Abstract
Background
Compared with White Americans, Black American men are at a significant increased risk of presenting with prostate cancer (PCa) and associated mortality, suggesting a link to African-ancestry. However, PCa status within Africa is largely unknown. We address the clinical presentation of PCa within Black South African men.
Methods
Over 1,000 participants with or without PCa have enrolled in the Southern African Prostate Cancer Study (SAPCS). Using genome-wide profiling we establish a unique within Africa population substructure. Adjusting for age, clinical variables were assessed, compared against Black Americans and between rural and urban localities while addressing potential socio-demographic confounders.
Results
We report a significant difference in the distribution of prostate specific antigen (PSA) levels skewed towards higher PSA levels in the PCa cases (83.0% present with a PSA ≥ 20 µg/L; median PSA = 98.8 µg/L) relative to men with no detectable PCa (18.5% present with a PSA ≥ 20 µg/L; median PSA = 9.1 µg/L). Compared with Black Americans, Black South Africans presented with significantly more aggressive disease defined by Gleason score >7 (17% and 36%, respectively) and PSA ≥ 20 µg/L (17.2% and 83.2%, respectively). We report exasperated disease aggression defined by Gleason score >7 (P = 0.0042) and poorly differentiated tumor grade (P < 0.0001) within rural versus urban localities.
Conclusion
Black South African men present with higher PSA levels and histopathological tumor grade compared with Black Americans, which is further escalated in men from rural localities. Our data suggests that lack of PSA testing may be contributing to an aggressive PCa disease phenotype within South African men.
Keywords: Prostate cancer, clinical presentation, African ancestry, Southern Africa, aggressive disease
Introduction
The global burden of PCa is expected to reach 1.7 million new cases and 499,000 new deaths by 2030 [1]. However, this burden is not equally distributed around the globe, with the most affluent countries having the highest incidence rates [2]. PCa is one of the most common malignancies in men, and the known risk factors include increasing age, a family history of PCa and African ancestry. Compared with European Americans, African American men are 1.7 times more likely to develop PCa [3,4], are generally younger at diagnosis, present with a more aggressive disease phenotype [5] and are approximately 2.5 times more likely of dying from the disease [6–8]. While factors related to socioeconomic status have been investigated, one cannot ignore the potential effect of genetic factors related to African ancestry. The significance of one's genetic inheritance is further supported by familial [9,10] and twin studies [11,12], while differences in ethnic or racial based prostate tumor biology is evident [13]. However, limited data are available pertaining to PCa status within Africa. Clinically aggressive disease phenotypes have been reported within selected populations from Western Africa [14–16], Eastern Africa [17], and within an ethnically admixed population from Southern Africa [18].
In contrast to affluent nations, PCa screening, including digital rectal examination and prostate specific antigen (PSA) testing, is not routinely practiced in many parts of Africa. Alternatively, traditional medicine may be preferential within some communities. The most likely contributors to the increased variation in PCa incidence rates (up to 25-fold) compared to mortality rates (up to 10-fold) observed globally [1] are disparities in cancer surveillance. Defining the impact of PCa within Africa is further impeded by several confounding issues including a dramatically decreased life-expectancy (reducing the impact of age-related diseases), limited access to health services, lack of national cancer registries, reduced PCa awareness, and both logistical and economical challenges associated with establishing and maintaining PCa research studies. A recent review addresses some of the issues associated with investigating PCa within Africa [19]. Although Africans present globally with the greatest between population matched [20] and within individual [21] genetic diversity, in contrast to African Americans with roughly 10–20% European genetic contribution [22,23], individuals from the African continent allow for exclusion of a non-African genetic admixture fraction. While establishing if genetics is a driver for the significant link between PCa status and African ancestry is paramount, such analysis cannot and should not be conducted in isolation of extensive environmental, life-style, and clinical information. Although not yet well established, evidence for environmental influences on PCa risk is mounting and particularly evident in populations of Eastern nations, who typically display low incidence rates, but are at an increased risk of PCa after migrating to Western nations and presumably adapting Western lifestyles [24,25]. Assessing epidemiological factors contributing to PCa status within Africa requires an additional level of complexity and challenges associated with social and cultural practices, and ultimately data interpretation.
In this study we describe the recruitment of patients with PCa within the Southern African Prostate Cancer Study (SAPCS), an ongoing collection of clinical and epidemiological data, as well as biological specimens, from Black South African men either with or without PCa. In addition we address factors that may confound data interpretation, in particular when associating the SAPCS to studies of Black Americans, by performing comprehensive genetic analysis of the population substructure. This study is to the best of our knowledge the first and largest of its kind for the region and highlights the significance of PCa within a population where this disease has largely been ignored. The inclusion of both rural and urban-based clinics allows for much needed assessment of PCa status within rural Africa and confounding environmental influences. This study and the future use and development of this cohort will continue to provide critical contributions to unraveling the link between PCa and African ancestry.
Participants and Methods
SAPCS Participant Recruitment
The SAPCS was initiated in 2008 with seed funding from the Medical Research Council (MRC) of South Africa to local researchers at the Universities of Limpopo (PAV) and Pretoria (MSRB), with support from international collaboration (VMH). The overall objective of the SAPCS is to assess the impact of PCa on Black South Africans by establishing a cohort for clinical, epidemiological and genetic analyses. Men have been recruited from local clinics (Fig. 1A) and status defined as case (with PCa) or control (absence of PCa) by urological examination, PSA testing and where necessary, histopathology. Control samples may include individuals diagnosed with benign prostatic hyperplasia (BPH). PCa status is further defined by Gleason score (sum of the two most common Gleason grades) and additionally in some cases, a three tiered grading system, reflecting the appearance of tumor cells relative to “normal,” was used to describe the tumor phenotype as either well, moderate or poorly differentiated (referred to throughout this manuscript as “tumor grade”). Men completed a purpose written questionnaire managed by a trained local research nurse and provided a blood sample for research purposes. All participants self-identified as Southern Bantu representing the Sepedi, Setswana, Xitsonga, Tshivenda, isiNdebele, isiZulu, isiXhosa, Sesotho and siSwati languages spoken by the Bapedi, Batswana, VaTsonga, VhaVenda, amaNdebele, amaZulu, amaXhosa, Basotho and siSwati people, respectively. Although we acknowledge that it is grammatically correct to use prefixes when referring to a Bantu language or people, for simplicity throughout this paper we have elected to use the English derived ethno-linguistic identifiers Pedi, Tswana, Tsonga, Venda, Ndebele, Zulu, Xhosa, Sotho, and Swati.
