Abstract
Rationale
Animal models suggest that neuroactive steroids contribute to alcohol’s acute effects. We previously reported that a common non-synonymous polymorphism, AKR1C3*2 in the gene encoding the enzyme 3α-HSD2/17β-HSD5 and a synonymous SNP, rs248793, in SRD5A1, which encodes 5α-reductase, were associated with alcohol dependence (AD).
Objectives
To investigate whether these polymorphisms moderate subjective effects of alcohol in humans and whether AKR1C3*2 affects neuroactive steroid synthesis.
Methods
65 Caucasian men (34 lighter and 31 heavier drinkers; mean age 26.2 y) participated in a double-blind laboratory study where they consumed drinks containing no ethanol or 0.8 g/kg of ethanol. Breath alcohol, heart rate (HR), and self-reported alcohol effects were measured at 40-min intervals and genotype was examined as a moderator of alcohol’s effects. Levels of the neuroactive steroid 5α-androstane-3α,17β-diol and its precursors, 3α,5α-androsterone and dihydrotestosterone, were measured at study entry using GC/MS.
Results
Initially, carriers of the AD-protective AK1C3*2 G-allele had higher levels of 5α-androstane-3α,17β-diol relative to the precursor 3α,5α-androsterone than C-allele homozygotes. AKR1C3*2 G-allele carriers exhibited greater increases in heart rate and stimulant and sedative effects of alcohol than C-allele homozygotes. The genotype effects on sedation were observed only in heavier drinkers. The only effect of the SRD5A1 SNP was to moderate HR. There were no interactive effects of the two SNPs.
Conclusions
The observed effects of variation in a gene encoding a neuroactive steroid biosynthetic enzyme on the rate of 17p–reduction of androsterone relative to androstanediol and on alcohol’s sedative effects may help to explain the association of AKR1C3*2 with AD.
Keywords: Psychiatric Genetics, 3α-HSD, 17β-HSD, Alcohol Dependence, Polymorphism
INTRODUCTION
Alcohol use disorders (AUD) are highly prevalent in the general population (Grant et al. 2004). Their etiology is complex, with a variety of environmental and hereditary factors contributing to their development and chronic course (Devor & Cloninger, 1989). Subjective responses to sedative and intoxicating effects of alcohol are proximal measures of the risk of AUD (Bauer & Hesselbrock, 1993; Schuckit, 1984; Schuckit et al., 1994; Schuckit et al., 2000). A low level of sedation/impairment in response to alcohol has been associated with a greater risk of AUD in longitudinal follow-up studies (Schuckit and Smith, 1996; Schuckit et al, 2004; King et al, 2013). Individuals with a low level of sedation/impairment may tolerate larger amounts of alcohol and may consume larger amounts to achieve the desired stimulant or pleasurable effects than those who experience these adverse effects of alcohol. This distinction is conceptualized in the differentiator model proposed by Newlin and Thompson (1990) and further developed by other groups (King et al, 2011; 2013; Ray et al, 2009). The model also differentiates risk groups based on their response to the pleasurable vs. sedative effects of alcohol. Factors that are thought to mediate individual differences in levels of response to alcohol include differences in cortisol, prolactin, and ACTH levels following alcohol consumption (Schuckit et al., 1987a, 1987b; Schuckit, et al., 1988); variation in alcohol metabolizing genes (Duranceaux et al. 2006); and components of the serotonin, GABAA, glutamate, and opioid signaling systems (Ray & Hutchison, 2004, Hu et al., 2005; Joslyn et al., 2010).
Another group of candidate mediators of the physiological response to alcohol are neuroactive steroids, which are endogenous, highly potent allosteric modulators of GABAA receptor function (Paul & Purdy, 1992). Neuroactive steroids have been shown to be involved in the acute effects of alcohol, as well as alcohol tolerance and dependence (Morrow et al., 2001; Milivojevic et al., 2011). In animal models, alcohol increases neuroactive steroids in the periphery and in the brain (Barbaccia et al., 1999; Morrow et al., 1999; VanDoren et al., 2000; Sanna et al., 2004; Paris and Frye, 2009). Neuroactive steroids have also been reported to influence alcohol intake (Martin-Garcia et al., 2007; Ford et al., 2008). In humans, plasma neuroactive steroids are increased following severe intoxication (Torres & Ortega, 2003, 2004), but not moderate intoxication (Nyberg et al. 2005; Holdstock et al. 2006; Pierucci-Lagha et al. 2006). Based on this literature, it appears that alcohol indirectly modulates GABAA receptor function by increasing neuroactive steroid levels.
Neuroactive steroid synthesis can be blocked with finasteride, an inhibitor of 5α-reductase (5AR), a key enzyme in the production of neuroactive steroids. In rodents, finasteride blocks both type I and type II 5AR isozymes. At clinical dosages in humans, finasteride blocks only the type II 5AR, the isoenzyme of 5AR that is most abundant in prostate and skin. Dutasteride, a 5AR inhibitor approved by the Food and Drug Administration to treat prostatic hyperplasia, inhibits both 5AR isoenzymes in humans at clinical dosages (Clark et al., 2004). Our group recently reported that dutasteride reduced sedative effects of alcohol in a clinical laboratory setting (Covault et al., 2014).
The enzyme 3α-hydroxysteroid dehydrogenase (3α-HSD) is also involved in neuroactive steroid biosynthesis. Genetic variation in a region of chromosome 10 containing the genes AKR1C1, AKR1C3 and AKR1C4, which encode 3 isoforms of 3α-HSD, was associated with variation in subjective alcohol response (Joslyn et al., 2010). More recently, we reported that both a non-synonymous single nucleotide polymorphism (SNP), rs12529 (C>G; His5Gln; AKR1C3*2) in exon 1 of AKR1C3, and a synonymous SNP, rs248793 (G>C; Arg30Arg), in exon 1 of SRD5A1, which encodes 5α-reductase, were associated with AD. We found that the minor allele of each gene (G and C, respectively) was protective (Milivojevic et al., 2011). Previously, the AKR1C3*2 minor G-allele was shown to protect against bladder cancer in a large case-control sample (n=2299) of Spanish subjects (Figueroa et al. 2008). To our knowledge there have been no other reports associating the SRD5A1 SNP with disease.
Here we use these two common genetic variants as tools in a pharmacogenetic study (i.e., to study moderation of alcohol’s effects by genotype) to further examine the involvement of neuroactive steroids in some of the acute effects of alcohol in humans. We hypothesized that the amino-acid coding AKR1C3*2 variant (His5Gln), which has been associated with reduced risk of both AD and bladder cancer, moderates the stimulant or sedative effects of alcohol. We examined this hypothesis using the self-reported Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993) in a group of male participants in a recently completed human laboratory study of the combined effects of alcohol and dutasteride (Covault et al., 2014). Further, we examined whether the synonymous SRD5A1 SNP moderates subjective alcohol effects alone or in combination with the AKR1C3*2 variant and whether the moderating effects of the AKR1C3*2 variant on alcohol responses differ between lighter and heavier drinkers. Finally, we examined whether AKR1C3*2 genotype predicted baseline synthesis of the GABAA receptor-modulating neuroactive steroid androstanediol from its precursor androsterone.
METHODS
Subjects
Because the allele frequency of the markers examined vary by race and have not been studied with respect to AD in non-Caucasians, we limited our examination of genotype effects on alcohol responses to the 65 Caucasian men who completed a 4-session alcohol/dutasteride study (Covault et al., 2014). Men were recruited by advertisement in the Greater Hartford, CT region, including at nearby colleges and universities. All subjects gave written informed consent to participate in the study as approved by the University of Connecticut Health Center Institutional Review Board and were paid for their participation. Following an initial telephone interview, potentially eligible participants were screened in person using the Timeline Follow-back Interview (Sobell & Sobell, 1992) to quantify alcohol use during the prior 90 days and the Structured Clinical Interview for DSM-IV (First et al., 1995) to identify the presence of Axis I psychiatric disorders and antisocial personality disorder. Additional screening evaluations included a medical history and physical examination with routine laboratory tests (liver and renal function tests, complete blood count, serum glucose, and urine drug screen).
