of microglia was measure by immunohistochemical (IHC) staining with an antibody directed against CD45 (A) or Iba-1 (C) on day 20 after of MOG35-55 injection. Bars = 50 μm. The number of CD45+ cells (B) and active microglia (D) was determined. Data were presented as mean ± SD; **p<0.01, ***p<0.001, compared with EAE mice; n = 5 for each group.
Figure 3. Effect of TD on the infiltration of CD4+ and CD8+ T lymphocytes in the spinal cord of EAE mice.
EAE in C57BL/6 mice was induced by injection of MOG35-55 with or without TD. On day 20 after MOG35-55 injection, CD4+ and CD8+T lymphocytes in the spinal cord were measured by IHC using specific antibodies (A and C). Bars = 20 μm. The number of CD4+ and CD8+T lymphocytes was quantified (B and D). Data were presented as mean ± SD; n = 5 for each group. ***p<0.001, compared with EAE mice.
TD potentiates antigen-stimulated proliferation of T lymphocytes
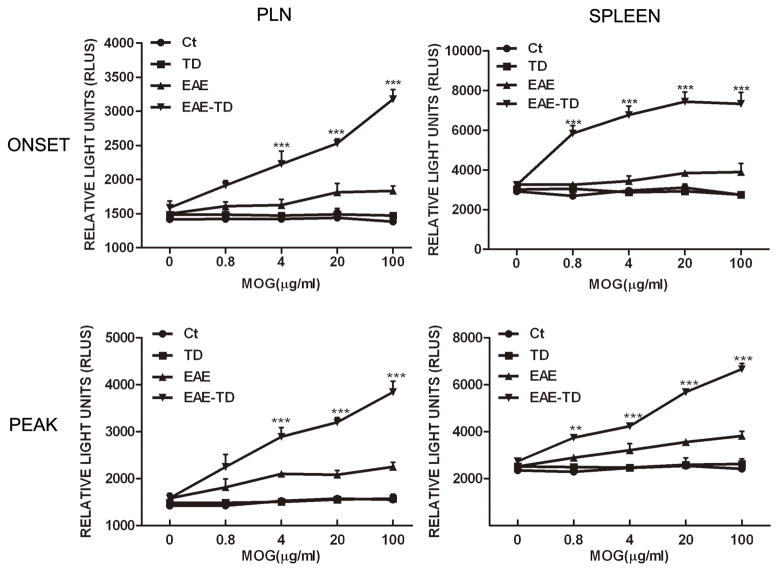
We hypothesized that TD increased the proliferation of T lymphocytes in response to the antigen. To test this hypothesis, we examined the antigen-stimulated proliferation of T lymphocytes isolated from the peripheral lymph node and spleen of control, TD mice, and EAE with/without TD mice on the onset and peak of EAE. Although other cells may be present, T lymphocytes are major cells in peripheral lymph node. MOG did not stimulate the proliferation of lymphocytes isolated from control and TD mice, but significantly increased the proliferation of lymphocytes isolated from EAE mice (Fig. 4). More importantly, the lymphocytes isolated from EAE mice plus TD mice displayed a much stronger and concentration-dependent response to MOG-mediated proliferation.
Figure 4. TD enhances the proliferation of T lymphocytes in response to MOG35–55.
EAE in C57BL/6 mice was induced by injection of MOG35-55 with or without TD. On the onset and peak of EAE, T lymphocytes were isolated from peripheral lymph node and spleen and challenged with MOG35–55 in vitro. Cell proliferation was determined as described under the Materials and Methods. Data were presented as mean ± SD; **p<0.01, ***p<0.001, compared with EAE group; n = 4 for each group.
TD aggravates EAE in adoptive transfer model
We hypothesized that the antigen-specific T cells isolated from EAE plus TD would cause more severe EAE. To test this hypothesis, we transferred encephalitogenic cells from mice treated with MOG with/without TD for 10 days to recipient mice that were irradiated sublethally. The encephalitogenic cells isolated from MOG-treated mice caused EAE which was shown by the clinical score and the loss of body weight; while the encephalitogenic cells isolated from MOG plus TD-treated mice induced more severe EAE in recipient mice which was indicated by higher clinical scores and a greater loss of body weight (Fig 5A). Next, we transferred encephalitogenic cells isolated MOG-treated mice to recipient mice that were treated with/without TD. In the recipient mice that were treated with TD, the encephalitogenic cells caused a quicker and more severe development of EAE as determined by the clinical scores (Fig. 5B). Consistently, the encephalitogenic cells induced greater body weight loss in the recipient mice that were treated with TD (Fig 5B).
Figure 5. Effect of TD on adoptive EAE model.