Fig 1.
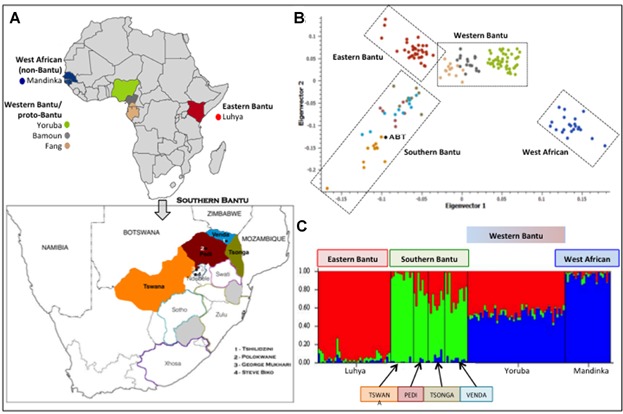
A: Geographical localities for continental populations used in this study to define ancestral contributions including west African Mandinka (non-Bantu), Yoruba, Bamoun and Fang (western Bantu/proto-Bantu) and east African Luhya (Eastern Bantu) and (inset A) significant Southern Bantu populations from the most northerly borders of South Africa contributing to the SAPCS, specifically the Pedi, Venda, Tsonga, and Tswana. Geographical distribution of minority contributing Southern Bantu populations, specifically Swati, Ndebele, Sotho, Zulu and Xhosa, is depicted including the recruitment localities (1 to 4). Merging genome-wide genotype data for the four prominent SAPCS Southern Bantu populations (n = 36) with publically available data for west (n = 22 non-Bantu, n = 80 Western Bantu) and east African populations (n = 35 Eastern Bantu) resulted in 23,183 overlapping autosomal markers, which were used to define within Southern African and between African population substructure depicted as (B) principal component analysis including the Southern Bantu Reference Genome (ABT) [21], and (C) STRUCTURE analysis assuming three contributing populations (K = 3).
The study has developed in stages starting with the most rural and least affluent of the South African Provinces, namely Limpopo (Polokwane and Tshilidzini Hospitals) where the Blacks represent 97% of the population [26]. Arguably the most challenging region for sample collection, initiating recruitment within Limpopo, allowed the team to evaluate potential limitations of the study design early in the process, whilst developing infrastructure and logistics. Ethics approval was granted from the Provincial Government of Limpopo (#32/2008) and subsequently also the University of Limpopo Medical Research Ethics Committee (#MREC/H/28/2009). Initially the study focused on the recruitment of the most predominant populations within the region, namely the Pedi, Tsonga, Venda, and Tswana. In 2010 the SAPCS was expanded to include Pretoria's Steve Biko Academic Hospital (SBAH, University of Pretoria) and the Medical University of South Africa's (MEDUNSA, University of Limpopo) Dr. George Mukhari Hospital near Ga-Runkuwa, north of Pretoria, within the Province of Gauteng. Additional approval was granted from the University of Pretoria Human Research Ethics Board (#43/2010) and the J. Craig Venter Institutional Review Board (IRB #2010–129). In contrast to Limpopo, Gauteng is not only the smallest province, it is the most populated and industrialized, attracting a diversity of peoples to this financial hub and calling for expansion to include all peoples defined as Black South Africans who represent 77% of the total provincial population [26]. By mid 2012 a total of 837 participants had been recruited allowing for preliminary epidemiological and genetic analyses [27].
SAPCS Expansion and Maintenance
Follow-up of SAPCS participants is documented by participating urologists on return visits. As all participating urologists are active members of the SAPCS, these updates have become routine. Repeat visits are however, largely dependent on disease severity and follow-up will most significantly impact the subset of participants undergoing treatment, with skewing towards aggressive disease. Patients diagnosed with PCa are requested to return to the clinic within 1 month of diagnosis for commencement of treatment. In cases with a PSA > 100 µg/L immediate treatment is recommended, including orchidectomy, radical prostatectomy, or androgen deprivation therapy. Given the relatively recent establishment of the SAPCS and the estimated life-span post-PCa diagnosis, we do not anticipate the immediate inclusion of follow-up data for analysis.
As recruitment has thus far focused on men presenting at urological clinics, a natural skewing towards cases over controls has become evident. To overcome this bias, as well as the potential confounding influence related to clinic based recruitment, including study participants presenting with benign urological complaints such as BPH, ethics approval has been granted from the South African National Blood Services (SANBS) Human Research Ethics Committee (#2012/11) to recruit additional controls via random population and age-matched sampling. In parallel the team initiated the acquisition of surplus prostate tissue from consented participants at time of needle biopsy. Ethics approvals were amended and collection started in the latter half of 2012. Recruitment was restricted to the Steve Biko Hospital in Pretoria with a total of 148 matched fresh frozen biopsy prostate tissues sampled to date. The total study recruitment by June 2013 is 1,068 participants. Analysis of clinical characteristics was performed for 917 participants.
SAPCS Genetic Substructure
Genomic DNA was isolated from whole blood using the QIAamp DNA Blood Mini kit or the FlexiGene DNA kit (QIAGEN). A total of 36 randomly selected participants (7 Pedi, 8 Tsonga, 11 Venda, and 10 Tswana), representing the major contributors to the study populations, were genotyped using the Illumina HumanOmni1-Quad Beadchips. The Illumina GenomeStudio software (version1.7.4) was used for data analysis with a GenTrain score of 0.5 as the minimum for inclusion. No subjects were related based on identity by descent estimations. We merged our data with the single sequenced Southern Bantu individual (ABT) from Tswana/Xhosa heritage [21] as well as published African-relevant data including the, Luhya (n = 35), Yoruba (n = 47), Bamoun (n = 18), Fang (n = 15), and Mandinka (n = 22) [28] (http://www-evo.stanford.edu/pubs.html). Total autosomal overlap after merging was 23,183 markers. Analytical methods included principal components analysis (PCA) and STRUCTURE analysis. For PCA we used the HelixTree module of the SVS (SNP and Variation Suite) 7.5 software available from Golden Helix (http://www.goldenhelix.com/), considering the first two eigenvectors. STRUCTURE 2.3.3 analyses [29] was performed using 10,000 burn-ins, followed by 10,000 iterations assuming K = 2–7 population clusters running five replications per ancestral estimation.