Subjects were included in the study if they were 21–45 years of age, reported drinking 3 or more standard drinks (SD) on at least one occasion during the past month, had a body mass index of 18.5 to 32.5 kg/m2, and weighed up to 235 lb. Exclusion criteria included a lifetime diagnosis of alcohol or drug dependence; alcohol abuse during the preceding 2 years or current nicotine dependence; a current, untreated medical condition; or current use of benzodiazepines, other psychotropic medications or medications known to influence steroid hormone levels or metabolism or that modify the effects of alcohol. Women were excluded in view of the potential teratogenic effects of dutasteride.
Study Design
The study used a double-blind, within-subject design, in which each subject received either 0 or 4 mg of dutasteride 2–4 days prior to participating in an alcohol laboratory session where they received either 3 drinks containing an alcohol mask or a total dose of 0.8 gr/kg of ethanol. Vodka was used as the ethanol source, and was mixed with the participants’ choice of martini mixer. The order of drug (placebo or 4 mg dutasteride) and alcohol (placebo or 0.8 mg/kg) was urn randomized to provide a balanced assignment of the 24 possible administration sequences. The groups included lighter drinkers [i.e., those with non-hazardous drinking, defined as ≤14 drinks/week and ≤1 heavy drinking day per month (HDD; defined as > 4 standard drinks in a day)] and heavier drinkers (those exceeding the limits of lighter drinkers). Our primary focus in this report is on the two sessions that were preceded by 0 mg of dutasteride.
Genotyping
DNA was extracted from peripheral blood samples using a commercial kit (Gentra Puregene, Qiagen, Valencia, CA). The AKR1C3*2 SNP (rs12529) was genotyped using a closed-tube fluorescent TaqMan 5’-nuclease allelic discrimination assay using MGB-probes (Vic-ATTCCAAACAGCAGTGTG and Fam-ATTCCAAACACCAGTGTG) and primers (GCTAGTCAGACAAGTGACAGGGAAT and CATGAAGTGGCCATCATTTAGCT) designed using Primer Express v3.0 software [Applied Biosystems Inc. (ABI) Foster City, CA]. Fluorescence plate reads and genotype calls were made using a 7500 Sequence Detection System following PCR amplification for 40 cycles at 95°C for 15 sec followed by 60°C for 60 sec. The SRD5A1 synonymous exon 1 SNP rs248793 was genotyped using HinfI (New England BioLabs, Ipswich, MA) restriction digest of PCR amplicons generated using primers TCGCCTACCTGCAGTGCGCC and TCGGAGCCTGTGGCTGGGCA followed by gel electrophoresis with the major G-allele producing a 57+44 bp product and the C-allele a 101 bp product. All samples were examined in duplicate. A discrepant result occurred for a single sample and was resolved by additional genotyping. There was no evidence of deviation from Hardy-Weinberg expectations for either marker (p’s > 0.2). We previously reported that the AKR1C3*2 minor G-allele and the SRD5A1 rs248793 minor C-allele were associated with a lower risk of alcohol dependence in a case-control study of 1,083 Caucasians (Milivojevic et al., 2011).
Physiological & Subjective Effects
Breath alcohol concentration (BrAC) and heart rate were measured at 40-min intervals throughout the laboratory session. Subjective effects were measured using the Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993), which was administered on a computer using SPSS data Builder version 3.0. The BAES is a 14-item unipolar adjective rating scale used to measure both the stimulant (i.e., elated, energized, excited, stimulated, talkative, up, vigorous) and sedative (i.e., difficulty concentrating, down, heavy head, inactive, sedated, slow thoughts, sluggish) effects of alcohol. Before alcohol ingestion, subjects were asked to rate on a scale of 0 (not at all) to 10 (extremely) the extent to which they experienced “alcohol-like” feelings at baseline. After alcohol ingestion, subjects were asked to rate the extent to which drinking alcohol produced these feelings.
Neuroactive Steroid Extraction and Analysis
Plasma levels of 5α-androstan-3α,17β-diol (androstanediol), 3α,5α-androsterone (androsterone), and 5α-dihydrotestosterone (DHT) were measured using gas chromatography/mass spectrometry (GC/MS) with electron capture-negative chemical ionization, as previously described (Milivojevic & Covault, 2013).
Steroid extraction and separation
Steroids were extracted using slightly modified methods described in Vallee et al. (2000). In brief, 0.3 ml of plasma, in parallel with a 6-level dilution series of neuroactive steroids standards diluted in 5% charcoal-stripped BSA (Sigma Aldrich, St. Louis, MO) were combined with 4 µl of an internal standard solution containing D3-androstanediol (16 pg/µl) for androstanediol and C13-(2)-testosterone (80 pg/µl) for DHT and androsterone (Cambridge Isotope Laboratories, Andover, MA) to control for variation in sample-to-sample extraction and derivatization efficiencies. 500µl of 75% methanol/water (v/v) was added to each sample, and vortex mixed. Samples were diluted with de-ionized water to a final concentration of 5% methanol and then applied to 200-mg C18 solid-phase columns (Varian Corp. Palo Alto, CA; previously conditioned with 6 ml of 100% methanol followed by 6 ml of 5% methanol). The columns were washed with 5% methanol thrice (9 ml total) and steroids eluted with 2 ml of 100% methanol into silinized tubes and dried in a rotary evaporator for approximately 2 hr.
Derivatization
Dried steroids were derivatized according to the method described by Ostlund et al. (1996), with slight modifications. Briefly, samples were reacted with the following reagents: 50 µl toluene was added to each sample, vortex mixed, and allowed to sit at room temperature for 5–10 min to solubilize the dried sample; 25 µl of 40% pyridine (pyridine/toluene; 40/60 v/v) was then added, vortex mixed, and allowed to sit at room temperature for 3 min; and was followed by the addition of 25 µl of 10% pentafluorobenzoyl chloride in toluene (PFBC; Sigma–Aldrich, St. Louis, MO), vortex mixed, and incubated for 10–12 min at room temperature. In batches of 8, the samples were then mixed with 0.6 ml of de-ionized water and vortex mixed three times for 10 sec each. The steroids were then extracted by adding 1 ml of hexane and vortex mixing for 20 sec, which was repeated with another 1 ml of hexane (2 ml total). The pooled hexane extract samples were dried in a rotary evaporator for approximately 2 hr. All solvents were anhydrous of analytical grade (Sigma-Aldrich; St. Louis, MO). Steroid standards were purchased from Steraloids (Newport, RI).
Instrumentation
Derivatized samples were analyzed by GC followed by electron capture-negative chemical ionization MS according to the method described in Kim et al. (2000), with slight modifications. The analysis was performed using a Hewlett-Packard (HP) 5890 gas chromatograph coupled to an HP 5988B mass spectrometer using select ion monitoring mode for primary m/z peaks for compounds of interest to increase sensitivity. A thin-film capillary column (30 m x 0.25 mm, 0.1 µm film thickness; Restek, Bellefonte, PA) was used to resolve the hydroxyl-reacted pentafluorobenzoyl steroid derivatives. The injector and transfer-line temperatures were maintained at 300 and 310°C, respectively. One minute following splitless injection (2 ul of final 12 ul sample) the GC oven temperature was raised from 150 to 230°C at 30°C/min, 230 to 250°C at 1°C/min, and 250 to 320°C at 30°C/min. The temperatures of mass spectrometer ion source and quadrupole were set at 200 and 100°C, respectively with a methane gas flow to produce 1.5 Torr.
The ratio of the molecular ion peak for each steroid relative to the corresponding area of the density labeled internal standard was calculated for each sample, and using a standard curve generated with each run, the ratio was converted to steroid concentrations. Each sample was assayed in duplicate and a sample average was used for data analysis.