A: EAE was induced in C57BL/6 mice by injection of MOG35-55 with or without TD. Encephalitogenic cells were collected from these mice as described under the Materials and Methods and injected into recipient mice (wild-type). The recipient mice were irradiated sublethally (500 rad) 16 hours before the injection. The clinical scores for EAE and body weight were determined as described under the Materials and Methods. B: EAE was induced in C57BL/6 mice by injection of MOG35-55. The encephalitogenic cells were prepared from EAE mice and injected into recipient mice with/without TD. The clinical scores for EAE and body weight were determined as described under the Materials and Methods. Data were presented as mean ± SD; *p<0.05, **p<0.01, ***p<0.001, compared with control group. n = 8 for each group. C: Lymph node cells were isolated from EAE mice with or without TD after 10 days of induction. The percentage of IFN-γ, IL-4 and IL-17 producing cells were measured by FACS. Data were presented as mean ± SD; *p<0.05, compared with EAE group. n = 4 for each group.
CD4+ T cells have been classified into distinct subsets namely Th1 (characterized by the production of interferon-γ), Th2 (characterized by the production of interleukin 4) and Th17 (characterized by the production of interleukin 17). IFN-γ-producing Th1 cells and IL-17-producing Th17 cells have been implicated in EAE induction. We examined the relative populations of Th1, Th2 and Th17 cells in the lymph node using flow cytometry after 10 days of induction. There was a significant increase in the interferon-γ producing cells (23% more) and interleukin producing cells (56% more) in EAE plus TD model compared to EAE only model (Fig. 5C). However, there was no difference in IL4 producing cells among all experimental groups (Fig. 5C).
CCL2 is involved in TD-induced exacerbation of EAE
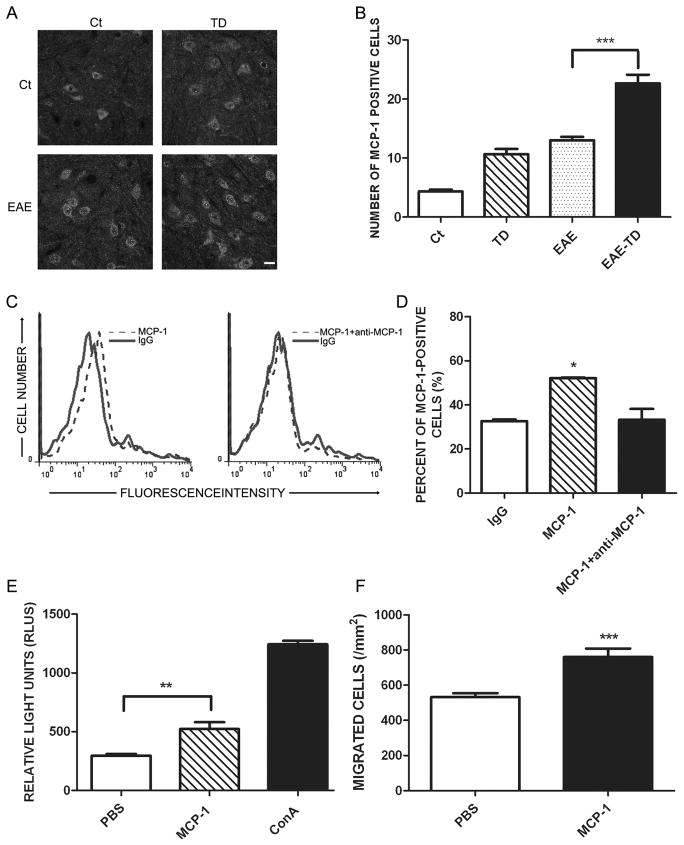
We have demonstrated that the expression of C-C Chemokine Ligand 2 (CCL2) was selectively induced by TD in the brain (52). We hypothesized that CCL2 may mediate TD-induced exacerbation of EAE. To test this hypothesis, we examined the expression of CCL2 in the spinal cord with IHC. Both MOG and TD treatment increased the number of cells expressing CCL2, but MOG plus TD further increased CCL2+ cells in comparison to MOG or TD-treated group alone (Figs 6A and B). To confirm that CCL2 can regulate T lymphocytes infiltration, we first determined whether CCL2 can directly bind to T cells. We treated lymph node T cells with IgG, CCL2 or CCL2 plus anti-CCL2 antibody for 60 min, and then determined the amount of CCL2 bound to T cells by flow cytometry. Anti-CCL2 antibody blocked the binding of CCL2 to T cells, indicating that CCL2 specifically bound to T cells (Figs 6C and D). More importantly, CCL2 increased the proliferation and migration of T lymphocytes in vitro (Figs. 6E and F).
Figure 6. Effect of TD on the expression of CCL2 in spinal cord of EAE mice.
A: EAE was induced in C57BL/6 mice by injection of MOG35-55 with or without TD. The expression of CCL2 in the spinal cord was determined by IHC on day 20 after MOG35-55 injection. Bar = 20 μm. B: The number of CCL2-positive cells was quantified as described under the Materials and Methods. n = 5 for each group. C: Lymph node cells were isolated and incubated with CCL2 with/without anti-CCL2 antibody. The binding of CCL2 to T lymphocytes was evaluated by FACS. D: The binding of CCL2 to T lymphocytes was quantified by FlowJo software. E: Peripheral T lymphocytes were isolated from lymph nodes and treated with CCL2 (20 μg/ml) for 72 hours. Cell proliferation was determined using ViaLight Plus Kit. ConA was used as positive control for the stimulation of T cell proliferation. F: T cells were treated with CCL2 (5μg/ml) for 4 hours. CCL2-induced T-cell migration was measured by a Transwell Migration assay. Data were presented as mean ± SD; *p<0.05, **p<0.01, ***p<0.001, compared with control group. The experiment was replicated four times.