Statistical Analyses
PS Power and Sample Size Calculation version 3.0.43 was used to determine the study power to achieve a significance of P < 0.05 and determine the optimal target study size of 1,500 cases and 1,500 controls. Pearson's Chi-square test with Yates' continuity correction was used to predict significance between clinical features of the US-White and US-Black PCa cases in the publically available US-based SEER (surveillance Epidemiology and End Results) registry and SAPCS samples. Fisher's exact test with two-tailed P-values was used to predict significance levels for clinical characteristics in the SAPCS case samples from rural (Limpopo) versus industrialized (Gauteng) collection locations. Logistic regression estimates using a generalized linear model were used to correct for the confounding factor of age.
Results
As the study was initiated in Limpopo, this province provides the largest study contribution at 643 men (70.1%) compared with 266 men (29.0%) from Gauteng. An additional 8 (0.9%) participants have no recorded collection location. Self-identified primary language grouping, considering both maternal and paternal heritage, identified the Pedi as the largest contributors (52.8%) followed by Tsonga (13.8%), Venda (12.1%), Tswana (8.2%), Ndebele (4.7%), Zulu (4.3%), Swati (2.0%), Sotho (1.1%), and Xhosa (1.1%). The Pedi, Tsonga, Venda, and Tswana therefore contribute 86.9% of the total study. Before the SAPCS can be used for case–control analysis it is essential to determine the genetic substructure of the contributing populations as to prevent false-positive associations as a result of extensive population stratification (existence of subpopulations within a population).
Population Substructure
While a single complete Southern Bantu genome (ABT from Tswana/Xhosa heritage) alluded to the extent of genetic diversity within a single individual [21], the genetic diversity between Southern Bantu populations is largely unknown. We therefore tested the genetic relationship (population substructure) of the most significant SAPCS contributing Southern Bantu language identifiers (Pedi, Tsonga, Venda, and Tswana) and extended Bantu peoples of Africa, including the Eastern Bantu Luhya, the Western Bantu (or Proto-Bantu) Yoruba, Bamoun and Fang, as well as the non-Bantu West African Mandinka speakers (Fig. 1). PCA and STRUCTURE analysis defined the Southern Bantu as genetically distinct from the west and east African populations, while establishing a common Southern Bantu ancestral genomic contribution. Additionally, we excluded for any out of Africa contribution (specifically European and/or Asian) within the Southern Bantu (data not presented). Genotype data has been made freely available at http://www.garvan.org.au/TKCC/HumanComparativeProstateCancerGenomicsLaboratory/SAPCS.
Clinical Presentation
Men presented at one of the four participating urological clinics largely as a result of a urological or associated complaint (Table 1). Unlike most western countries, only 3.3% (30/920) presented as a result of a predetermined elevated PSA level, while 1.9% (17/920) presented for an age-related check-up and 3.6% (33/920) of men provided no reason for their visit. The most common complaint was associated with urinary flow (740/920, 80.4%), including difficulty passing urine (665/920, 72.3%), dysuria (72/920, 7.8%), or obstruction/poor flow (13/920, 1.4%). While dysuria was significantly associated with a positive PCa diagnosis (9.5% (56/592) and 4.9% (16/328) of cases and controls, respectively present with this symptom; P = 0.0145), urinary obstruction was negatively associated with diagnosis (present in 0.5% (3/592) and 3.0% (10/328) of PCa cases and controls respectively; P = 0.0028). Although urinary retention was the most common complaint, there was no observable difference between cases and controls. No other presenting symptom was found in our study to be associated with a positive diagnosis for PCa.
TABLE I.
SAPCS Clinical Characteristics by Locality
| Characteristic | Cases |
Controls |
Significance levels |
|||||
|---|---|---|---|---|---|---|---|---|
| Total Na |
Limpopob |
Gautengc |
Total Na |
Limpopob |
Gautengc |
Case vs. controld | Limpopo vs. Gautenge | |
| n = 576 (%) | n = 389 (%) | n = 181 (%) | n = 341 (%) | n = 254 (%) | n = 85 (%) | |||
| Reason for attending clinicf | NA | NA | NA | NA | P = 0.06 | NA | ||
| Urological complaintsg | 509 (86.0) | 288 (87.8) | ||||||
| Incidental high PSA | 19 (3.2) | 11 (3.4) | ||||||
| Erectile dysfunction | 2 (0.3) | 0 (0) | ||||||
| Swollen/injured scrotal region (glands/testis/scrotum) | 8 (1.4) | 6 (1.8) | ||||||
| Back or leg pain | 24 (4.1) | 3 (0.9) | ||||||
| General check-up | 13 (2.2) | 4 (1.2) | ||||||
| No reason given | 17 (2.9) | 16 (4.9) | ||||||
| Age | P = 0.48 | P = 0.0046 | ||||||
| Mean | 71 | 71.8 | 69.2 | 70.6 | 70.8 | 69.8 | ||
| Median | 71 | 72 | 69 | 70 | 71 | 68 | ||
| Range | 49–101 | 49–101 | 50–98 | 45–99 | 45–99 | 47–93 | ||
| n | 516 | 367 | 148 | 314 | 241 | 73 | ||
| PSA | P < 0.0001 | P = 0.19 | ||||||
| <4 µg/L | 9 (1.8) | 6 (1.7) | 3 (2.1) | 74 (24.5) | 58 (24.4) | 16 (25.0) | ||
| ≥4<10 µg/L | 22 (4.5) | 11 (3.2) | 11 (7.9) | 88 (29.1) | 65 (27.3) | 23 (35.9) | ||
| ≥10<20 µg/L | 52 (10.7) | 35 (10.1) | 17 (12.1) | 84 (27.8) | 67 (28.2) | 17 (26.6) | ||
| ≥20<98 µg/L | 160 (32.9) | 123 (35.5) | 37 (26.4) | 46 (15.2) | 40 (16.8) | 6 (9.4) | ||
| ≥98 µg/L | 244 (50.1) | 171 (49.4) | 72 (51.4) | 10 (3.3) | 8 (3.4) | 2 (3.1) | ||
| Gleason score | NA | P = 0.0042h | ||||||
| <7 | 137 (39.6) | 99 (39.9) | 38 (39.2) | N/A | ||||
| 7 | 86 (24.9) | 51 (20.6) | 35 (36.1) | |||||
| >7 | 123 (35.5) | 98 (39.5) | 24 (24.7) | |||||
| Tumor grade | NA | P < 0.0001h | ||||||
| Well differentiated | 71 (23.4) | 47 (20.2) | 24 (34.8) | N/A | ||||
| Mod differentiated | 154 (50.7) | 110 (47.2) | 42 (60.9) | |||||
| Poor differentiated | 79 (26.0) | 76 (32.6) | 3 (4.3) | |||||
Bolding of P-value indicates statistical significance.