Data Analysis
Linear mixed-effects models were used to test the effect of AKR1C3*2 genotype on alcohol-induced change in BrAC, heart rate (HR), and the BAES stimulant and sedative subjective response subscales. Six time points were included in the analysis relative to the first alcohol drink: 40, 80, 120, 160, 210, and 240 with the pre-alcohol time 0 response used as a co-variate in the analyses. Time was included as a linear covariate for BrAC and BAES scores as the change in these measures was a unidirectional decline beginning with the first observation following alcohol, while for HR time was included as a categorical factor as the shape of change in HR over time was more complex. Secondary models were used to examine the main and interactive effects of SRD5A1 genotype or baseline drinking group with AKR1C3*2 genotype. General linear model analysis was used to examine the relationship of AKR1C3*2 genotype, androsterone, and DHT concentrations as predictors of androstanediol concentration. For the primary analysis of AKR1C3*2 moderation of alcohol’s effects on BrAC, HR, BAES stimulation and sedation scale scores, and androstanediol concentration, we controlled for multiple testing by using p<0.01 (0.05/5) as a threshold for statistical significance. All statistical analyses were conducted with SPSS v15.
RESULTS
Subjects
All subjects were Caucasian men (n=65) whose average age was 26.2 y (SD=6.7). During the 90 days prior to study enrollment, subjects reported an average of 2.3 drinking days (DD) per week, with 0.7 heavy drinking days (HDD) per week (i.e., > 4 standard drinks in a day), consuming an average of 8.1 standard drinks (SD) per week. Because subjective responses to alcohol have been reported to differ in heavier and lighter drinkers (King et al. 2002; Gilman et al. 2012), we used baseline drinking reports to characterize subjects as non-hazardous or lighter drinkers (LDs), n=34 (<15 SD/week and not more than 1 HDD per month during past 90 days consistent with safe drinking guidelines) and hazardous or heavier drinkers (HDs), n=31, who either drank heavily more than once per month or consumed ≥15 drinks per week. Drinking measures from the 90-day TLFB for the two baseline drinking groups are shown in Table 1. Twenty-five subjects (10 HDs) were AKR1C3*2 C-allele homozygotes, 26 (13 HDs) were heterozygotes, and 14 (8 HDs) were G-allele homozygotes. The minor G-allele frequency was 0.415. There was no distortion in the distribution of AKR1C3*2 genotypes comparing lighter and heavier drinkers [χ2(2)=1.2; p=0.56]. Sixteen subjects (8 HDs) were SRD5A1 G-allele homozygotes, 36 (16 HDs) were heterozygotes, and 13 (7 HDs) were C-allele homozygotes. The minor C-allele frequency was 0.477. There was no difference in the distribution of SRD5A1 genotypes comparing lighter and heavier drinkers [χ2(2)=0.4; p=0.83]. The distributions of subjects by AKR1C3*2 and SRD5A1 genotype or by AKR1C3*2 genotype and baseline drinking group are shown in Table 2.
Table 1.
90-day TLFB measures of alcohol use among 65 Caucasian men [mean (sd)].
| Group | N | DD/wk | HDD/wk | drinks/wk | drinks/HDD |
|---|---|---|---|---|---|
| Entire sample | 65 | 2.3 (1.6) | 0.7 (0.9) | 8.1 (7.1) | 6.3 (1.5) |
| Lighter drinkers | 34 | 2.2 (1.8) | 0.08 (0.09) | 4.4 (3.6) | 5.8 (0.9) |
| Heavier drinkers |
31 | 2.3 (1.3) | 1.4 (0.9) | 12.0 (7.9) | 6.6 (1.7) |
DD: drinking days; HDD: heavy drinking days.
Table 2.
Cross-tabulation of AKR1C3*2 and SRD5A1 genotypes or baseline drinker group.
| SRD5A1 genotype | Drinker | Group | |||||
|---|---|---|---|---|---|---|---|
| n= | GG 16 |
GC 36 |
CC 13 |
Lighter 34 |
Heavier 31 |
||
| AKR1C3*2 | |||||||
| CC homozygotes | n=25 | 5 | 16 | 4 | 15 | 10 | |
| GC heterozygotes | n=26 | 9 | 11 | 6 | 13 | 13 | |
| GG homozygotes | n=14 | 2 | 9 | 3 | 6 | 8 | |
AKR1C3*2 genotype and acute alcohol effects in the absence of dutasteride
Breath Alcohol Concentration
BrAC ascended steeply after the initiation of drinking, peaking at approximately 0.08 g/l at 40 min, the first post-drinking time point, after which it descended linearly to approximately 0.02 g/l at 300 min. There was no effect of genotype or the interaction of genotype with time on the BrAC curve (p’s>0.2).
Heart Rate and BAES self report alcohol effects
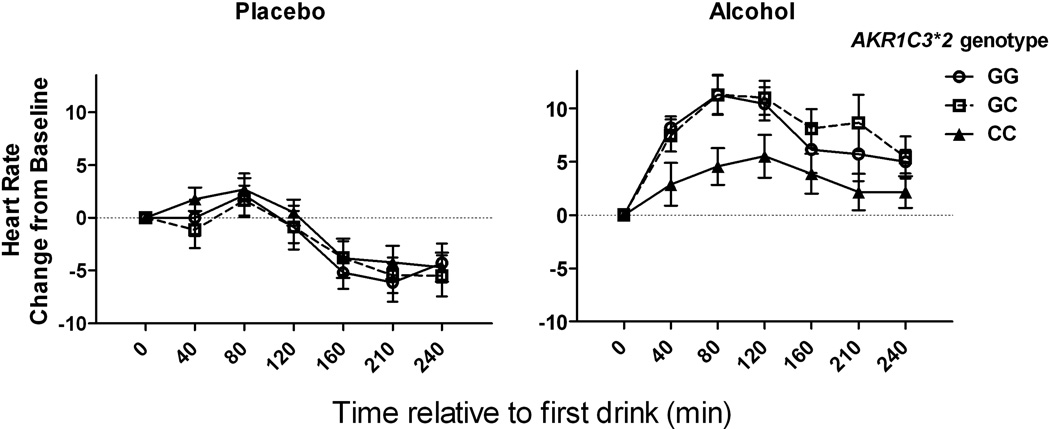
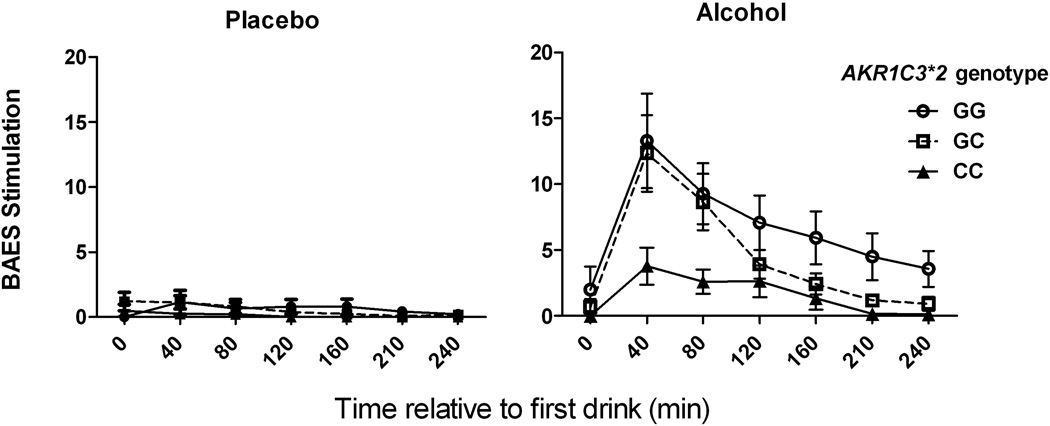
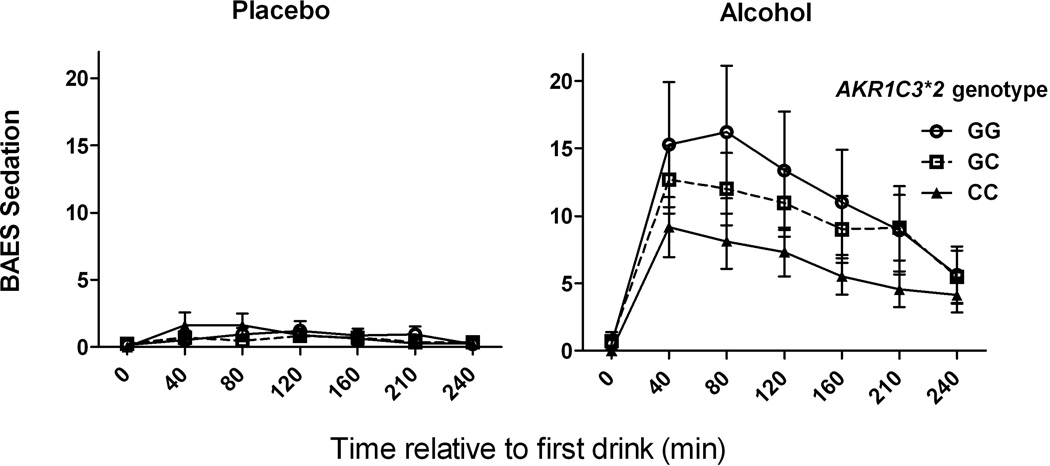
There was a significant interaction of AKR1C3*2 genotype and alcohol on the change in heart rate and BAES stimulation and BAES sedation scores (Table 3). Post-hoc contrasts identified significant alcohol by genotype interactions (<0.001) comparing homozygotes of the AD-associated C allele with subjects with either 1 or 2 minor alleles for all three outcomes, but not when contrasting heterozygotes and minor allele homozygotes. As illustrated in Figures 1, 2, 3, carriers of the minor G-allele had greater responses to alcohol on each of the measures than homozygotes for the AD-associated C allele.