To confirm the involvement of CCL2, we treated mice with bindarit, an inhibitor of CCL2 synthesis. Bindarit inhibited the expression of CCL2 in the spinal cord of (Supplementary Fig. 2C and D). Bindarit had therapeutic effects on mice of EAE plus TD, lowering the clinical scores and alleviating the loss of body weight (Supplementary Fig. 2A and B). Mice suffered EAE plus TD showed a rapid progression of EAE, reaching a maximum mean clinical score of 2.5. Bindarit-treated mice with EAE plus TD had a slower progression and a maximum mean clinical score of 1.5.
TD increases the expression CCR2 in T lymphocytes
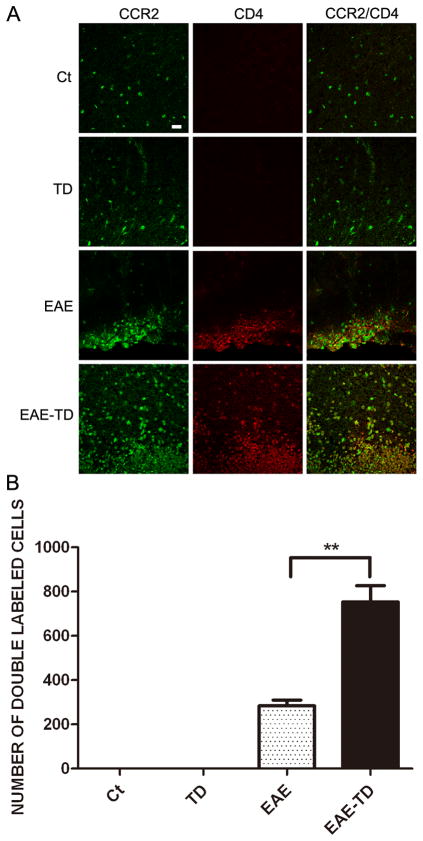
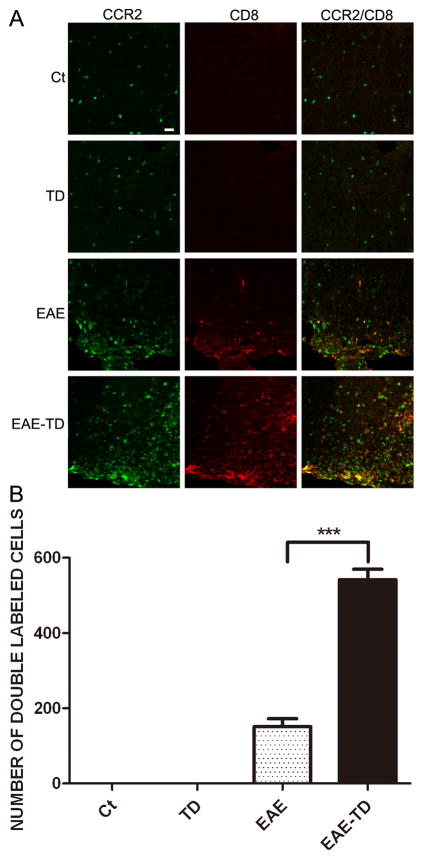
CCR2 is a chemokine receptor that responds predominantly to CCL2. We examined the expression of CCR2 in lymphocytes isolated from lymph nodes by flow cytometry. The expression of CCR2 on lymphocytes was confirmed by isolated T cells from LN (Supplementary Figure 3). Approximately 30% of lymphocytes expressed CCR2 and CCR2 was observed in both CD4+ and CD8+ cells (data not shown). We next examined the effect of MOG and TD on CCR2 expression in CD4+ and CD8+ T lymphocytes in the spinal cord. MOG significantly increased the number of CCR2-positive CD4+ or CD8+ T lymphocytes in the spinal cord and TD potentiated the effect of MOG (Figs 7 and 8).
Figure 7. Effect of TD on CCR2-positive CD4 cells in spinal cord of EAE mice.
A: EAE was induced in C57BL/6 mice by injection of MOG35-55 with or without TD. The expression of CCR2 on CD4+ T lymphocytes on the lumbar spinal cord of EAE mice was examined with double immunofluorescent staining using antibodies directed against CCR2 or CD4. Bars = 20 μm. B: CCR2+ and CD4+ cells were quantified as described under the Materials and Methods. Data were presented as mean ± SD; **p<0.01, compared with EAE group. n = 5 for each group.
Figure 8. Effect of TD on CCR2-positive CD8 cells in spinal cord of EAE mice.
The notations are as Figure 8. Data were presented as mean ± SD; ***p<0.001, compared with EAE group. n = 5 for each group.