Total N includes all case and control samples, inclusive of samples where collection location was not recorded.
Province of Limpopo: rural collection locations including urological clinics in Polokwane (central Limpopo) and Tshilidzini Hospitals (northern Limpopo).
Province of Gauteng: more industrialized collection locations including Steve Biko Academic Hospital (SBAH, University of Pretoria) and the Medical University of South Africa's (MEDUNSA, University of Limpopo) Dr George Mukhari Hospital near Ga-Runkuwa (north of Pretoria).
P-values for case–control analysis comparing the distribution of defined characteristics in total cases versus total controls were generated using Fisher's exact test for dichotamous variables and Welch two sample t-test for continuous variables.
P-values comparing the distribution of defined characteristics in all samples collected in Limpopo versus all samples collected in Gauteng were generated as described for case-control analysis.
Reason for attending clinic is reported for all study samples present in the SAPCS database as of July 2012, which includes 592 case samples and 328 controls. Reports are received per study population, without report of clinic location.
Urological complaints include: retention/LUTS, dysuria, obstructed flow, Haematuria, Nocturia, incontinence, increased frequency, and Urinary tract infection.
The more aggressive disease phenotypes (Gleason score >7 and tumor grade = Poor) were more significantly associated with cases collected from the rural Limpopo clinics compared to Gauteng.
Mean age at presentation was 71.0 years for the cases and 70.6 years for the controls. The age of study participants ranged from 45 to 101 years. Clinical characteristics have been made available for 917 of the 1,068 participants, 576 (62.8%) presenting with histopahtologically confirmed PCa (defined as a case), and 341 (37.2%) showing no detectable PCa (defined as a control). PSA levels have been made available for 487 cases and 302 controls (Fig. 2A). While the majority of controls presented with a PSA level of less than 10.0 µg/L (162/302, 53.6%) and 81.5% with less than 20.0 µg/L (246/302), a staggering 83.0% (404/487) of cases presented with a PSA greater than or equal to 20.0 µg/L (50.1% equal or over 98 µg/L), which is significantly different to the study controls (P < 0.0001). Frequency distribution at all PSA ranges, namely <4.0 µg/L, 4.0–9.9 µg/L, 10.0–19.9 µg/L, 20.0–97.9 µg/L, and greater than or equal to 98 µg/L, were significantly different between SAPCS cases and controls (all P < 0.0001).
Fig 2.
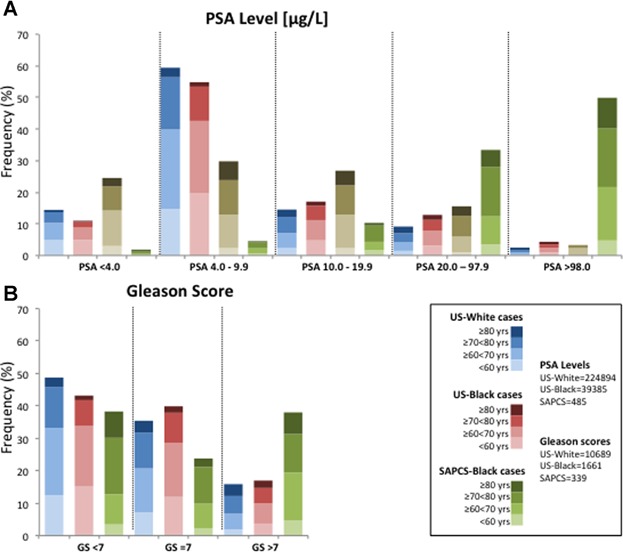
Clinical presentation of prostate cancer in the SAPCS cases (green) and urological controls (brown) relative to White (blue) and Black (red) populations sourced from the US-based SEER 18 Registries, with appropriate age distributions depicted as increasing color intensity with age. A: The distribution of serum PSA levels between US and SAPCS case samples for all serum PSA levels tested (PSA < 4 µg/L, PSA = 4.0–9.9 µg/L, PSA = 20–97.9 µg/L and PSA ≥ 98 µg/L). B: Gleason score (GS) distribution in SEER case samples and the SAPCS case samples for each GS < 7, GS = 7, and GS > 7.
PCa status was defined via histological tissue sampling, either via ultrasound guided prostate biopsy sampling of on average 10–12 cores per patient (69.9%) or trans urethral resection of the prostate (TURP, 6.6%). To-date only nine study participants diagnosed with PCa (1.7%) have undergone radical prostatectomy (RP). Gleason score and tumor grades were available for 346 (60.1%) and 304 (52.8%) cases, respectively. While 35.5% (123/346) of men presented with a Gleason score greater or equal to 8, we observed an increase to 43.6% (151/346) when considering Gleason score 7 (4 + 3). A total of 26% (79/304) of men presented with a poorly differentiated tumor.