Table 3.
Mixed Model results using AKR1C3*2 genotype as predictor of alcohol effects.
| Change in Heart Rate | BAES Stimulation | BAES Sedation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | df | F | p-value | df | F | p-value | df | F | p-value |
| Alcohol | 1, 700 | 317.4 | <0.001 | 1, 707 | 153.8 | <0.001 | 1, 708 | 133.8 | <0.001 |
| Time | 5, 700 | 16.5 | <0.001 | 1, 707 | 82.8 | <0.001 | 1, 708 | 30.1 | <0.001 |
| Alcohol x Time | 5, 700 | 3.1 | 0.010 | 1, 707 | 59.4 | <0.001 | 1, 708 | 18.9 | <0.001 |
| AKR1C3*2 genotype1 | 2, 62 | 0.9 | 0.401 | 2, 62 | 5.2 | 0.008 | 2, 62 | 1.0 | 0.379 |
| Alcohol x AKR1C3*2 | 2, 700 | 19.6 | <0.0012 | 2, 712 | 12.9 | <0.0012 | 2, 708 | 9.0 | <0.0012 |
AKR1C3*2 genotype coded as the number of minor G-alleles (0=25, 1=26, 2=14)
Alcohol x genotype significant model term results are bolded (p<0.01).
Fig. 1. Change in heart rate as a function of alcohol and AKR1C3*2 genotype.
Left panel: Heart rate change from baseline following the placebo beverage. Right panel: Heart rate change from baseline following alcohol. Heart rate was measured approximately every 40 min and displayed as a change from baseline. C-allele homozygotes had a smaller alcohol-induced increase in heart rate than G-allele carriers. Data are displayed as mean ± S.E.M. of change in heart rate.
Fig. 2. Biphasic Alcohol Effects Scale (BAES) stimulation as a function of alcohol and AKR1C3*2 genotype.
Placebo beverage (left panel) and Alcohol (right panel) laboratory sessions. CC genotype subjects had lower self-reported levels of stimulation following alcohol than G-allele carriers. Data are displayed as mean ± S.E.M. of BAES stimulation score (sum of 7 items).
Fig. 3. Biphasic Alcohol Effects Scale (BAES) sedation as a function of alcohol and AKR1C3*2 genotype.
Placebo (left panel) and Alcohol (right panel) laboratory sessions. CC genotype subjects had lower self-reported levels of sedation following alcohol than G-allele carriers. Data are displayed as mean ± S.E.M. of BAES sedation score (sum of 7 items).
SRD5A1 genotype and interaction with AKR1C3*2 on acute alcohol responses
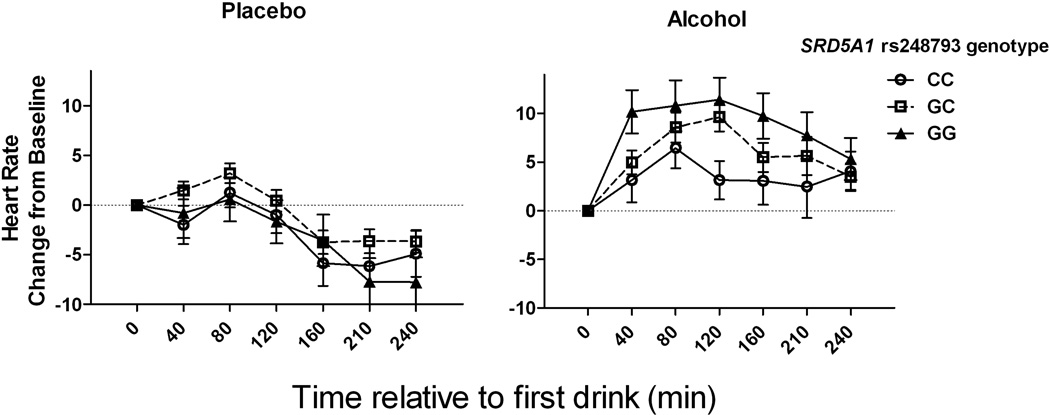
To model the interaction of AKR1C3*2 with SRD5A1 genotype (or the interaction of AKR1C3*2 and baseline drinking group in the next section), a dichotomous G-carrier coding status was used for AKR1C3*2 genotype comparing CC homozygotes with combination of heterozygotes and minor G-allele homozygotes, to preserve power and avoid spurious results based on small cell sizes. This coding is consistent with an apparent dominant effect of the G-allele with respect to the greater responses to alcohol on the three acute alcohol effect measures. Results from linear mixed model analysis including both AKR1C3*2 and SRD5A1 genotypes as predictors are shown in Table 4. There were no significant interaction effects of the two gene variants for any of the three alcohol response measures. As shown in Figure 4, there was a statistically significant interaction of alcohol and SRD5A1 genotype on HR, such that homozygotes for the AD-associated G allele had larger increases in HR following alcohol than carriers of the minor C allele. Post-hoc alcohol x SRD5A1 genotype contrasts were significant for GG vs. GC or CC (p<0.001) but not GC vs. CC genotypes (p=0.71). The pattern of alcohol-induced change in HR in homozygotes for the AD-associated allele was opposite that seen for AKR1C3*2, where homozygotes for the AD-associated C allele had a lower HR response (Figure 1) and the SRD5A1 SNP, where homozygotes for the AD-associated G allele had a higher HR response (Figure 4).
Table 4.