Discussion
Multiple sclerosis (MS) is considered a T lymphocyte-mediated CNS autoimmune disease that results from the interplay between environmental factors and a susceptible genetic background (15, 53–54). Epidemiological studies show that environmental influences, such as diet, play an important role in the development of MS (5). Vitamin deficiency has been implicated in the pathogenesis of MS. For example, there is a correlation between vitamin D deficiency and MS (7–8). Vitamin D supplements have been considered a potential therapeutic strategy for multiple sclerosis patients (9–10). MS and vitamin B12 deficiency share some common inflammatory, neurodegenerative and pathophysiological characteristics and a decreased level of vitamin B12 has been demonstrated in MS patients (55–56). The supplementation with vitamin B12 improved clinical outcomes in MS patients (13). In an animal model of MS, both nicotinic acid and fumaric acid esters have been shown to improve neurological function, and this effect was accompanied by a significant reduction in inflammatory infiltrates (57). Fumaric esters have recently been evaluated in Phase III trials for MS (58–60) and nicotinic acid has been shown to have neuroprotective and anti-inflammatory effects during acute ischemic stroke (61).
Thiamine (vitamin B1) deficiency (TD) produces a mild impairment of oxidative metabolism and TD in animals has been used to model the diminished metabolism and reduced activity of the thiamine-dependent mitochondrial enzymes that occur in the brain of aging-related neurodegenerative disorders (40, 62). In this study, we show that TD exacerbates experimental autoimmune encephalomyelitis (EAE) which is a widely used model to study the pathogenesis of MS (63). Two models of EAE were used in our study; an active immunization model was achieved by subcutaneous injection of myelin antigen (MOG) and an adoptive transfer model which transferred purified MOG-specific T cells obtained from EAE mice into naive mice. In both active and adoptive transfer EAE models, TD drastically increases the progression of EAE which is indicated by a significant worse clinical score and a more severe loss of body weight (Figs 1 and 5). The mice of TD alone showed neurological alterations, such as clumsy gait or poor righting ability after 25 days.
The clinical severity of EAE is directly associated with T cell activation (64). We show that TD increases the number of infiltrated cells and active microglia in the spinal cord. We further demonstrate that TD increases CD4+ and CD8+ T lymphocytes in the spinal cord. These results indicated that TD exacerbates the development of EAE by inducing T activation. Neuro-antigen-specific CD4+ T cells can initiate and sustain neuroinflammation and pathology in EAE (15). Depending on the major cytokines produced, CD4+ T cells have been classified into distinct subsets namely Th1, Th2 and Th17. Recently, many studies have been undertaken to identify the T cell subsets that are involved in tissue-specific autoimmune diseases. Interferon-γ-expressing Th1 cells were initially considered to be the effector CD4+ T cell subset that induced EAE. Later, Th17 cells were also reported to cause more severe EAE; animal models that were deficient in IL-17A or the IL-17RA receptor were more resistant to EAE (65–67). We demonstrate that TD significantly increases the number of CD4+ cells and specifically the number of Th1 and Th17 cells but not Th2 cells (Figs 3 and 5). These results are consistent with the role of these T cells in EAE. TD also increases the number of CD8+T cells (Fig. 3). The role of CD8+T cells in EAE is unclear, although some studies indicate a potential pathogenic action for CNS-targeted CD8+T cells (68–69).
A critical event in the pathogenesis of EAE is the entry of both antigen-specific T lymphocytes and antigen-nonspecific mononuclear cells into the CNS. Chemokines are a key mediator that regulate the transmigration of T cells and monocytes across the blood–brain barrier (BBB) (70). CCL2, one of the first chemokines to be characterized, regulates the activity of monocytes, dendritic cells, and NK cells and plays an important role in innate immunity (35); it is also associated with pathological inflammation (71). CCR2 is a C-C chemokine receptor that responds predominantly to CCL2. In murine EAE, the expression of CCL2 mRNA in the brain and spinal cord was upregulated and may mediate the onset of EAE (31–32). We have previously demonstrated that neuronal CCL2 plays an important role in TD-induced microglia recruitment/activation and neurodegeneration in the brain (52). We show here that TD increases the expression of CCL2 and CCR2 in spinal cord of EAE in mice (Figs 6–8). In humans, CCR2 is expressed in monocytes and CD4+ T cells in the circulation (72). Our results show that CCR2 is expressed in both CD4+ and CD8+ T cells in C57BL6 mice. Our data indicate that CCL2 can directly activate T cells as it binds to T cells and promotes the proliferation and migration of T cells in vitro (Fig. 6). Bindarit, CCL2 synthesis inhibitor, suppresses the expression of CCL2 in the spinal cord and has therapeutic effects on EAE plus TD mice (Supplementary Fig. 2). These indicate that the involvement of CCL2.