Global Perspective
Placing the clinical presentation of the SAPCS into global perspective, we correlate age, PSA levels, and Gleason scores at PCa presentation with currently the largest and most comprehensive publically available dataset for men of European (White) and African ancestry (Black) with PCa, namely the US-based SEER registry. As previously reported for the United States, Black men in the SEER study present with PCa at a younger age than Whites by roughly 3 years, and are more likely to present with a higher PSA level [30]. Age at diagnosis has dramatically reduced over time as a direct consequence of PSA screening, leading to earlier stage at diagnosis. Lack of PSA screening contributes to increased overall age at PCa diagnosis within the SAPCS compared with the SEER study (mean 71.0 years, range 49–101 years) with significant age differences (P = 0.0046) when comparing urban (mean 69.2 years) and rural localities (mean 71.8 years).
While baseline PSA levels can fluctuate from patient to patient [31], as observed within the SEER study, US-White (n = 224,894) and US-Black (n = 39,385) men are more likely to present with a PSA level equal or greater than 4.0 µg/L (85.6% and 89.1%, respectively), which is even more dramatic when considering the SAPCS study (98.1%). We observe a significant increase in frequency of baseline PSA levels equal or greater than 20 µg/L within the SAPCS compared to both US-based populations (Fig. 2A), (both P < 0.0001). In contrast, we observe a significant decrease in frequency of SAPCS cases presenting with baseline PSA levels less than 20 µg/L compared to US populations, although this effect is more dramatic for PSA levels less than 10 µg/L (P < 0.0001) than for baseline PSA ranging between 10 and 19.9 µg/L (P = 0.0111 and P = 0.0001 for comparison with US-White and US-Black SEER cases, respectively).
We observe a significant difference in the distribution of men with a Gleason score equal or greater than 7 between both the US-White (n = 3,785 (35.4%) and 5,481 (15.9%) out of 10,687, respectively) and US-Black cases (n = 662 (39.9%) and 282 (17.0%) out of 1661, respectively) compared to SAPCS (n = 82 (24.6%) and 131 (36.0%) out of 345, respectively) case samples (all P < 0.0001) (Fig. 2B). Alternatively, the US-White (n = 5,206/10,687 (48.7%)) and US-Black (n = 717/1661 (43.2%)) populations are more likely to present with a Gleason score less than 7 compared to the SAPCS (n = 135/342 (39.5%)), although this difference is only significant between the US-White and SAPCS cases samples (P = 0.0009). Additionally, we observe a significant difference in the distribution of age between the different population groups for measures of Gleason score. The proportion of US cases aged <70 years presenting with a GS < 7 or =7 is significantly greater than the proportion of SAPCS men matched for age and Gleason score (P < 0.0001).
Associated Localities and Socio-Demographic Confounders
Significant differences in clinical presentation were noted based on locality, defined as rural Limpopo versus urban Gauteng (Fig. 3). Men from Limpopo presented with more aggressive PCa disease phenotype based on histophathological analysis including Gleason score greater than 7 (P = 0.0042) or poorly differentiated tumor (P < 0.0001). While the median age for men presenting at the urban-based clinics is 3 years earlier than the rural clinics for both the cases (69 and 72 years, respectively) and controls (68 and 71 years, respectively), PSA levels were only slightly increased in the cases from Limpopo (P = 0.0609) with no significant differences noted for the rural versus urban presenting control subjects (P = 0.2866).
Fig 3.
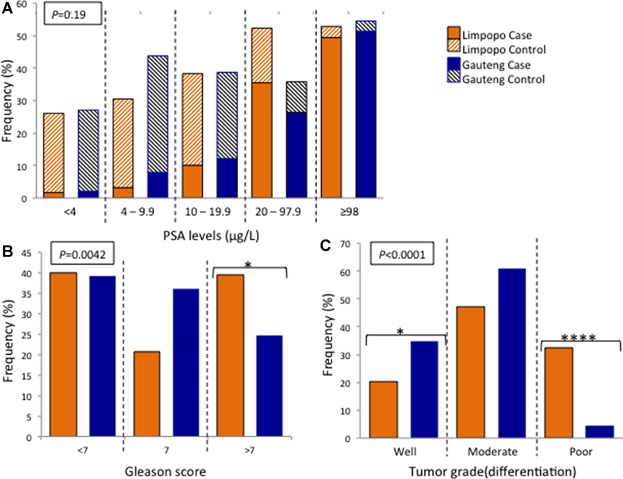
Distribution of clinical prostate cancer characteristics based on collection location and defined as rural (Limpopo, orange) or urban (Gauteng, blue), for (A) serum PSA levels (including both prostate cancer cases and urological controls), (B) Gleason score, and (C) tumor grade.
As well as clinical characteristics, statistics for several epidemiological factors, including demographic, lifestyle, and environmental factors were collected for each study participant. To assess the impact of rural versus urban locality on epidemiological and clinical outcomes, we test for significance with over-all PCa status. Several epidemiological factors reached statistical significance (defined as an un-adjusted P < 0.05) in case–control analysis [27], which we also show to be associated with rural versus urban collection locations, bringing into question the source of association. Such parameters include population group, occupation, present sexual capacity, acne, and aspirin usage. It is possible the observed association with disease status for these measurements is partially attributed to a difference of frequency in Limpopo versus Gauteng (Table 1). Similarly, characteristics associated with disease status but not collection location are more likely to be accurate predictors of disease, including a family history of cancer (excluding PCa), the presence of diabetes, erectile dysfunction, balding pattern, and clinically significant PSA levels. Alternatively several factors were found to be solely associated with collection location, potentially indicative of a confounding factor for future analysis. Such factors include age, allergic conditions and previous exposure to STDs, age of first sexual encounter, presence of chest hair and male breasts, balding age, and consumption of red meat.