Mixed Model results using AKR1C3*2 and SRD5A1 rs248793 genotypes1 as predictors of alcohol effects.
| Change in Heart Rate | BAES Stimulation | BAES Sedation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | df | F | p-value | df | F | p-value | df | F | p-value |
| Alcohol | 1, 697 | 208.7 | <0.001 | 1, 704 | 125.4 | <0.001 | 1, 705 | 110.9 | <0.001 |
| Time | 5, 697 | 17.0 | <0.001 | 1, 704 | 83.2 | <0.001 | 1, 704 | 30.0 | <0.001 |
| Alcohol x Time | 5, 697 | 3.2 | 0.008 | 1, 704 | 59.6 | <0.001 | 1, 704 | 18.9 | <0.001 |
| AKR1C3*2 | 1, 59 | 1.0 | 0.325 | 1, 59 | 5.4 | 0.024 | 1, 59 | 0.75 | 0.391 |
| Alcohol x AKR1C3*2 | 1, 697 | 37.9 | <0.0012 | 1, 704 | 17.7 | <0.0012 | 1, 706 | 10.3 | 0.0012 |
| SRD5A1 genotype | 2, 59 | 0.9 | 0.408 | 2, 58 | 0.05 | 0.954 | 2, 59 | 0.12 | 0.887 |
| Alcohol x SRD5A1 | 2, 697 | 10.6 | <0.0012 | 2, 704 | 0.04 | 0.960 | 2, 706 | 0.96 | 0.377 |
| AKR1C3*2 x SRD5A1 | 2, 59 | 0.2 | 0.819 | 2, 58 | 0.46 | 0.631 | 2, 59 | 0.12 | 0.884 |
| Alc x AKR1C3*2 x SRD5A1 | 2, 697 | 1.9 | 0.147 | 2, 704 | 3.5 | 0.029 | 2, 706 | 0.28 | 0.760 |
AKR1C3*2 genotype coded as 0 for CC (n=25) or 1 for G-carriers (n=40) and for SRD5A1 genotype the number of minor C-alleles as 0 (n=16), 1 (n=36) or 2 (n= 13).
Alcohol x genotype significant model term results are bolded (p<0.01).
Fig. 4. Change in heart rate as a function of alcohol and SRD5A1 rs248793 genotype.
Left panel: Heart rate change from baseline following the placebo beverage. Right panel: Heart rate change from baseline following alcohol. AD-associated risk allele (G) homozygotes had a greater alcohol induced-increase in HR than minor C-allele carriers. Data are displayed as mean ± S.E.M. of change in heart rate.
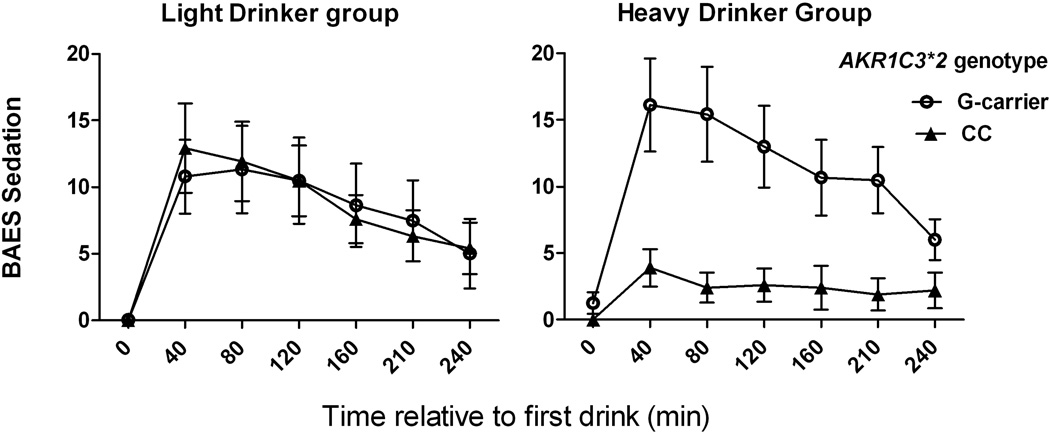
Interaction of AKR1C3*2 genotype and baseline drinking on sedative effects of alcohol
Our sample included 34 lighter drinkers and 31 heavier drinkers. We tested whether the moderation by AKR1C3*2 genotype of subjective effects differed by drinker status based on prior observations that subjective effects of alcohol differ based on drinker status (King et al. 2002; Gilman et al. 2012). Table 5 presents mixed model results that include AKR1C3*2 genotype and baseline drinker status as factors. We found a significant 3-way interaction of alcohol x AKR1C3*2 genotype x drinker status (p<0.001) on the BAES sedation measure, such that among homozygotes for the AD-associated C-allele, heavier drinkers reported a low level of sedation, but lighter drinkers did not (Figure 5).
Table 5.
Mixed Model results using AKR1C3*2 genotype1 and drinker group as predictors of alcohol effects.
| Change in Heart Rate | BAES Stimulation | BAES Sedation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | df | F | p-value | df | F | p-value | df | F | p-value |
| Alcohol | 1, 699 | 254.9 | <0.001 | 1, 706 | 128.9 | <0.001 | 1, 707 | 113.2 | <0.001 |
| Time | 5, 699 | 16.8 | <0.001 | 1, 706 | 82.8 | <0.001 | 1, 706 | 31.0 | <0.001 |
| Alcohol x Time | 5, 704 | 3.1 | 0.009 | 1, 706 | 59.4 | <0.001 | 1, 706 | 19.6 | <0.001 |
| AKR1C3*2 | 1, 61 | 1.4 | 0.236 | 1, 61 | 8.3 | 0.005 | 1, 61 | 2.2 | 0.147 |
| Alcohol x AKR1C3*2 | 1, 699 | 43.3 | <0.0012 | 1, 707 | 23.8 | <0.0012 | 1, 708 | 24.2 | <0.0012 |
| Heavier drinker | 1, 61 | 0.06 | 0.816 | 1, 60 | 0.09 | 0.764 | 1, 61 | 0.27 | 0.607 |
| Alcohol x Heavier Drk | 1, 699 | 12.8 | <0.001 | 1, 713 | 0.05 | 0.830 | 1, 712 | 7.0 | 0.008 |
| AKR1C3*2 x Heavier Drk | 1, 61 | 0.2 | 0.819 | 1, 61 | 1.5 | 0.230 | 1, 61 | 3.2 | 0.080 |
|
Alc x AKR1C3*2 x Heavier Drk |
1, 699 | 0.8 | 0.373 | 1, 708 | 3.3 | 0.070 | 1, 712 | 17.3 | <0.0012 |
AKR1C3*2 genotype coded as 0 for CC (n=25) or 1 for G-carrier (n=40).
Alcohol x genotype significant model term results are bolded (p<0.01).
Fig. 5. AKR1C3*2 genotype moderation of acute sedative effects of alcohol are seen in heavier but not lighter drinkers.
BAES sedation scores following alcohol administration for 34 lighter drinkers (left panel) and 31 heavier drinkers (right panel). CC genotype subjects had lower levels of sedation following alcohol only in the heavier drinker group. Data are displayed as mean ± S.E.M. of BAES sedation score (sum of 7 items).
AKR1C3*2 genotype effects in the presence of dutasteride
To examine the moderating effect of AKR1C3*2 genotype on the pharmacological inhibition of 5AR, we examined data from all 4 sessions, using dutasteride pretreatment, alcohol vs. placebo alcohol, and AKR1C3*2 G-carrier vs. CC genotype as factors. After correction for multiple comparisons, there were no significant 3-way interactions on HR or BAES stimulation or sedation scores. There was a nominally significant 3-way interaction effect on HR, such that the effect of genotype was reduced following dutasteride pre-treatment (p=0.019). The previously identified alcohol x AKR1C3*2 genotype interactions remained significant for all three outcomes (p<0.001).