Taken together, our results suggest that TD upregulates CCL2 and CCR2, and through its interaction with CCR2, CCL2 enhances the proliferation and recruitment of encephalitogenic T cells into the spinal cord which causes more pathological inflammation. The mechanisms underlying TD-induced CCL2/CCR2 upregulation are currently unknown. TD causes oxidative stress. Oxidative stress is detrimental to neurons and may promote inflammatory activities and the production/secretion of CCL2. Although a direct regulation cannot be ruled out, it is more likely TD modulates CCL2 expression through an indirect mechanism, such as oxidative stress. Regardless of the mechanisms, CCL2/CCR2 signaling plays an important role in TD-potentiated EAE.
Supplementary Material
Acknowledgments
We would like to thank Jacqueline A. Frank for reading this manuscript. This research was supported by the Ministry of Science and Technology of China (2010CB912000), National Natural Science Foundation of China (30801031 to ZJ, 31271142), China Postdoctoral Science Foundation (to ZJ), the Program of Clinical Research Center, Institute for Nutritional Sciences and Xuhui Central Hospital (CRC20100010). Dr. J. Luo was supported by grants from NIH/NIAAA (AA015407 and AA019693).
Abbreviations
- MS
Multiple Sclerosis
- EAE
Experimental Autoimmune Encephalomyelitis
- CNS
Central Nervous System
- TD
Thiamine Deficiency
- MOG
Myelin Oligodendroglial Glycoprotein
- CCL2
C-C Chemokine Ligand 2
- DMSO
Dimethyl sulfoxide
- CCR2
C-C Chemokine Receptor 2
- WKS
Wernicke–Korsakoff’s syndrome
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 2.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504–512. [PubMed] [Google Scholar]
- 3.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 4.Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, Edkins S, Gray E, Booth DR, Potter SC, Goris A, Band G, Oturai AB, Strange A, Saarela J, Bellenguez C, Fontaine B, Gillman M, Hemmer B, Gwilliam R, Zipp F, Jayakumar A, Martin R, Leslie S, Hawkins S, Giannoulatou E, D’Alfonso S, Blackburn H, Martinelli Boneschi F, Liddle J, Harbo HF, Perez ML, Spurkland A, Waller MJ, Mycko MP, Ricketts M, Comabella M, Hammond N, Kockum I, McCann OT, Ban M, Whittaker P, Kemppinen A, Weston P, Hawkins C, Widaa S, Zajicek J, Dronov S, Robertson N, Bumpstead SJ, Barcellos LF, Ravindrarajah R, Abraham R, Alfredsson L, Ardlie K, Aubin C, Baker A, Baker K, Baranzini SE, Bergamaschi L, Bergamaschi R, Bernstein A, Berthele A, Boggild M, Bradfield JP, Brassat D, Broadley SA, Buck D, Butzkueven H, Capra R, Carroll WM, Cavalla P, Celius EG, Cepok S, Chiavacci R, Clerget-Darpoux F, Clysters K, Comi G, Cossburn M, Cournu-Rebeix I, Cox MB, Cozen W, Cree BA, Cross AH, Cusi D, Daly MJ, Davis E, de Bakker PI, Debouverie M, D’Hooghe B, Dixon MK, Dobosi R, Dubois B, Ellinghaus D, Elovaara I, Esposito F, Fontenille C, Foote S, Franke A, Galimberti D, Ghezzi A, Glessner J, Gomez R, Gout O, Graham C, Grant SF, Guerini FR, Hakonarson H, Hall P, Hamsten A, Hartung HP, Heard RN, Heath S, Hobart J, Hoshi M, Infante-Duarte C, Ingram G, Ingram W, Islam T, Jagodic M, Kabesch M, Kermode AG, Kilpatrick TJ, Kim C, Klopp N, Koivisto K, Larsson M, Lathrop M, Lechner-Scott JS, Leone MA, Leppa V, Liljedahl U, Bomfim IL, Lincoln RR, Link J, Liu J, Lorentzen AR, Lupoli S, Macciardi F, Mack T, Marriott M, Martinelli V, Mason D, McCauley JL, Mentch F, Mero IL, Mihalova T, Montalban X, Mottershead J, Myhr KM, Naldi P, Ollier W, Page A, Palotie A, Pelletier J, Piccio L, Pickersgill T, Piehl F, Pobywajlo S, Quach HL, Ramsay PP, Reunanen M, Reynolds R, Rioux JD, Rodegher M, Roesner S, Rubio JP, Ruckert IM, Salvetti M, Salvi E, Santaniello A, Schaefer CA, Schreiber S, Schulze C, Scott RJ, Sellebjerg F, Selmaj KW, Sexton D, Shen L, Simms-Acuna B, Skidmore S, Sleiman PM, Smestad C, Sorensen PS, Sondergaard HB, Stankovich J, Strange RC, Sulonen AM, Sundqvist E, Syvanen AC, Taddeo F, Taylor B, Blackwell JM, Tienari P, Bramon E, Tourbah A, Brown MA, Tronczynska E, Casas JP, Tubridy N, Corvin A, Vickery J, Jankowski J, Villoslada P, Markus HS, Wang K, Mathew CG, Wason J, Palmer CN, Wichmann HE, Plomin R, Willoughby E, Rautanen A, Winkelmann J, Wittig M, Trembath RC, Yaouanq J, Viswanathan AC, Zhang H, Wood NW, Zuvich R, Deloukas P, Langford C, Duncanson A, Oksenberg JR, Pericak-Vance MA, Haines JL, Olsson T, Hillert J, Ivinson AJ, De Jager PL, Peltonen L, Stewart GJ, Hafler DA, Hauser SL, McVean G, Donnelly P, Compston A. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrie RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol. 2004;3:709–718. doi: 10.1016/S1474-4422(04)00933-0. [DOI] [PubMed] [Google Scholar]