Discussion
Although still in collection phase, the SAPCS is the largest and most comprehensive resource currently available for investigating the impact of PCa within Southern Africa. The cohort is constantly evaluated and evolving to address specific limitations and needs, while tailoring study design for an African setting. The study has faced many challenges during almost 6 years since initiation. At the national level these include minimal resources (including available funding to support clinical and nursing staff) and lack of emphasis on age-related diseases. Within more rural located clinics challenges include vast distances to the nearest clinic, shortage of trained urologists per capita, as well as significant limitations in essential resources and infrastructure. The latter was highlighted in the past year when medical supplies required for routine PCa screening was terminated to the most rural province of South Africa and largest contributor to this study. The team has overcome these challenges generating a unique resource and foundation for further development and regional expansion, with the goal to contribute significantly to defining global disparities in PCa and observed link to African-ancestry. An overwhelming lack of African-based PCa studies calls for regular data driven assessment of the SAPCS. Additionally, as genomic diversity is greatest within Africa we assess the genetic substructure of the SAPCS representative populations as potential confounders of genetic associations, while exclude for non-African contributions. We address the clinical presentation of PCa within South Africa, including comparative analysis between urban and rural locality.
Studies associating African-ancestry with PCa risk and adverse outcomes have been performed in African American men shown to have on average a European ancestral contribution of up to 20% [23]. The African ancestral contribution to African Americans has more recently been defined as predominantly of west African origin (82%), specifically 63% defined by the proto-Bantu Yoruba speakers and 19% by the more westerly non-Bantu speaking Mandinka people [32]. To truly understand the link to African-ancestry observed within African Americans it is essential that non-migrant African populations are included within PCa studies, both within and outside the African-ancestral contributions to African American people. Analysis of the genetic constitution of the Southern Bantu peoples represented within the SAPCS, defines a pure African derived population lacking significant non-African contribution. Having migrated from the proto-Bantu homeland (believed to be in the Nigerian-Cameroon region) no earlier than 5,000 years ago marking the estimated beginnings of Bantu expansion [33], the Southern Bantu are believed to have migrated down the eastern coast of Africa making their home in present day South Africa beginning 1,500 years ago [34]. Our data suggests that the Southern Bantu peoples represent a unique Bantu-derived ancestral cluster, independent from the Eastern Bantu and Western Bantu peoples, while lacking non-Bantu West African ancestral contributions. The latter in contrast to African American ancestral contributions. The SAPCS allows not only for a pure African-based dataset (non African contribution) but also importantly provides an independent ancestral alternative to African American studies. Preliminary studies from our group have shown that known PCa risk alleles are not predictive within the SAPCS and we call for genome-wide approaches to identify African-specific risk alleles [27].
Clinical diagnosis is significantly impacted by lack of clinical screening and PCa awareness. While PSA testing has been routine practice in most western countries for around 20 years, this is not the case within the northerly regions of South Africa. This is likely contributing to the roughly 5 years earlier median age for diagnosis of PCa in the United States (66 years based on the SEER report 2006–2010, all races/ethnic groups; http://www.seer.cancer/gov/statfacts) compared to the SAPCS. Interestingly, a previous study performed in the Western Cape region of South Africa reported the mean age of PCa presentation to be significantly higher in Whites (n = 291; 69.7 years) compared with regionally-matched Blacks (n = 71; 68.9 years) [18]. The significantly older age at PCa presentation in the SAPCS (71.0 years) may be driven by lack of PSA testing and reflects more closely that reported in Uganda (n = 210; 70.6 years) [35]. Remarkably, only 3% of the men in the SAPCS presented as a result of a known elevated PSA. Additionally, 42.4% (350/825) of men reported that their primary health care was based on traditional and not western medical practices. Clinical presentation was predominantly associated with urinary flow issues, specifically difficulty passing urine. The only clinical presentation in our study associated with PCa status and therefore a likely symptom for PCa was a report of pain or burning during urination (dysuria). Although men without any clinically defined PCa, presented with a median age only 1 year younger than men with PCa, PSA levels at time of diagnosis were significantly elevated for cases with the vast majority, specifically 83.0%, presenting with a PSA greater or equal to 20 µg/L. This concurs with elevated serum PSA levels at PCa presentation reported for Black South Africans compared with non-Black men from the Western Cape region [18]. In contrast 81.5% of the SAPCS urological controls presented with a PSA less than 20 µg/L. As men are population-matched, it is unlikely that the extreme elevation in PSA levels observed within the cases from this study is purely population-specific and more likely indicative of presentation at diagnosis of more aggressive disease. This is further supported by the observation that PSA levels below 4.0 µg/L were significantly increased in the SAPCS control group compared to both US-White and US-Black patients at time of diagnosis (P < 0.0001).
Histopathological evidence for aggressive PCa disease at presentation in Southern Africa men includes the observation of increased Gleason scores and poor tumor differentiation. In affluent countries most men will present at diagnosis with a Gleason score of 7 or 6. Gleason scores greater than 7 are reported for 15.9% of US-White and 17% of US-Black cases (SEER study), while slightly higher frequencies have been reported in the UK, specifically 22% UK-White and 21% UK-Black PCa cases [36]. In contrast 35.5% of the SAPCS present with a Gleason score greater than 7, further supported by the observation of poorly differentiated tumors in 26% of the cases. Although Gleason score distribution between African nations is reportedly varied, there is a trend towards a higher percentage of men presenting with a Gleason score greater than 7, specifically 62% Sudan (n = 234), 46% Uganda (n = 197), 31% Botswana (n = 13), 30% Western Cape South Africa (n = 155), and 26% Ghana (n = 440) [35]. In the same study, the presentation of White South African men with a Gleason score greater than 7 was reportedly 28% (n = 158). Treatment is also significantly impacted by disease presentation. Removal of the prostate is arguably the “gold standard” for treating localized PCa in the presence of negative surgical margins. However, less than 2% of patients within the SAPCS have undergone RP. While curative therapy in the form of RP/radiotherapy was reported for 12% of Black South African patients within the Western Cape study [18], the mean age of RP in the United States at around 63 years [37] is at least 8 years prior to average clinic presentation for the SAPCS. One can speculate that socioeconomic factors, such as PSA screening, is a significant driver of disparities in clinical appearance of PCa both within South Africa and across the globe, calling for caution when making comparative analyses between the SAPCS and SEER study.