Genotype effects on baseline neuroactive steroids in the absence of dutasteride or alcohol
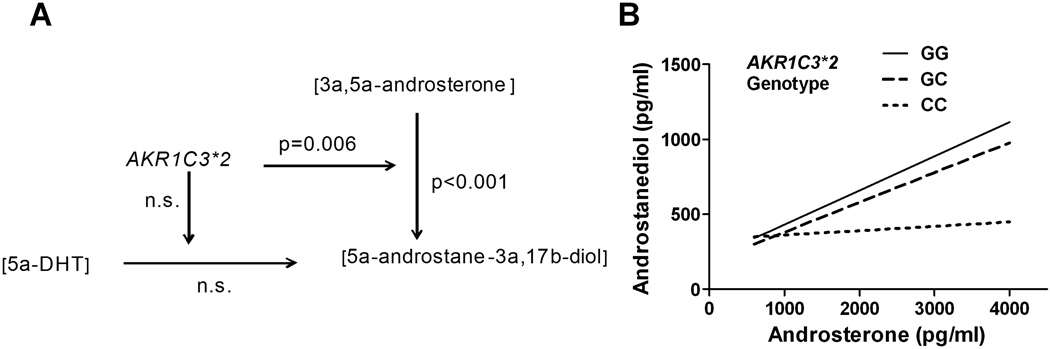
The AKR1C3 gene product has both 3α-reductase and 17β-reductase activities and is expressed in the brain and peripheral organs. The 17β-reductase activity contributes to the generation of the neuroactive steroid 5α-androstane-3α,17β-diol from 3α,5α-androsterone. Plasma 5α-androstane-3α,17β-diol can also be generated from DHT via 3α-reduction (principally via the liver-specific AKR1C4 gene product 3α-HSD type 1). We used GC/MS to measure levels of DHT, 3α,5α-androsterone, and 5α-androstane-3α,17β-diol from plasma obtained at study entry for 71 Caucasian men (65 completers plus 6 partial study completers) to examine whether there were AKR1C3*2 genotype interactions with the amount of either precursor (DHT or 3α,5α-androsterone) relative to levels of 5α-androstane-3α,17β-diol. Using a general linear model analysis in which DHT, androsterone, genotype, DHT x genotype and androsterone x genotype were predictors of androstanediol levels, we found neither a main effect of DHT concentration or a genotype x DHT interaction. However, there was a main effect of androsterone [Wald χ2(1) =14.9; p<0.001] and an interaction of androsterone x AKR1C3 genotype [Wald χ2(1) =10.4; p=0.006], such that, at progressively higher androsterone concentrations, AD-protective G-allele carriers had greater increases in androstanediol than C-allele homozygotes (see Figure 6).
Fig. 6. Interaction of AKR1C3*2 genotype with androstanediol precursors.
Study baseline plasma androsterone, DHT and androstanediol levels were measured using GC/MS. There was a significant main effect of androsterone level and interaction of androsterone with genotype in predicting androstanediol levels, panel A. The simple slopes for the GLM parameter estimates for each of the three genotypes are shown in panel B.
Discussion
In this study, we examined the effects of an amino acid coding polymorphism (His5Gln), AKR1C3*2 in the neuroactive steroid synthetic enzyme 3a–HSD Type 2 (17β-HSD type 5), on physiological and subjective responses to acute alcohol ingestion in a sample of non-dependent drinkers. We also tested whether there was an effect of a synonymous polymorphism in SRD5A1 or an interaction effect of that polymorphism with AKR1C3*2 on alcohol-induced subjective effects. We had previously reported that the minor allele at each of these two polymorphisms were less frequent in controls than in alcohol dependent subjects (Milivojevic et al., 2011) and that the reduction in risk associated with the minor allele of each gene was enhanced by the presence of the minor allele at the other gene. Additionally, we examined whether the AKR1C3*2 polymorphism was associated with a change in the levels of the potent neuroactive steroid, androstanediol, relative to its precursors androsterone and DHT via the actions of the gene products responsible for 17β-reductase or 3α-reductase activities.
We found that the AKR1C3*2 minor G-allele that was protective for AD was associated with larger increases in HR and greater stimulant and sedative effects of alcohol than two copies of the AD-associated C allele. Of particular interest, the AKR1C3*2 genotype’s moderation of sedative effects was observed only in heavier drinkers. In contrast, for the SRD5A1 SNP, we observed only moderation of the alcohol-induced change in HR, with minor C-allele carriers having a lower response than individuals with two copies of the AD-linked G-allele. In contrast to our report of an interaction of the two markers on the risk of AD (Milivojevic et al., 2011), we did not see an interaction of the AKR1C3*2 and SRD5A1 alleles on any of the three acute alcohol effect measures.
Additionally, the presence of the AD-protective AKR1C3*2 G allele was associated with higher levels of the GABAA receptor-modulating neuroactive steroid androstanediol relative to its precursor androsterone at study entry. In addition to 3α-HSD activity, the human AKR1C3 gene product also has 17β-HSD activity (Lin et al. 1997) and is also referred to as 17β-HSD type 5 (Adamski & Jakob, 2001). A separate gene product, 17β-reductase type 3, catalyzes the conversion of androstenedione to testosterone in the testis (Luu-The et al., 1995), while the AKR1C3 encoded 17β-reductase type 5 catalyzes this conversion in other organs (Dufort et al., 1999). Testosterone is further reduced to 5α-dihydrotestosterone (DHT) by 5α-reductase, which is then reduced by 3α-HSD to androstanediol, a potent positive allosteric modulator of GABAA receptors (Carver & Reddy, 2013; Reddy, 2008). Additional functions of 17β-HSD type 5 include the synthesis of androstanediol from androsterone. The physiological basis for the reduced subjective effects of alcohol in AKR1C3*2 C-allele homozygotes may be the reduced production of the neuroactive steroid androstanediol. In the current study, at baseline, subjects homozygous for the AD-associated AKR1C3*2 C allele had lower levels of the GABAA receptor neuroactive steroid agonist androstanediol relative to its precursor androsterone and exhibited lower subjective responses to alcohol than G-allele carriers. The AKR1C3*2 variation could affect the enzyme’s catalytic efficiency, substrate specificity, or affinity related to either its NADPH-dependent 17β-HSD or 3α-HSD activities, any of which could modify levels of neuroactive steroids relative to their precursors. Thus, C-allele homozygotes may have a reduced production of the neuroactive steroid androstanediol in response to alcohol. Because genotype did not predict the subjects’ breath alcohol concentration, the observed effects of AKR1C3*2 genotype do not appear to be attributable to differences in ethanol metabolism.
The moderation of alcohol-associated increases in heart rate, BAES stimulation, and sedation by the AKR1C3*2 SNP and of heart rate by the SRD5A1 SNP is consistent with the hypothesis that these alcohol responses are in part mediated by changes in neuroactive steroid concentrations. The moderation of only one of these three effects, sedation, by dutasteride blockade of 5AR (Covault et al., 2014) suggests that natural genetic variation in neuroactive steroid biosynthetic enzymes may provide tools to develop a more complete picture of the role of neuroactive steroids in alcohol’s effects in humans than is possible using pharmacological blockade of 5α-reductase. Interestingly, the pattern of genotype effects for each of the three measures was unique (HR moderation by both markers, AKR1C3*2 genotype effects on BAES stimulation in both lighter and heavier drinkers but genotype effects on BAES sedation only in heavier drinkers), suggesting that there is not a common neuroactive steroid compound and or locus of action that underlies neuroactive steroid effects for these different alcohol responses. The variation in genotype effects on the three measures examined may reflect different local brain neuroactive steroid changes in response to acute alcohol. This is consistent with observations in rat models showing that alcohol increases local cellular levels of the progesterone-derived neuroactive steroid allopregnanolone in some regions and decreases it in others (Cook et al., 2014a). Additionally, while alcohol-induced increases of allopregnanolone in frontal cortex were dependent on peripheral adrenal sources of steroids, alcohol-induced increases of allopregnanolone in subcortical areas and decreases in the nucleus accumbens were due to local steroid metabolism and occurred in adrenalectomized animals (Cook et al., 2014b). With respect to the interaction of AKR1C3*2 genotype and heavy drinking on BAES sedation, it is of interest to note that chronic ethanol administration in rats decreases localized levels of mRNA for the 5AR and 3α-HSD enzymes (Cagetti et al, 2004).