- 6.Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012;8:602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agranoff BW, Goldberg D. Diet and the geographical distribution of multiple sclerosis. Lancet. 1974;2:1061–1066. doi: 10.1016/s0140-6736(74)92163-1. [DOI] [PubMed] [Google Scholar]
- 8.Simpson S, Jr, Blizzard L, Otahal P, Van der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82:1132–1141. doi: 10.1136/jnnp.2011.240432. [DOI] [PubMed] [Google Scholar]
- 9.Niino M. Vitamin D and its immunoregulatory role in multiple sclerosis. Drugs Today (Barc) 2010;46:279–290. doi: 10.1358/dot.2010.46.4.1476498. [DOI] [PubMed] [Google Scholar]
- 10.Kriegel MA, Manson JE, Costenbader KH. Does vitamin D affect risk of developing autoimmune disease?: a systematic review. Semin Arthritis Rheum. 2011;40:512–531. e518. doi: 10.1016/j.semarthrit.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, He ZY, Liu HN. Meta-analysis of the relationship between homocysteine, vitamin B(1)(2), folate, and multiple sclerosis. J Clin Neurosci. 2011;18:933–938. doi: 10.1016/j.jocn.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Najafi MR, Shaygannajad V, Mirpourian M, Gholamrezaei A. Vitamin B(12) Deficiency and Multiple Sclerosis; Is there Any Association? Int J Prev Med. 2012;3:286–289. [PMC free article] [PubMed] [Google Scholar]
- 13.Verma R, Praharaj HN. Unusual association of Arnold-Chiari malformation and vitamin B12 deficiency. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-03-2012-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moghaddasi M, Mamarabadi M, Mohebi N, Razjouyan H, Aghaei M. Homocysteine, vitamin B12 and folate levels in Iranian patients with Multiple Sclerosis: A case control study. Clin Neurol Neurosurg. 2013 doi: 10.1016/j.clineuro.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Simmons SB, Pierson ER, Lee SY, Goverman JM. Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol. 2013 doi: 10.1016/j.it.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bramow S, Frischer JM, Lassmann H, Koch-Henriksen N, Lucchinetti CF, Sorensen PS, Laursen H. Demyelination versus remyelination in progressive multiple sclerosis. Brain. 2010;133:2983–2998. doi: 10.1093/brain/awq250. [DOI] [PubMed] [Google Scholar]
- 17.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 18.Lassmann H, Wisniewski HM. Chronic relapsing experimental allergic encephalomyelitis: clinicopathological comparison with multiple sclerosis. Arch Neurol. 1979;36:490–497. doi: 10.1001/archneur.1979.00500440060011. [DOI] [PubMed] [Google Scholar]
- 19.Bernard CC, Leydon J, Mackay IR. T cell necessity in the pathogenesis of experimental autoimmune encephalomyelitis in mice. Eur J Immunol. 1976;6:655–660. doi: 10.1002/eji.1830060912. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda T, Tsumita T, Nagai Y, Mitsuzawa E, Ohtani S. Experimental allergic encephalomyelitis (EAE) in mice. I. Induction of EAE with mouse spinal cord homogenate and myelin basic protein. Jpn J Exp Med. 1975;45:423–427. [PubMed] [Google Scholar]
- 21.Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol. 1995;25:1951–1959. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- 22.Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;2:175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 23.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 24.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 25.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allavena P, Bianchi G, Zhou D, van Damme J, Jilek P, Sozzani S, Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur J Immunol. 1994;24:3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- 28.Kuna P, Reddigari SR, Rucinski D, Oppenheim JJ, Kaplan AP. Monocyte chemotactic and activating factor is a potent histamine-releasing factor for human basophils. J Exp Med. 1992;175:489–493. doi: 10.1084/jem.175.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 32.Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM. Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am J Pathol. 1997;150:617–630. [PMC free article] [PubMed] [Google Scholar]
- 33.Juedes AE, Hjelmstrom P, Bergman CM, Neild AL, Ruddle NH. Kinetics and cellular origin of cytokines in the central nervous system: insight into mechanisms of myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J Immunol. 2000;164:419–426. doi: 10.4049/jimmunol.164.1.419. [DOI] [PubMed] [Google Scholar]