Assessing for rural versus urban localities within the SAPCS, we found a significant increase in aggressive presenting disease defined by Gleason score and tumor differentiation for men residing in the population-matched rural setting. Age at presentation was, however, 3 years older in Limpopo, once again likely indicative of lack of PCa awareness and screening within rural communities. After adjusting for age, the observed association between Gleason score and clinic location was lost, however, the association with tumor grade was maintained. We see a significant decrease in the less aggressive, well differentiated tumor in the more rural setting of Limpopo compared to Gauteng, with a risk ratio of 0.06 and 95% CI = 0.005–0.726 (P = 0.03). And although limited by sample size (n = 76 and 3 for Limpopo and Gauteng, respectively), we observe a significant increase in the aggressive, poorly differentiated tumor in Limpopo with a risk ratio of 13.9 95% CI = 4.50–61.15 (P = 0.006).
Conclusions
The presentation of highly aggressive PCa disease (tumor grade) and elevated serum PSA levels at time of diagnosis within Black men from the most northerly regions of South Africa concurs with previous studies focused on populations from the most southerly region of the country [18,35], which is further compounded when considering clinical presentation for Black men from western countries. We further show that Black men from the rurally located Province of Limpopo are on average 2.6 and 2.9 years older at time of PCa diagnosis than Black men Gauteng and Western Cape, respectively. As a consequence, curative treatment for PCa is severely limited within the study region. We postulate that the lack of PCa awareness and PSA screening are the largest contributors to presentation of aggressive PCa disease within rural African communities.
Acknowledgments
We thank the University of Limpopo for facilitating and supporting this research project and the Garvan Institute of Medical Research for hosting the SAPCS webpage and database. The authors are grateful to the study participants, the study nurses Mrs. G.W. Schulenburg (University of Limpopo) and Sr. H.M. Mahango (Polokwane Clinic), and Dr. Munro Marx and Unistel Medical Laboratories (South Africa) for providing DNA extractions. This research was supported by grants from the Medical Research Council (MRC) of South Africa (to MSRB, PAV and VMH), Unistel Medical Laboratories, South Africa (to PAV and VMH), the Cancer Institute of New South Wales, Australia (to EAT and VMH), National Health and Medical Research Council of Australia (Fellowship to EAT), National Institute of Health (NIH) Grant #CA170081 (to VMH), the J. Craig Venter Family Fund, CA, U.S.A (to VMH) and the Petre Foundation Australia (to VMH).
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Delongchamps NB, Singh A, Haas GP. Epidemiology of prostate cancer in Africa: Another step in the understanding of the disease. Curr Probl Cancer. 2007;31(3):226–236. doi: 10.1016/j.currproblcancer.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H, Part I. Cancer in Indigenous Africans–burden, distribution, and trends. Lancet Oncol. 2008;9(7):683–692. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- 5.Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183(5):1792–1796. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters N, Armstrong K. Racial differences in prostate cancer treatment outcomes: A systematic review. Cancer Nurs. 2005;28(2):108–118. doi: 10.1097/00002820-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. The worldwide epidemiology of prostate cancer: Perspectives from autopsy studies. Can J Urol. 2008;15(1):3866–3871. [PMC free article] [PubMed] [Google Scholar]
- 8.Bigler SA, Pound CR, Zhou X. A retrospective study on pathologic features and racial disparities in prostate cancer. Prostate Cancer. 2011;2011:239460. doi: 10.1155/2011/239460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YC, Page JH, Chen R, Giovannucci E. Family history of prostate and breast cancer and the risk of prostate cancer in the PSA era. Prostate. 2008;68(14):1582–1591. doi: 10.1002/pros.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas JA, Gerber L, Moreira DM, Hamilton RJ, Bañez LL, Castro-Santamaria R, Andriole GL, Isaacs WB, Xu J, Freedland SJ. Prostate cancer risk in men with prostate and breast cancer family history: Results from the REDUCE study (R1) J Intern Med. 2012;272(1):85–92. doi: 10.1111/j.1365-2796.2011.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 12.Cnattingius S, Lundberg F, Sandin S, Grönberg H, Iliadou A. Birth characteristics and risk of prostate cancer: The contribution of genetic factors. Cancer Epidemiol Biomarkers Prev. 2009;18(9):2422–2426. doi: 10.1158/1055-9965.EPI-09-0366. [DOI] [PubMed] [Google Scholar]
- 13.Martin DN, Starks AM, Ambs S. Biological determinants of health disparities in prostate cancer. Curr Opin Oncol. 2013;25(3):235–241. doi: 10.1097/CCO.0b013e32835eb5d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odedina FT, Akinremi TO, Chinegwundoh F, Roberts R, Yu D, Reams RR, Freedman ML, Rivers B, Green BL, Kumar N. Prostate cancer disparities in Black men of African descent: A comparative literature review of prostate cancer burden among Black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer. 2009;4:S2. doi: 10.1186/1750-9378-4-S1-S2. (Suppl 1): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akinremi TO, Ogo CN, Olutunde AO. Review of prostate cancer research in Nigeria. Infect Agent Cancer. 2011;6:S8. doi: 10.1186/1750-9378-6-S2-S8. (Suppl 2): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabore FA, Zango B, Sanou A, Yameogo C, Kirakoya B. Prostate cancer outcome in Burkina Faso. Infect Agent Cancer. 2011;6:S6. doi: 10.1186/1750-9378-6-S2-S6. (Suppl 2): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasike RW, Magoha GA. Descriptive case series of patients presenting with cancer of the prostate and their management at Kenyatta National Hospital, Nairobi. East Afr Med J. 2007;84:S31–S35. doi: 10.4314/eamj.v84i9.9559. (9 Suppl): [DOI] [PubMed] [Google Scholar]