The response to alcohol is biphasic, such that individuals consuming alcohol simultaneously experience the opposing effects of stimulation and sedation, although on a somewhat different time course (Hendler et al., 2011). Stimulation generally occurs first, increases rapidly with the BrAC, and then quickly diminishes. Sedation, on the other hand, rises more gradually and has a more prolonged effect, declining more gradually than stimulation (Addicott et al., 2007). The alcohol administration paradigm used in this study resulted in a rapid ascending limb that peaked at the time of our first post-alcohol assessment and so prevented us from examining BAES stimulation and sedation responses on the ascending BrAc limb.
Although our results provide little insight into potential mechanisms by which the SRD5A1 SNP may moderate risk of AD, the results for AKR1C3*2 and BAES sedation in the heavier drinking group are of particular interest. Among heavier drinkers, risk-allele (C) homozygotes reported minimal sedative effects, which contrasts with the effects in the lighter drinker group, where C-allele homozygotes reported higher levels of sedation, which were similar to those of G-allele carriers. These data are consistent with the hypothesis that the development of tolerance to the sedative effects of alcohol may occur more readily in risk-allele (C) homozygotes than in protective G-allele carriers, who reported similar levels of sedation in the lighter and heavier drinker groups. Heavier drinkers are at greater risk of developing AUD and it is among this group that others have examined acute alcohol response as a predictor of drinking (King et al, 2013).
Early models of alcohol response as predictors of the subsequent development of AUD (Schukit 1984; 1994) focused on a low response to alcohol, as measured by the Subjective High Assessment Scale (SHAS), which focuses on the sedative and unpleasant effects of alcohol. More recent studies also emphasize greater subjective stimulation and pleasurable effects of alcohol on the ascending BrAC curve as risk factors for AUD (Newlin and Thomson, 1990). Refinements of this differentiator model derive from the observation that increased stimulation and decreased sedation at the peak BrAC both predict subsequent binge drinking (King et al., 2011) and AUD symptom count assessed over a 6-year follow-up period (King et al., 2013). In a follow-up study of 104 heavy social drinkers, lower BAES sedation at peak BrAC was the strongest predictor of AUD symptom count over time (King et al, 2013). Furthermore, using a combination of subjective responses following alcohol administration in a sample of heavy drinkers, including the SHAS, BAES stimulation and sedation and Profile of Mood States (POMS), exploratory factor analysis yielded a three-factor model that captured 1) alcohol-induced stimulation as a positive reinforcer, 2) sedative and unpleasant effects of alcohol as aversive and 3) alcohol-induced alleviation of tension and negative mood as negative reinforcement to drinking (Ray et al., 2009). In that study, the stimulant effects factor was unrelated to drinking quantity, frequency, or alcohol problems. In contrast, the sedative and unpleasant effects factor on which the SHAS and BAES sedation measures loaded equally, was negatively correlated with drinking quantity, such that individuals who reported higher sensitivity to the sedative and unpleasant effects of alcohol consumed fewer drinks (Ray et al., 2009). Reduced sedation following alcohol is a consistent predictor of the subsequent development of an AUD in each of these models and suggests that the genotype effects of AKR1C3*2 in relation to BAES sedation, among the heavier drinker group (Figure 5) could contribute to the risk of progression from heavy drinking to AUD.
The present study was limited in that we only examined genotype effects in Caucasian men, thus we are unable to generalize the findings to other populations. Studies of the moderation by AKR1C3*2 genotype of alcohol’s effects in a variety of population groups and in women are needed to inform our understanding of the gene’s role in moderating the acute effects of alcohol and the risk for AUD. An important consideration in the interpretation of current findings is that we used the BAES to measure the level of response to alcohol, whereas many of the early studies examining the subjective response to alcohol used the SHAS. However, similar results have been reported using the SHAS and the BAES in more recent human alcohol administration studies, particularly for the sedative effects of alcohol, where the two measures are highly correlated (Ray et al., 2009).
In summary, these findings provide examples of the potential functional relevance of the AD risk vs. protective alleles of the AKR1C3*2 non-synonymous C>G SNP. Specifically, AKR1C3*2 genotype moderated the subjective responses to alcohol and affected neuroactive steroid synthesis, such that the minor, protective G allele was associated with a higher level of androstanediol relative to its precursor androsterone at baseline. The moderating effect of the AKR1C3*2 genotype on BAES sedation in heavier but not light drinkers is of particular interest in light of prior studies identifying low levels of sedation in response to alcohol in heavier drinkers as a risk factor for developing AUD symptoms (Schuckit, 1994; Ray et al. 2009; King et al. 2013). The form of the interaction of heavier vs. lighter drinking x AKR1C3*2 genotype on BAES sedation suggests that, compared with AD-risk C-allele homozygotes, protective G-allele carriers may be less likely to develop tolerance to the sedating effects of alcohol (compare LD and HD in Figure 5) which could protect against the development of AUD.
These findings provide further support for the role of neuroactive steroids in the neurobiology of alcohol’s effects in humans. Further examination of associations of the AKR1C3*2 non-synonymous C>G SNP with alcohol consumption in the natural environment and in experimental paradigms are novel approaches with which to explore the involvement of neuroactive steroids in human drinking behavior and AUD risk.
Acknowledgments
Supported by NIH grants R01 AA015606 (to JC), K24 AA13736 (to HRK), P60 AA03510 (University of Connecticut Alcohol Research Center), and M01 RR06192 (University of Connecticut General Clinical Research Center). The authors thank Timothy Pond, Linda Burian, Kaitlin Miller and Pamela Fall for their expert technical assistance in the conduct of this study.
Footnotes
Financial disclosures: VM, RF, and JC have no disclosures to make. HK has been a consultant or advisory board member for the following pharmaceutical companies: Alkermes, Lilly, Lundbeck, Pfizer, and Roche. He is also a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which is supported by Lilly, Lundbeck, AbbVie, Ethypharm, and Pfizer.