- 34.Sun D, Tani M, Newman TA, Krivacic K, Phillips M, Chernosky A, Gill P, Wei T, Griswold KJ, Ransohoff RM, Weller RO. Role of chemokines, neuronal projections, and the blood-brain barrier in the enhancement of cerebral EAE following focal brain damage. J Neuropathol Exp Neurol. 2000;59:1031–1043. doi: 10.1093/jnen/59.12.1031. [DOI] [PubMed] [Google Scholar]
- 35.Sagar D, Lamontagne A, Foss CA, Khan ZK, Pomper MG, Jain P. Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood-brain barrier through paracellular transmigration and ERK activation. J Neuroinflammation. 2012;9:245. doi: 10.1186/1742-2094-9-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poliani PL, Brok H, Furlan R, Ruffini F, Bergami A, Desina G, Marconi PC, Rovaris M, Uccelli A, Glorioso JC, Penna G, Adorini L, Comi G, t Hart B, Martino G. Delivery to the central nervous system of a nonreplicative herpes simplex type 1 vector engineered with the interleukin 4 gene protects rhesus monkeys from hyperacute autoimmune encephalomyelitis. Hum Gene Ther. 2001;12:905–920. doi: 10.1089/104303401750195872. [DOI] [PubMed] [Google Scholar]
- 37.Karpus WJ, Kennedy KJ. MIP-1alpha and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J Leukoc Biol. 1997;62:681–687. [PubMed] [Google Scholar]
- 38.Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hakim AM, Pappius HM. The effect of thiamine deficiency on local cerebral glucose utilization. Ann Neurol. 1981;9:334–339. doi: 10.1002/ana.410090404. [DOI] [PubMed] [Google Scholar]
- 40.Ke ZJ, DeGiorgio LA, Volpe BT, Gibson GE. Reversal of thiamine deficiency-induced neurodegeneration. J Neuropathol Exp Neurol. 2003;62:195–207. doi: 10.1093/jnen/62.2.195. [DOI] [PubMed] [Google Scholar]
- 41.Ke ZJ, Gibson GE. Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochem Int. 2004;45:361–369. doi: 10.1016/j.neuint.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Zhang G, Ding H, Chen H, Ye X, Li H, Lin X, Ke Z. Thiamine nutritional status and depressive symptoms are inversely associated among older Chinese adults. J Nutr. 2013;143:53–58. doi: 10.3945/jn.112.167007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L, Huang D, Matsui M, He TT, Hu T, Demartino J, Lu B, Gerard C, Ransohoff RM. Severe disease, unaltered leukocyte migration, and reduced IFN-gamma production in CXCR3-/- mice with experimental autoimmune encephalomyelitis. J Immunol. 2006;176:4399–4409. doi: 10.4049/jimmunol.176.7.4399. [DOI] [PubMed] [Google Scholar]
- 44.Marusic S, Leach MW, Pelker JW, Azoitei ML, Uozumi N, Cui J, Shen MW, DeClercq CM, Miyashiro JS, Carito BA, Thakker P, Simmons DL, Leonard JP, Shimizu T, Clark JD. Cytosolic phospholipase A2 alpha-deficient mice are resistant to experimental autoimmune encephalomyelitis. J Exp Med. 2005;202:841–851. doi: 10.1084/jem.20050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Wang B, Fan Z, Shi X, Ke ZJ, Luo J. Thiamine deficiency induces endoplasmic reticulum stress in neurons. Neuroscience. 2007;144:1045–1056. doi: 10.1016/j.neuroscience.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q, Yang G, Li W, Fan Z, Sun A, Luo J, Ke ZJ. Thiamine deficiency increases beta-secretase activity and accumulation of beta-amyloid peptides. Neurobiol Aging. 2011;32:42–53. doi: 10.1016/j.neurobiolaging.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Cioli V, Ciarniello MG, Guglielmotti A, Luparini MR, Durando L, Martinelli B, Catanese B, Fava L, Silvestrini B. A new protein antidenaturant agent, bindarit, reduces secondary phase of adjuvant arthritis in rats. J Rheumatol. 1992;19:1735–1742. [PubMed] [Google Scholar]
- 48.Ge S, Shrestha B, Paul D, Keating C, Cone R, Guglielmotti A, Pachter JS. The CCL2 synthesis inhibitor bindarit targets cells of the neurovascular unit, and suppresses experimental autoimmune encephalomyelitis. J Neuroinflammation. 2012;9:171. doi: 10.1186/1742-2094-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia YF, Liu LP, Zhong CP, Geng JG. NF-kappaB activation for constitutive expression of VCAM-1 and ICAM-1 on B lymphocytes and plasma cells. Biochem Biophys Res Commun. 2001;289:851–856. doi: 10.1006/bbrc.2001.6067. [DOI] [PubMed] [Google Scholar]
- 50.Liu LP, Xia YF, Yang L, DiDonato JA, DiCorleto PE, Zhong CP, Geng JG. B lymphocytes and plasma cells express functional E-selectin by constitutive activation of NF-kappaB. Biochem Biophys Res Commun. 2001;286:281–291. doi: 10.1006/bbrc.2001.5344. [DOI] [PubMed] [Google Scholar]
- 51.Bruce B, Khanna G, Ren L, Landberg G, Jirstrom K, Powell C, Borczuk A, Keller ET, Wojno KJ, Meltzer P, Baird K, McClatchey A, Bretscher A, Hewitt SM, Khanna C. Expression of the cytoskeleton linker protein ezrin in human cancers. Clin Exp Metastasis. 2007;24:69–78. doi: 10.1007/s10585-006-9050-x. [DOI] [PubMed] [Google Scholar]