- 18.Heyns CF, Fisher M, Lecuona A, van der Merwe A. Prostate cancer among different racial groups in the Western Cape: Presenting features and management. S Afr Med J. 2011;101(4):267–270. doi: 10.7196/samj.4420. [DOI] [PubMed] [Google Scholar]
- 19.Rebbeck TR, Devesa SS, Chang BL, Bunker CH, Cheng I, Cooney K, Eeles R, Fernandez P, Giri VN, Gueye SM, Haiman CA, Henderson BE, Heyns CF, Hu JJ, Ingles SA, Isaacs W, Jalloh M, John EM, Kibel AS, Kidd LR, Layne P, Leach RJ, Neslund-Dudas C, Okobia MN, Ostrander EA, Park JY, Patrick AL, Phelan CM, Ragin C, Roberts RA, Rybicki BA, Stanford JL, Strom S, Thompson IM, Witte J, Xu J, Yeboah E, Hsing AW, Zeigler-Johnson CM. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of african descent. Prostate Cancer. 2013;2013:560857. doi: 10.1155/2013/560857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henn BM, Cavalli-Sforza LL, Feldman MW. The great human expansion. Proc Natl Acad Sci USA. 2012;109(44):17758–17764. doi: 10.1073/pnas.1212380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster SC, Miller W, Ratan A, Tomsho LP, Giardine B, Kasson LR, Harris RS, Petersen DC, Zhao F, Qi J, Alkan C, Kidd JM, Sun Y, Drautz DI, Bouffard P, Muzny DM, Reid JG, Nazareth LV, Wang Q, Burhans R, Riemer C, Wittekindt NE, Moorjani P, Tindall EA, Danko CG, Teo WS, Buboltz AM, Zhang Z, Ma Q, Oosthuysen A, Steenkamp AW, Oostuisen H, Venter P, Gajewski J, Zhang Y, Pugh BF, Makova KD, Nekrutenko A, Mardis ER, Patterson N, Pringle TH, Chiaromonte F, Mullikin JC, Eichler EE, Hardison RC, Gibbs RA, Harkins TT, Hayes VM. Complete Khoisan and Bantu genomes from southern Africa. Nature. 2010;463(7283):943–947. doi: 10.1038/nature08795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parra EJ, Marcini A, Akey J, Martinson J, Batzer MA, Cooper R, Forrester T, Allison DB, Deka R, Ferrell RE, Shriver MD. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet. 1998;63(6):1839–1851. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tishkoff SA, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, Froment A, Hirbo JB, Awomoyi AA, Bodo JM, Doumbo O, Ibrahim M, Juma AT, Kotze MJ, Lema G, Moore JH, Mortensen H, Nyambo TB, Omar SA, Powell K, Pretorius GS, Smith MW, Thera MA, Wambebe C, Weber JL, Williams SM. The genetic structure and history of Africans and African Americans. Science. 2009;324(5930):1035–1044. doi: 10.1126/science.1172257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63(6):963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control. 2007;14(1):78–85. doi: 10.1177/107327480701400111. [DOI] [PubMed] [Google Scholar]
- 26.Lehohla P. In: Census2011 Census in brief, Statistics South Africa. Africa SS, editor. Pretoria, South Africa: Statistics South Africa; 2012. p. 105. In: editor. [Google Scholar]
- 27.Tindall EA, Bornman MR, van Zyl S, Segone AM, Monare LR, Venter PA, Hayes VM. Addressing the contribution of previously described genetic and epidemiological risk factors associated with increased prostate cancer risk and aggressive disease within men from South Africa. BMC Urol. 2013;13:74. doi: 10.1186/1471-2490-13-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henn BM, Gignoux CR, Jobin M, Granka JM, Macpherson JM, Kidd JM, Rodríguez-Botigué L, Ramachandran S, Hon L, Brisbin A, Lin AA, Underhill PA, Comas D, Kidd KK, Norman PJ, Parham P, Bustamante CD, Mountain JL, Feldman MW. Hunter-gatherer genomic diversity suggests a southern African origin for modern humans. Proc Natl Acad Sci USA. 2011;108(13):5154–5162. doi: 10.1073/pnas.1017511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao YH, Demissie K, Shih W, Mehta AR, Stein MN, Roberts CB, Dipaola RS, Lu-Yao GL. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101(18):1280–1283. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA. Prevalence of prostate cancer among men with a prostate-specific antigen level <or =4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 32.Zakharia F, Basu A, Absher D, Assimes TL, Go AS, Hlatky MA, Iribarren C, Knowles JW, Li J, Narasimhan B, Sidney S, Southwick A, Myers RM, Quertermous T, Risch N, Tang H. Characterizing the admixed African ancestry of African Americans. Genome Biol. 2009;10(12):R141. doi: 10.1186/gb-2009-10-12-r141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diamond J, Bellwood P. Farmers and their languages: The first expansions. Science. 2003;300(5619):597–603. doi: 10.1126/science.1078208. [DOI] [PubMed] [Google Scholar]
- 34.Newman JL. The peopling of Africa: A geographic interpretation. USA: Yale University Press; 1995. p. 241. p. [Google Scholar]
- 35.Jalloh M, Friebel TM, Sira Thiam F, Niang L, Sy C, Siby T, Fernandez P, Mapulanga V, Maina Doodu S, Mante S, Yeboah E, Kyei M, Ankomah R, Amegbor J, Adusei B, Yegbe P, Watya S, Kaggwa S, Haiman C, Henderson BE, Narashimhamurthy M, Abuidris D, Mohamadani AA, Mohamed E, Mansoor MO, Elgaili EM, Elballal A, Zeigler-Johnson CM, Heyns CF, Gueye SM, Rebbeck TR. Evaluation of 4,672 routine prostate biopsies performed in six African countries. J Afr Cancer. 2013;5(3):144–154. [Google Scholar]
- 36.Evans S, Metcalfe C, Patel B, Ibrahim F, Anson K, Chinegwundoh F, Corbishley C, Gillatt D, Kirby R, Muir G, Nargund V, Popert R, Wilson P, Persad R, Ben-Shlomo Y. Clinical presentation and initial management of black men and white men with prostate cancer in the United Kingdom: The PROCESS cohort study. Br J Cancer. 2010;102(2):249–254. doi: 10.1038/sj.bjc.6605461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollenbeck BK, Dunn RL, Miller DC, Daignault S, Taub DA, Wei JT. Volume-based referral for cancer surgery: Informing the debate. J Clin Oncol. 2007;25(1):91–96. doi: 10.1200/JCO.2006.07.2454. [DOI] [PubMed] [Google Scholar]