REFERENCES
- Adamski J, Jakob FJ. A guide to 17beta-hydroxysteroid dehydrogenases. Molecular and cellular endocrinology. 2001;171:1–4. doi: 10.1016/s0303-7207(00)00383-x. [DOI] [PubMed] [Google Scholar]
- Addicott MA, Marsh-Richard DM, Mathias CW, Dougherty DM. The biphasic effects of alcohol: comparisons of subjective and objective measures of stimulation, sedation, and physical activity. Alcoholism, clinical and experimental research. 2007;31:1883–1890. doi: 10.1111/j.1530-0277.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases "GABAergic" neurosteroids in alcohol-preferring rats. Eur J Pharmacol. 1999;384:R1–R2. doi: 10.1016/s0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. EEG, autonomic and subjective correlates of the risk for alcoholism. J Stud Alcohol. 1993;54:577–589. doi: 10.15288/jsa.1993.54.577. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompained by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46:570–579. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230:151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89:2179–2184. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- Cook JB, Dumitru AM, O'Buckley TK, Morrow AL. Ethanol administration produces divergent changes in GABAergic neuroactive steroid immunohistochemistry in the rat brain. Alcohol Clin Exp Res. 2014a;38:90–99. doi: 10.1111/acer.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JB, Nelli SM, Neighbors MR, Morrow DH, O'Buckley TK, Maldonado-Devincci AM, Morrow AL. Ethanol alters local cellular levels, o. f. (3a5a)-3-hydroxypregnan-20-one (3a5a-THP) independent of the adrenals in subcortical brain regions. Neuropsychopharm. 2014b doi: 10.1038/npp.2014.46. EPub 19Mar 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Pond T, Feinn R, Arias A, Oncken C, Kranzler H. Dutasteride reduces alcohol's sedative effects in men in a human laboratory setting and reduces drinking in the natural environment. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3487-4. Epub 21 Feb 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor EJ, Cloninger CR. Genetics of alcoholism. Annu Rev Genet. 1989;23:19–36. doi: 10.1146/annurev.ge.23.120189.000315. [DOI] [PubMed] [Google Scholar]
- Dufort I, Rheault P, Huang XF, Soucy P, Luu-The V. Characteristics of a highly labile human type 5 17beta-hydroxysteroid dehydrogenase. Endocrinology. 1999;140:568–574. doi: 10.1210/endo.140.2.6531. [DOI] [PubMed] [Google Scholar]
- Duranceaux NC, Schuckit MA, Eng MY, Robinson SK, Carr LG, Wall TL. Associations of variations in alcohol dehydrogenase genes with the level of response to alcohol in non-Asians. Alcohol Clin Exp Res. 2006;30(9):1470–1478. doi: 10.1111/j.1530-0277.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- Figueroa JD, Malats N, Garcia-Closas M, Real FX, Silverman D, Kogevinas M, Chanock S, Welch R, Dosemeci M, Lan Q, Tardon A, Serra C, Carrato A, Garcia-Closas R, Castano-Vinyals G, Rothman N. Bladder cancer risk and genetic variation in AKR1C3 and other metabolizing genes. Carcinogenesis. 2008;29:1955–1962. doi: 10.1093/carcin/bgn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID - I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Ford MM, Yoneyama N, Strong MN, Fretwell A, Tanchuck M, Finn DA. Inhibition of 5alpha-reduced steroid biosynthesis impedes acquisition of ethanol drinking in male C57BL/6J mice. Alcoholism, clinical and experimental research. 2008;32:1408–1416. doi: 10.1111/j.1530-0277.2008.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Crouss T, Hommer DW. "Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns". Neuropsychopharmacology. 2012;37(2):467–477. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug and alcohol dependence. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hendler RA, Ramchandani VA, Gilman J, Hommer DW. Stimulant and Sedative Effects of Alcohol. Curr Top Behav Neurosci. 2011 doi: 10.1007/7854_2011_135. [DOI] [PubMed] [Google Scholar]
- Holdstock L, Penland SN, Morrow AL, de Wit H. Moderate doses of ethanol fail to increase plasma levels of neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one-like immunoreactivity in healthy men and women. Psychopharmacology. 2006;186:442–450. doi: 10.1007/s00213-005-0187-0. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism, clinical and experimental research. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Joslyn G, Ravindranathan A, Brush G, Schuckit M, White RL. Human variation in alcohol response is influenced by variation in neuronal signaling genes. Alcoholism, clinical and experimental research. 2010;34:800–812. doi: 10.1111/j.1530-0277.2010.01152.x. [DOI] [PubMed] [Google Scholar]
- Kim YS, Zhang H, Kim HY. Profiling neurosteroids in cerebrospinal fluids and plasma by gas chromatography/electron capture negative chemical ionization mass spectrometry. Analytical biochemistry. 2000;277:187–195. doi: 10.1006/abio.1999.4384. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit L, Holdstock L, Schuster A. "Biphasic alcohol response differs in heavy versus light drinkers". Alcohol Clin Exp Res. 2002;26(6):827–835. [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68(4):389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D. Alcohol Challenge Responses Predict Future Alcohol Use Disorder Symptoms: A 6-Year Prospective Study. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.001. Epub Oct 2013 ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3 alpha-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3 alpha/17 beta-HSD activity and cellular distribution.". Mol Endocrinol. 1997;11(13):1971–1984. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- Luu-The V, Zhang Y, Poirier D, Labrie F. Characteristics of human types 1, 2 and 3 17 beta-hydroxysteroid dehydrogenase activities: oxidation/reduction and inhibition. J Steroid Biochem Mol Biol. 1995;55(5–6):581–587. doi: 10.1016/0960-0760(95)00209-x. [DOI] [PubMed] [Google Scholar]
- Martin-Garcia E, Darbra S, Pallares M. Intrahippocampal allopregnanolone decreases voluntary chronic alcohol consumption in non-selected rats. Progress in neuro-psychopharmacology & biological psychiatry. 2007;31:823–831. doi: 10.1016/j.pnpbp.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism, clinical and experimental research. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Milivojevic V, Covault J. Alcohol exposure during late adolescence increases drinking in adult Wistar rats, an effect that is not reduced by finasteride. Alcohol Alcohol. 2013;48:28–38. doi: 10.1093/alcalc/ags105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic V, Kranzler HR, Gelernter J, Burian L, Covault J. Variation in genes encoding the neuroactive steroid synthetic enzymes 5alpha-reductase type 1 and 3alpha-reductase type 2 is associated with alcohol dependence. Alcoholism, clinical and experimental research. 2011;35:946–952. doi: 10.1111/j.1530-0277.2010.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcoholism, clinical and experimental research. 1999;23:1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thompson JB. Alcohol challeng with sons of alcoholics: A critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Andersson A, Zingmark E, Wahlstrom G, Backstrom T, Sundstrom-Poromaa I. The effect of a low dose of alcohol on allopregnanolone serum concentrations across the menstrual cycle in women with severe premenstrual syndrome and controls. Psychoneuroendocrinology. 2005;30:892–901. doi: 10.1016/j.psyneuen.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Ostlund RE, Jr, Hsu FF, Bosner MS, Stenson WF, Hachey DL. Quantification of cholesterol tracers by gas chromatography--negative ion chemical ionization mass spectrometry. Journal Mass Spectrom. 1996;31:1291–1296. doi: 10.1002/(SICI)1096-9888(199611)31:11<1291::AID-JMS424>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Frye CA. Alcohol Dose-Dependently Enhances 3a-Androstanediol Formation in Frontal Cortex of Male Rats Concomitant with Aggression. The Open Psychopharm J. 2009;2:1–10. [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, Marx CE, Shampine LJ, Kranzler HR. Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of alcohol. Psychopharmacology. 2006;186:451–461. doi: 10.1007/s00213-005-0231-0. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcoholism, clinical and experimental research. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, Leventhal A, Hutchison KE. Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcoholism, clinical and experimental research. 2009;33:2154–2161. doi: 10.1111/j.1530-0277.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Mass spectrometric assay and physiological-pharmacological activity of androgenic neurosteroids. Neurochemistry international. 2008;52:541–553. doi: 10.1016/j.neuint.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry. 1984;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53(3):202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Arch Gen Psychiatry. 1987a;44:942–945. doi: 10.1001/archpsyc.1987.01800230022005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch C. Serum prolactin levels in sons of alcoholics and control subjects. Am J Psychiatry. 1987b;144:854–859. doi: 10.1176/ajp.144.7.854. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Risch SC, Gold EO. Alcohol consumption, ACTH level, and family history of alcoholism. Am J Psychiatry. 1988;145:1391–1395. doi: 10.1176/ajp.145.11.1391. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K. Response to alcohol in daughters of alcoholics: a pilot study and a comparison with sons of alcoholics. Alcohol Alcohol. 2000;35:242–248. doi: 10.1093/alcalc/35.3.242. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG, Brown SA. Testing the level of response to alcohol: social information processing model of alcoholism risk--a 20-year prospective study. Alcohol Clin Exp Res. 2004;28(12):1881–1889. doi: 10.1097/01.alc.0000148111.43332.a5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Pierson J, Hesselbrock V, Bucholz KK, Kramer J, Kuperman S, Dietiker C, Brandon R, Chan G. The ability of the Self-Rating of the Effects of Alcohol (SRE) Scale to predict alcohol-related outcomes five years later. J Stud Alcohol Drugs. 2007;68:371–378. doi: 10.15288/jsad.2007.68.371. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: Psychosocial and Biochemical Methods. Humana Press; 1992. pp. 41–42. [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology. 2003;28:1207–1209. doi: 10.1038/sj.npp.1300170. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology. 2004;172:352–355. doi: 10.1007/s00213-003-1662-0. [DOI] [PubMed] [Google Scholar]
- Vallee M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Analytical biochemistry. 2000;287:153–166. doi: 10.1006/abio.2000.4841. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]