- 52.Yang G, Meng Y, Li W, Yong Y, Fan Z, Ding H, Wei Y, Luo J, Ke ZJ. Neuronal MCP-1 mediates microglia recruitment and neurodegeneration induced by the mild impairment of oxidative metabolism. Brain Pathol. 2011;21:279–297. doi: 10.1111/j.1750-3639.2010.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Methner A, Zipp F. Multiple sclerosis in 2012: Novel therapeutic options and drug targets in MS. Nat Rev Neurol. 2013;9:72–73. doi: 10.1038/nrneurol.2012.277. [DOI] [PubMed] [Google Scholar]
- 54.Scalfari A, Knappertz V, Cutter G, Goodin DS, Ashton R, Ebers GC. Mortality in patients with multiple sclerosis. Neurology. 2013;81:184–192. doi: 10.1212/WNL.0b013e31829a3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller A, Korem M, Almog R, Galboiz Y. Vitamin B12, demyelination, remyelination and repair in multiple sclerosis. J Neurol Sci. 2005;233:93–97. doi: 10.1016/j.jns.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5:949–960. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- 57.Cordiglieri C, Odoardi F, Zhang B, Nebel M, Kawakami N, Klinkert WE, Lodygin D, Luhder F, Breunig E, Schild D, Ulaganathan VK, Dornmair K, Dammermann W, Potter BV, Guse AH, Flugel A. Nicotinic acid adenine dinucleotide phosphate-mediated calcium signalling in effector T cells regulates autoimmunity of the central nervous system. Brain. 2010;133:1930–1943. doi: 10.1093/brain/awq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Limmroth V. Multiple sclerosis: oral BG12 for treatment of relapsing-remitting MS. Nat Rev Neurol. 2013;9:8–10. doi: 10.1038/nrneurol.2012.231. [DOI] [PubMed] [Google Scholar]
- 59.Oh J, O’Connor PW. Safety, tolerability, and efficacy of oral therapies for relapsing-remitting multiple sclerosis. CNS Drugs. 2013;27:591–609. doi: 10.1007/s40263-013-0080-z. [DOI] [PubMed] [Google Scholar]
- 60.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 61.Shehadah A, Chen J, Zacharek A, Cui Y, Ion M, Roberts C, Kapke A, Chopp M. Niaspan treatment induces neuroprotection after stroke. Neurobiol Dis. 2010;40:277–283. doi: 10.1016/j.nbd.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE. Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J Neuropathol Exp Neurol. 1999;58:946–958. doi: 10.1097/00005072-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Ji Z, Ke ZJ, Geng JG. SAP suppresses the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. Immunol Cell Biol. 2011 doi: 10.1038/icb.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reiseter BS, Miller GT, Happ MP, Kasaian MT. Treatment of murine experimental autoimmune encephalomyelitis with a myelin basic protein peptide analog alters the cellular composition of leukocytes infiltrating the cerebrospinal fluid. J Neuroimmunol. 1998;91:156–170. doi: 10.1016/s0165-5728(98)00171-4. [DOI] [PubMed] [Google Scholar]
- 65.Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e15531. doi: 10.1371/journal.pone.0015531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schulze-Topphoff U, Casazza S, Varrin-Doyer M, Pekarek K, Sobel RA, Hauser SL, Oksenberg JR, Zamvil SS, Baranzini SE. Tob1 plays a critical role in the activation of encephalitogenic T cells in CNS autoimmunity. J Exp Med. 2013 doi: 10.1084/jem.20121611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huber M, Heink S, Pagenstecher A, Reinhard K, Ritter J, Visekruna A, Guralnik A, Bollig N, Jeltsch K, Heinemann C, Wittmann E, Buch T, Prazeres da Costa O, Brustle A, Brenner D, Mak TW, Mittrucker HW, Tackenberg B, Kamradt T, Lohoff M. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest. 2013;123:247–260. doi: 10.1172/JCI63681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ji Q, Castelli L, Goverman JM. MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8(+) T cells. Nat Immunol. 2013;14:254–261. doi: 10.1038/ni.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ortega SB, V, Kashi P, Tyler AF, Cunnusamy K, Mendoza JP, Karandikar NJ. The disease-ameliorating function of autoregulatory CD8 T cells is mediated by targeting of encephalitogenic CD4 T cells in experimental autoimmune encephalomyelitis. J Immunol. 2013;191:117–126. doi: 10.4049/jimmunol.1300452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 71.Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 72.Kivisakk P, Trebst C, Liu Z, Tucky BH, Sorensen TL, Rudick RA, Mack M, Ransohoff RM. T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking. Clin Exp Immunol. 2002;129:510–518. doi: 10.1046/j.1365-2249.2002.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.